Affinity Membranes and Monoliths for Protein Purification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Chromatographic Materials
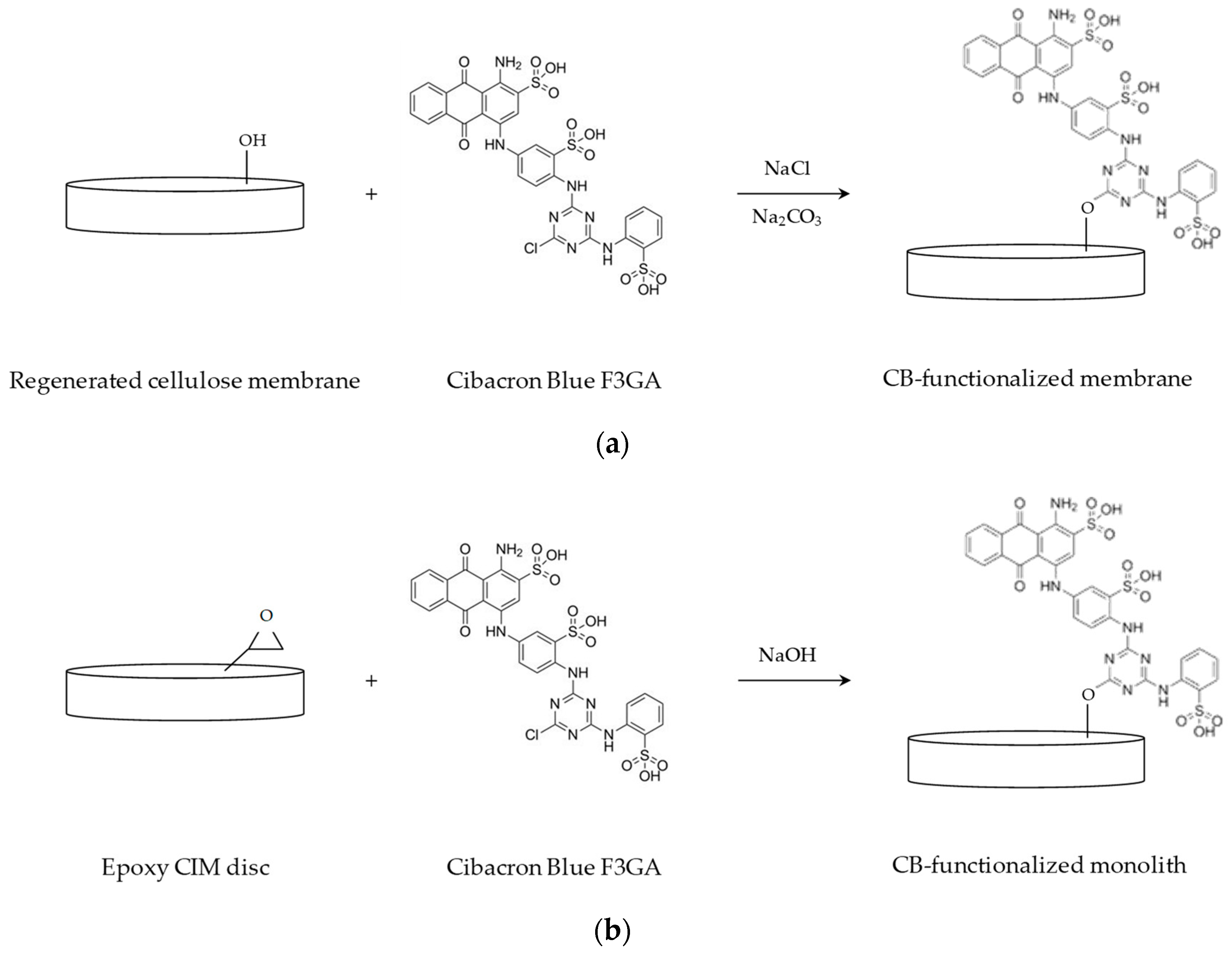
2.2. Membrane Functionalization
2.3. Monolith Functionalization
2.4. Chromatographic Materials Characterization
3. Results and Discussion
3.1. Membrane Ligand Density
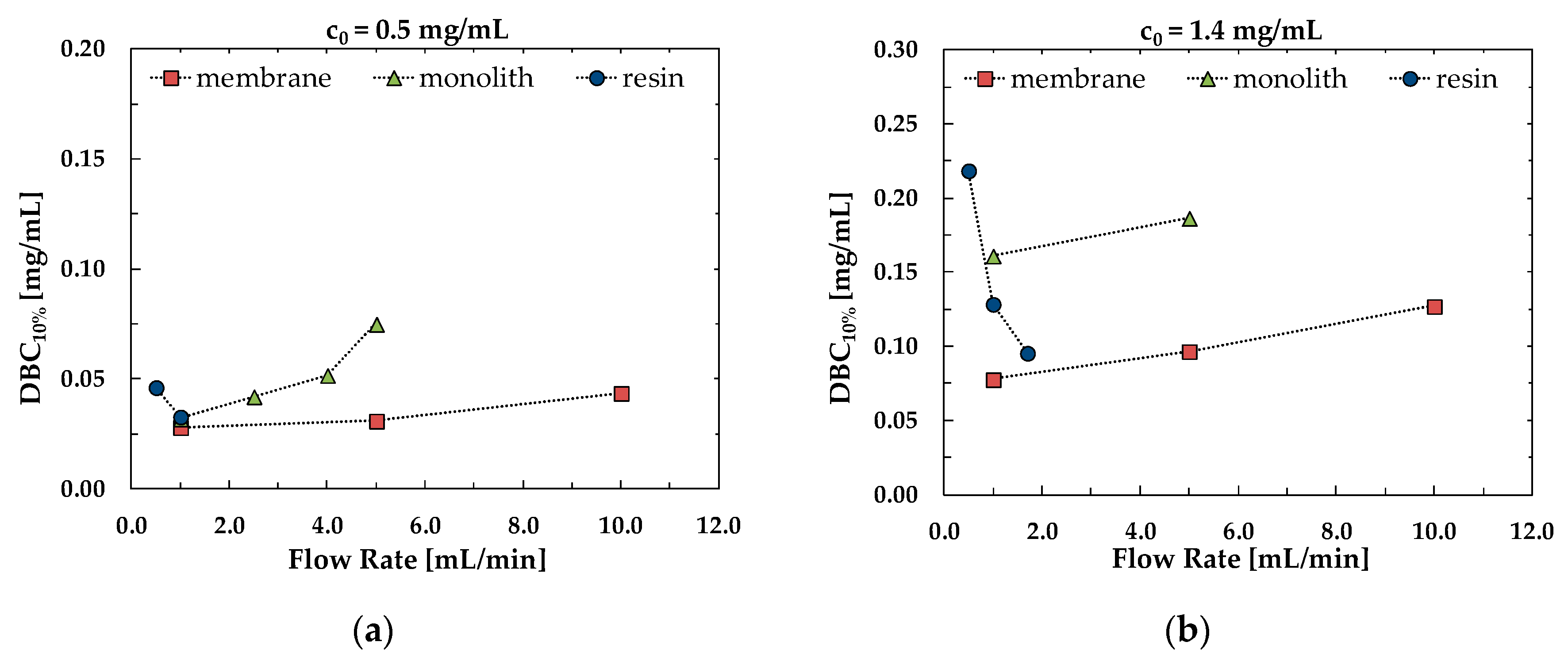
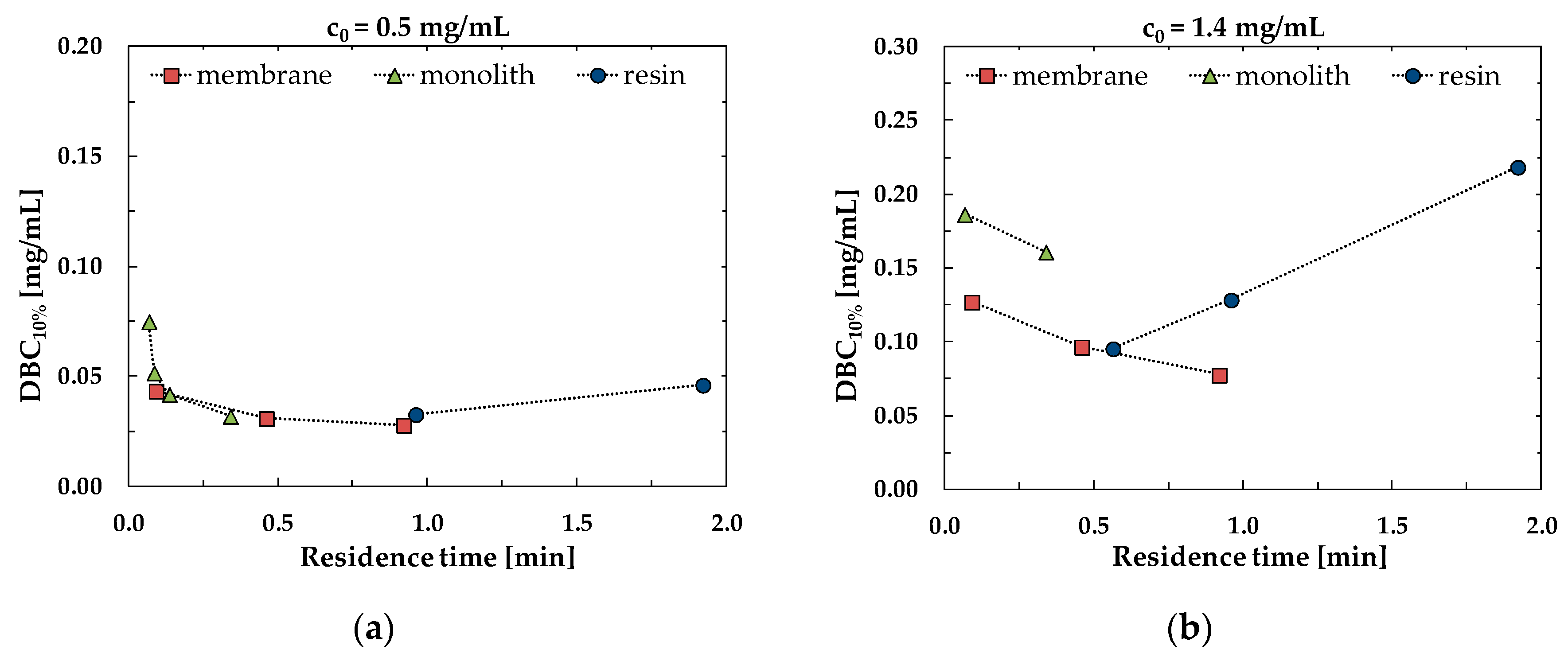
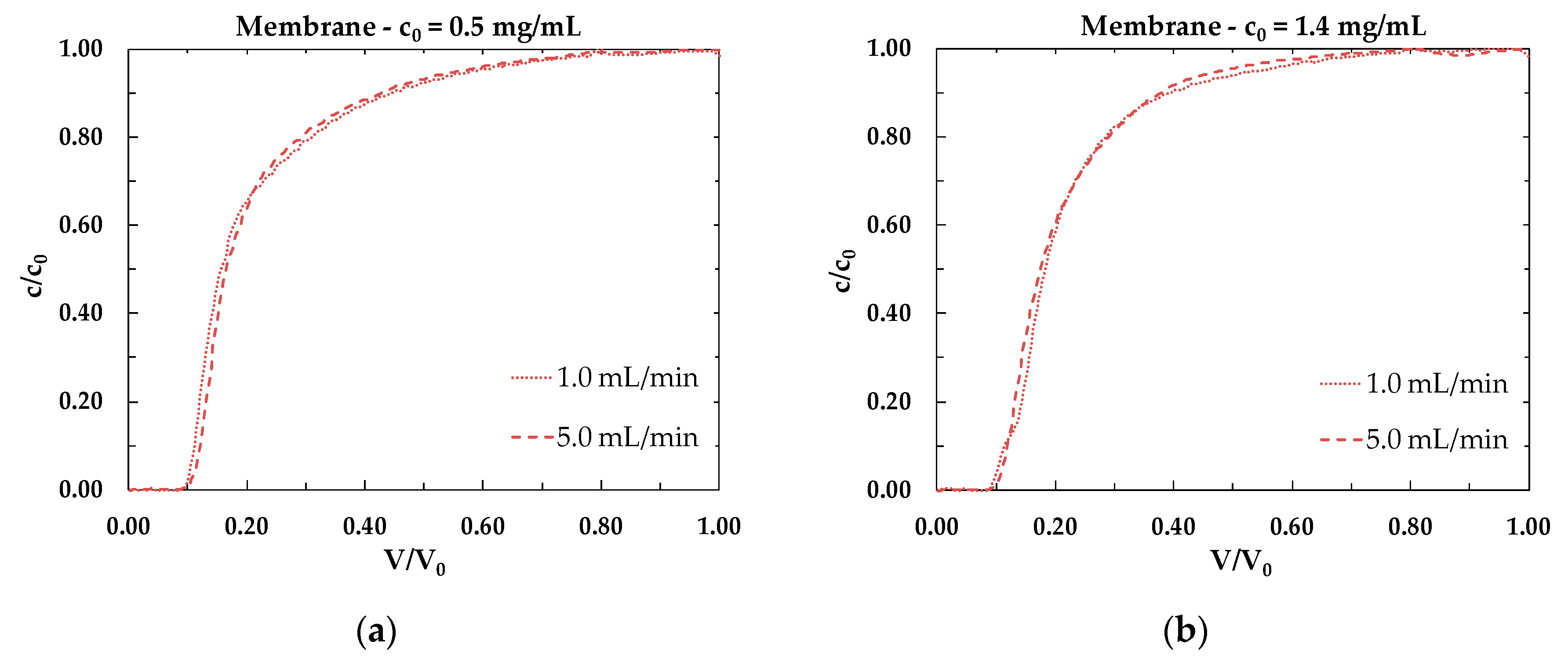
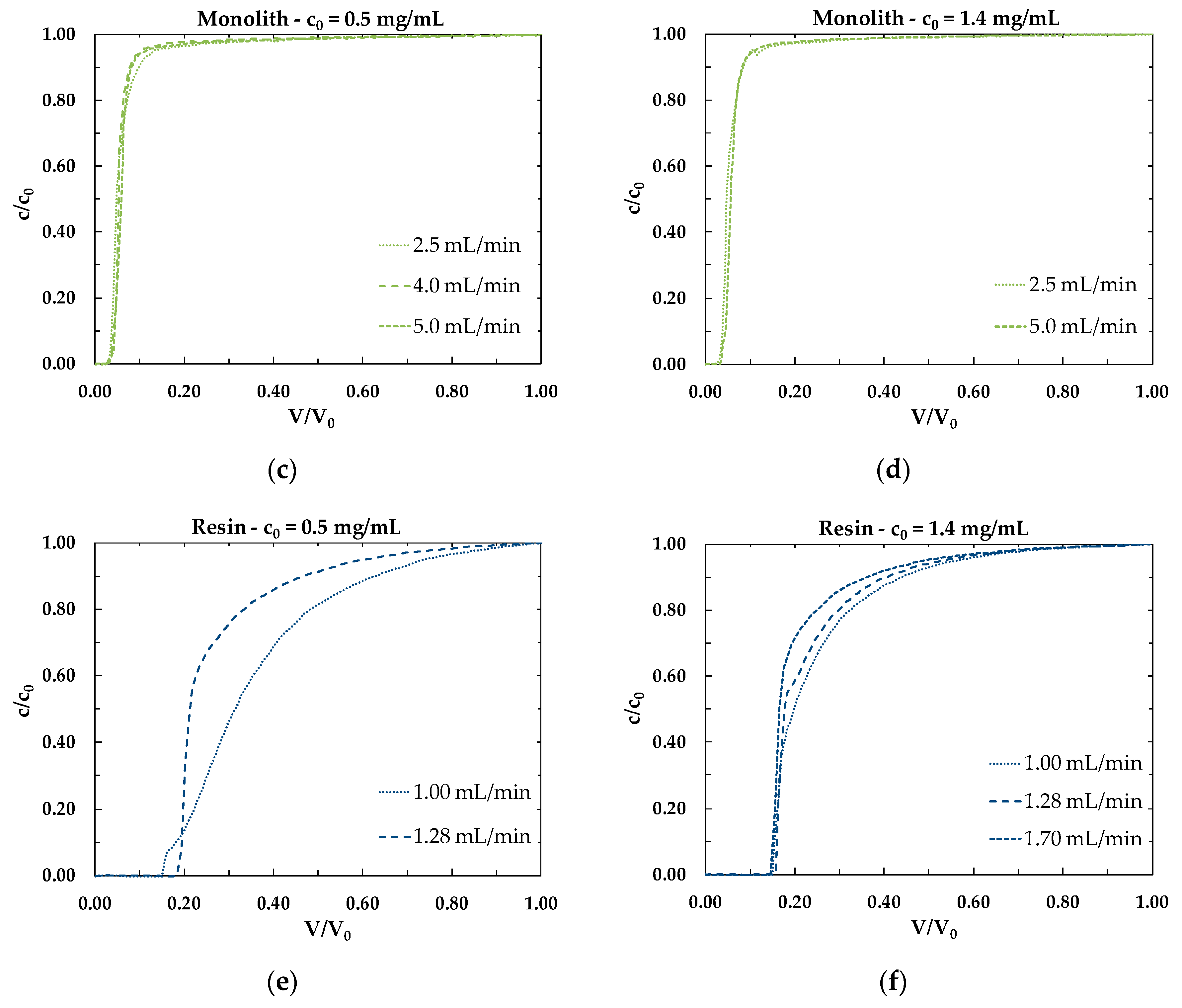
3.2. Dynamic Binging Capacity
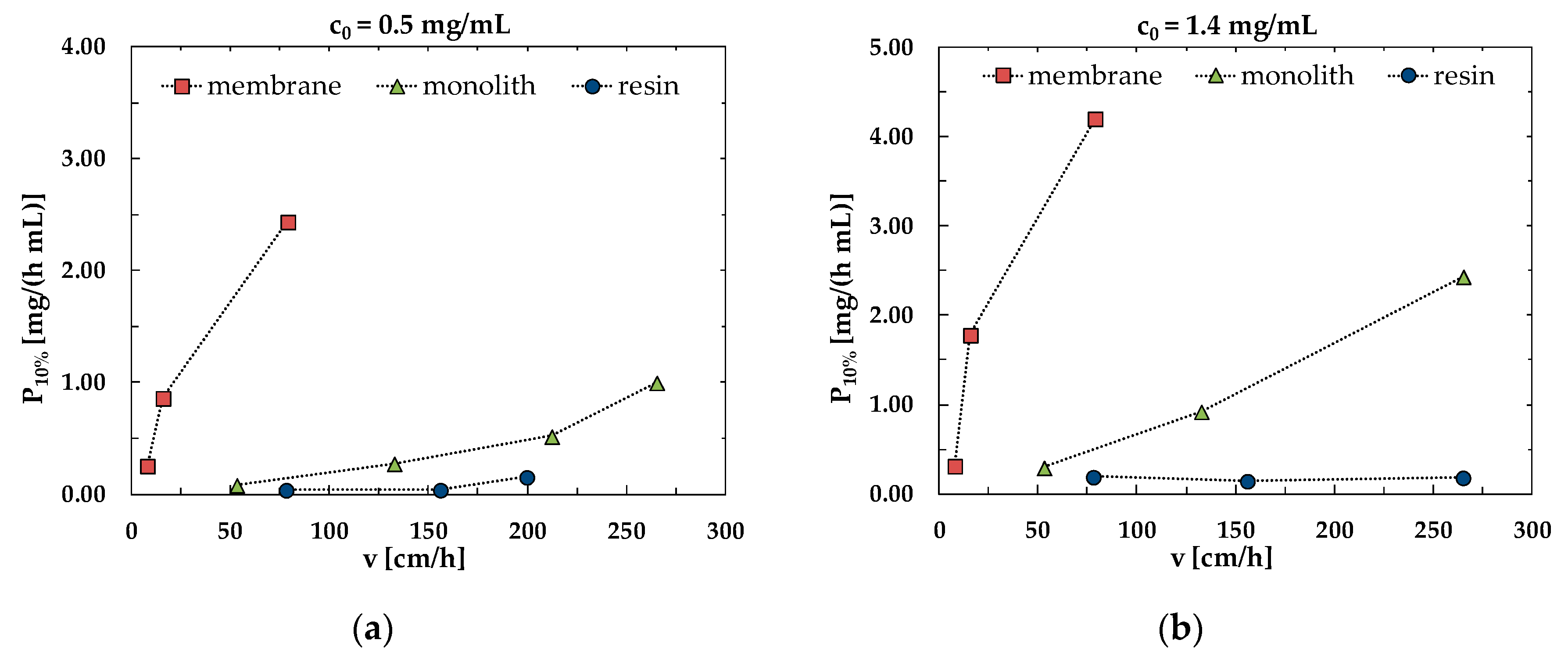
3.3. Productivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schügerl, K. Separation and Purification Techniques in Biotechnology; Chemie Ing. Tech.: Berlin, Germany, 1990. [Google Scholar]
- van Reis, R.; Zydney, A. Bioprocess membrane technology. J. Membr. Sci. 2007, 297, 16–50. [Google Scholar] [CrossRef]
- Tejeda-Mansir, A.; Montesinos, R.M.; Guzmán, R. Mathematical analysis of frontal affinity chromatography in particle and membrane configurations. J. Biochem. Biophys. Methods 2001, 49, 1–28. [Google Scholar] [CrossRef]
- Afeyan, N.B.; Gordon, N.F.; Mazsaroff, I.; Varady, L.; Fulton, S.P.; Yang, Y.B.; Regnier, F.E. Flow-through particles for the high-performance liquid chromatographic separation of biomolecules: Perfusion chromatography. J. Chromatogr. A 1990, 519, 1–29. [Google Scholar] [CrossRef]
- Boi, C. Membrane adsorbers as purification tools for monoclonal antibody purification. J. Chromatogr. B 2007, 848, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Umatheva, U.; Alforque, L.; Shirataki, H.; Ogawa, S.; Kato, C.; Ghosh, R. An annular-flow, hollow-fiber membrane chromatography device for fast, high-resolution protein separation at low pressure. J. Membr. Sci. 2019, 590, 117305. [Google Scholar] [CrossRef]
- Ye, J.; Wang, X.; Chu, J.; Yao, D.; Zhang, Y.; Meng, J. Electrospun poly(styrene-co-maleic anhydride) nanofibrous membrane: A versatile platform for mixed mode membrane adsorbers. Appl. Surf. Sci. 2019, 484, 62–71. [Google Scholar] [CrossRef]
- Dimartino, S. Studio Sperimentale e Modellazione della Separazione di Proteine con Membrane di Affinità. Ph.D. Thesis, University of Bologna, Bologna, Italy, 2009. [Google Scholar]
- Ghosh, R. Protein separation using membrane chromatography: Opportunities and challenges. J. Chromatogr. A 2002, 952, 13–27. [Google Scholar] [CrossRef]
- Ramos-de-la-Peña, A.M.; González-Valdez, J.; Aguilar, O. Protein A chromatography: Challenges and progress in the purification of monoclonal antibodies. J. Sep. Sci. 2019, 42, 1816–1827. [Google Scholar] [CrossRef]
- Zou, H.; Luo, Q.; Zhou, D. Affinity membrane chromatography for the analysis and purification of proteins. J. Biochem. Biophys. Methods 2001, 49, 199–240. [Google Scholar] [CrossRef]
- Madadkar, P.; Mahansaria, R.; Mukherjee, J.; Ghosh, R. Enhancing the efficiency of disc membrane chromatography modules by using a flow directing layer. J. Membr. Sci. 2019, 580, 154–160. [Google Scholar] [CrossRef]
- Boi, C. Membrane chromatography for biomolecule purification. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Charcosset, C., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Chapter 6; ISBN 9780128136065. [Google Scholar]
- Brämer, C.; Ekramzadeh, K.; Lammers, F.; Scheper, T.; Beutel, S. Optimization of continuous purification of recombinant patchoulol synthase from Escherichia coli with membrane adsorbers. Biotechnol. Prog. 2019. [Google Scholar] [CrossRef] [PubMed]
- Roper, D.K.; Lightfoot, E.N. Separation of biomolecules using adsorptive membranes. J. Chromatogr. A 1995, 702, 3–26. [Google Scholar] [CrossRef]
- Yang, H.; Viera, C.; Fischer, J.; Etzel, M.R. Purification of a large protein using ion-exchange membranes. Ind. Eng. Chem. Res. 2002, 41, 1597–1602. [Google Scholar] [CrossRef]
- Endres, H.N.; Johnson, J.A.C.; Ross, C.A.; Welp, J.K.; Etzel, M.R. Evaluation of an ion-exchange membrane for the purification of plasmid DNA. Biotechnol. Appl. Biochem. 2003, 37, 259–266. [Google Scholar] [CrossRef]
- Velali, E.; Stute, B.; Leuthold, M.; von Lieres, E. Model-based performance analysis and scale-up of membrane adsorbers with a cassettes format designed for parallel operation. Chem. Eng. Sci. 2018, 192, 103–113. [Google Scholar] [CrossRef]
- Boi, C.; Dimartino, S.; Sarti, G.C. Performance of a new protein A affinity membrane for the primary recovery of antibodies. Biotechnol. Prog. 2008, 24, 640–647. [Google Scholar] [CrossRef]
- Yu, D.; McLean, M.D.; Hall, J.C.; Ghosh, R. Purification of a human immunoglobulin G1 monoclonal antibody from transgenic tobacco using membrane chromatographic processes. J. Chromatogr. A 2008, 1187, 128–137. [Google Scholar] [CrossRef]
- Ma, Z.; Ramakrishna, S. Electrospun regenerated cellulose nanofiber affinity membrane functionalized with protein A/G for IgG purification. J. Membr. Sci. 2008, 319, 23–28. [Google Scholar] [CrossRef]
- Vogg, S.; Müller-Späth, T.; Morbidelli, M. Current status and future challenges in continuous biochromatography. Curr. Opin. Chem. Eng. 2018, 22, 138–144. [Google Scholar] [CrossRef]
- Vlakh, E.G.; Tennikova, T.B. Preparation of methacrylate monoliths. J. Sep. Sci. 2007, 30, 2801–2813. [Google Scholar] [CrossRef]
- Xie, S.; Allington, R.W.; Fréchet, J.M.J.; Svec, F. Porous polymer monoliths: An alternative to classical beads. Adv. Biochem. Eng. Biotechnol. 2002, 76, 87. [Google Scholar] [PubMed]
- Bedair, M.; El Rassi, Z. Affinity chromatography with monolithic capillary columns: I. Polymethacrylate monoliths with immobilized mannan for the separation of mannose-binding proteins by capillary electrochromatography and nano-scale liquid chromatography. J. Chromatogr. A 2004, 1044, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, A.; Hahn, R. Polymethacrylate monoliths for preparative and industrial separation of biomolecular assemblies. J. Chromatogr. A 2008, 1184, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Podgornik, A.; Krajnc, N.L. Application of monoliths for bioparticle isolation. J. Sep. Sci. 2012, 35, 3059–3072. [Google Scholar] [CrossRef] [PubMed]
- Etzel, M.R. Layered stacks. In Journal of Chromatography Library Vol 67, Monolithic Materials: Preparation, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2003; pp. 213–234. ISBN 9780199682676. [Google Scholar]
- Sproß, J.; Sinz, A. Monolithic media for applications in affinity chromatography. J. Sep. Sci. 2011, 34, 1958–1973. [Google Scholar] [CrossRef] [PubMed]
- Guichon, G.; Felinger, A.; Shirazi, D.G.; Katti, A.M. Fundamentals of Preparative and Nonlinear Chromatography; Elsevier: San Diego, CA, USA, 2006. [Google Scholar]
- Brandt, S.; Goffe, R.A.; Kessler, S.B.; O’Connor, J.L.; Zale, S.E. Membrane-based affinity technology for commercial scale purifications. Nat. Biotechnol. 1988, 6, 779–782. [Google Scholar] [CrossRef]
- Strancar, A.; Podgornik, A.; Barut, M.; Necina, R. Short monolithic columns as stationary phases for biochromatography. Adv. Biochem. Eng. Biotechnol. 2002, 76, 49–85. [Google Scholar]
- Svec, F.; Tennikova, T.B.; Deyl, Z. Monolithic Materials: Preparation, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2003; ISBN 9780874216561. [Google Scholar]
- Podgornik, A.; Štrancar, A. Convective Interaction Media® (CIM)—Short layer monolithic chromatographic stationary phases. Biotechnol. Annu. Rev. 2005, 11, 281–333. [Google Scholar]
- Hahn, R.; Panzer, M.; Hansen, E.; Mollerup, J.; Jungbauer, A. Mass transfer properties of monoliths. Sep. Sci. Technol. 2002, 37, 1545–1565. [Google Scholar] [CrossRef]
- Urthaler, J.; Schlegl, R.; Podgornik, A.; Strancar, A.; Jungbauer, A.; Necina, R. Application of monoliths for plasmid DNA purification: Development and transfer to production. J. Chromatogr. A 2005, 1065, 93–106. [Google Scholar] [CrossRef]
- Orr, V.; Zhong, L.; Moo-Young, M.; Chou, C.P. Recent advances in bioprocessing application of membrane chromatography. Biotechnol. Adv. 2013, 31, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Iberer, G.; Hahn, R.; Jungbauer, A. Monoliths as stationary phases for separation of biopolymers: The fourth generation of chromatography sorbents. LC GC 1999, 11, 998–1005. [Google Scholar]
- Boi, C.; Malavasi, A.; Carbonell, R.G.; Gilleskie, G. A direct comparison between membrane adsorber and packed column chromatography performance. J. Chromatogr. A 2019. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, A. Chromatographic media for bioseparation. J. Chromatogr. A 2005, 1065, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Denizli, A.; Pişkin, E. Dye-ligand affinity systems. J. Biochem. Biophys. Methods 2001, 49, 391–416. [Google Scholar] [CrossRef]
- Lowe, C.R.; Pearson, J.C. Affinity chromatography on immobilized dyes. Methods Enzymol. 1984, 104, 97–113. [Google Scholar] [PubMed]
- Gallant, S.R.; Koppaka, V.; Zecherle, N. Dye ligand chromatography. Methods Mol. Biol. 2007, 421, 61–70. [Google Scholar]
- Scopes, R.K. Strategies for enzyme isolation using dye-ligand and related adsorbents. J. Chromatogr. B Biomed. Sci. Appl. 1986, 376, 131. [Google Scholar] [CrossRef]
- Nash, D.C.; Chase, H.A. Modification of polystyrenic matrices for the purification of proteins II. Effect of the degree of glutaraldehyde-poly(vinyl alcohol) crosslinking on various dye ligand chromatography systems. J. Chromatogr. A 1997, 776, 55–63. [Google Scholar] [CrossRef]
- Boyer, P.M.; Hsu, J.T. Effects of ligand concentration on protein adsorption in dye-ligand adsorbents. Chem. Eng. Sci. 1992, 47, 241–251. [Google Scholar] [CrossRef]
- Zeng, X.; Ruckenstein, E. Supported chitosan-dye affinity membranes and their protein adsorption. J. Membr. Sci. 1996, 117, 271–278. [Google Scholar]
- Andac, C.A.; Andac, M.; Denizli, A. Predicting the binding properties of cibacron blue F3GA in affinity separation systems. Int. J. Biol. Macromol. 2007, 41, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Sundaram, T.K.; Kernick, M.; Wilkinson, A.E. Purification of bacterial malate dehydrogenases by selective elution from a triazinyl dye affinity column. Biochim. Biophys. Acta BBA Protein Struct. Mol. Enzymol. 1982, 708, 17–25. [Google Scholar] [CrossRef]
- Boyer, P.M.; Hsu, J.T. Adsorption equilibrium of proteins on a dye-ligand adsorbent. Biotechnol. Tech. 1990, 4, 61–66. [Google Scholar] [CrossRef]
- Müller-Schulte, D.; Manjini, S.; Vijayalakshmi, M.A. Comparative affinity chromatographic studies using novel grafted polyamide and poly(vinyl alcohol) media. J. Chromatogr. A 1991, 539, 307–314. [Google Scholar] [CrossRef]
- Tuncel, A.; Denizli, A.; Purvis, D.; Lowe, C.R.; Pişkin, E. Cibacron Blue F3G-A-attached monosize poly(vinyl alcohol)-coated polystyrene microspheres for specific albumin adsorption. J. Chromatogr. A 1993, 634, 161–168. [Google Scholar] [CrossRef]
- Camli, S.T.; Senel, S.; Tuncel, A. Cibacron blue F3G-A-attached uniform and macroporous poly(styrene-co-divinylbenzene) particles for specific albumin adsorption. J. Biomater. Sci. Polym. Ed. 1999, 10, 875–889. [Google Scholar] [CrossRef]
- Nie, H.L.; Zhu, L.M. Adsorption of papain with Cibacron Blue F3GA carrying chitosan-coated nylon affinity membranes. Int. J. Biol. Macromol. 2007, 40, 261–267. [Google Scholar] [CrossRef]
- Arica, M.Y.; Denizli, A.; Salih, B.; Piskin, E.; Hasirci, V. Catalase adsorption onto Cibacron Blue F3GA and Fe(III)-derivatized poly(hydroxyethyl methacrylate) membranes and application to a continuous system. J. Membr. Sci. 1997, 129, 65–76. [Google Scholar] [CrossRef]
- Suen, S.Y.; Chen, R.L.; Tsai, Y. Da Comparison of lysozyme adsorption to immobilized Cibacron Blue 3GA using various membrane supports. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 223–239. [Google Scholar] [CrossRef]
- Kassab, A.; Yavuz, H.; Odabaşi, M.; Denizli, A. Human serum albumin chromatography by Cibacron Blue F3GA-derived microporous polyamide hollow-fiber affinity membranes. J. Chromatogr. B Biomed. Sci. Appl. 2000, 742, 123–132. [Google Scholar] [CrossRef]
- Champluvier, B.; Kula, M.R. Dye-ligand membranes as selective adsorbents for rapid purification of enzymes: A case study. Biotechnol. Bioeng. 1992, 40, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Uzun, L.; Yavuz, H.; Say, R.; Ersöz, A.; Denizli, A. Poly(ethylene dimethacrylate-glycidyl methacrylate) monolith as a stationary phase in dye-affinity chromatography. Ind. Eng. Chem. Res. 2004, 43, 6507–6513. [Google Scholar] [CrossRef]
- Demiryas, N.; Tüzmen, N.; Galaev, I.Y.; Pişkin, E.; Denizli, A. Poly(acrylamide-allyl glycidyl ether) cryogel as a novel stationary phase in dye-affinity chromatography. J. Appl. Polym. Sci. 2007, 105, 1808–1816. [Google Scholar] [CrossRef]
- Wu, C.Y.; Suen, S.Y.; Chen, S.C.; Tzeng, J.H. Analysis of protein adsorption on regenerated cellulose-based immobilized copper ion affinity membranes. J. Chromatogr. A 2003, 996, 53–70. [Google Scholar] [CrossRef]
- Denizli, A.; Şenel, S.; Arica, M.Y. Cibacron blue F3GA and Cu(II) derived poly (2-hydroxyethylmethacrylate) membranes for lysozyme adsorption. Colloids Surf. B Biointerfaces 1998, 11, 113–122. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Zeng, X. Albumin separation with Cibacron Blue carrying macroporous chitosan and chitin affinity membranes. J. Membr. Sci. 1998, 142, 13–26. [Google Scholar] [CrossRef]
| Packed Column | Membrane | Monolith | |||
|---|---|---|---|---|---|
| d (cm) | h (cm) | d (cm) | h (cm) | d (cm) | h (cm) |
| 0.7 | 2.5 | 2.6 | 0.0240 | 1.2 | 0.3 |
| Packed Column | Membrane | Monolith | ||||||
|---|---|---|---|---|---|---|---|---|
| F (mL/min) | v (cm/h) | τ (min) | F (mL/min) | v (cm/h) | τ (min) | F (mL/min) | v (cm/h) | τ (min) |
| 0.5 | 78.0 | 1.92 | 0.5 | 7.9 | 0.92 | 1.0 | 53.1 | 0.34 |
| 1.0 | 155.9 | 0.96 | 1.0 | 15.8 | 0.46 | 2.5 | 132.6 | 0.14 |
| 1.2 | 199.6 | 0.75 | 5.0 | 78.9 | 0.09 | 4.0 | 212.2 | 0.09 |
| 1.7 | 265.0 | 0.56 | 10.0 | 157.8 | 0.05 | 5.0 | 265.3 | 0.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalli, E.; Silva, J.S.; Boi, C.; Sarti, G.C. Affinity Membranes and Monoliths for Protein Purification. Membranes 2020, 10, 1. https://doi.org/10.3390/membranes10010001
Lalli E, Silva JS, Boi C, Sarti GC. Affinity Membranes and Monoliths for Protein Purification. Membranes. 2020; 10(1):1. https://doi.org/10.3390/membranes10010001
Chicago/Turabian StyleLalli, Eleonora, Jouciane S. Silva, Cristiana Boi, and Giulio C. Sarti. 2020. "Affinity Membranes and Monoliths for Protein Purification" Membranes 10, no. 1: 1. https://doi.org/10.3390/membranes10010001
APA StyleLalli, E., Silva, J. S., Boi, C., & Sarti, G. C. (2020). Affinity Membranes and Monoliths for Protein Purification. Membranes, 10(1), 1. https://doi.org/10.3390/membranes10010001







