Shaping Antitumor Immunity with Peptide Vaccines: Implications of Immune Modulation at the Vaccine Site
Abstract
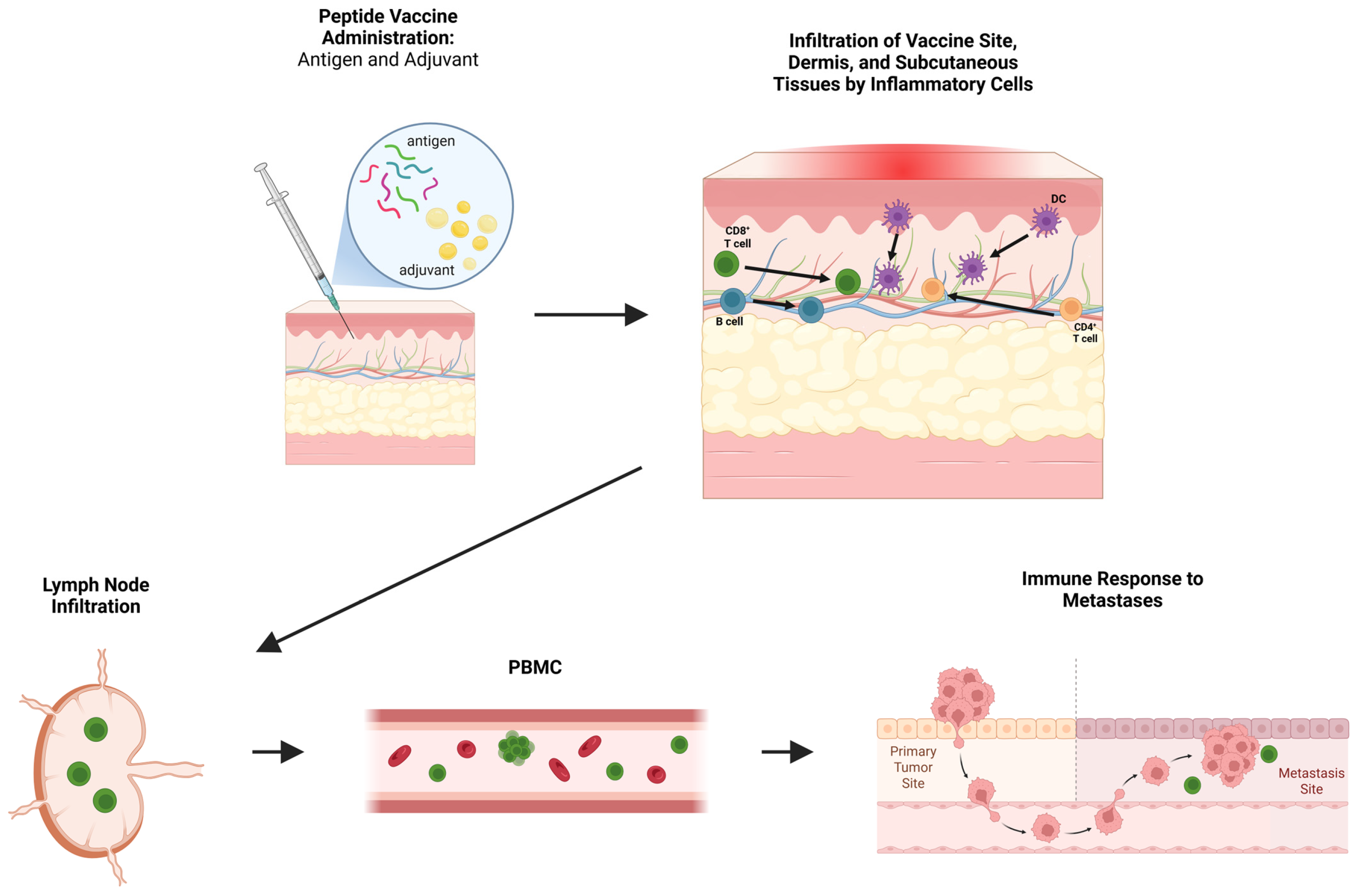
1. Introduction
2. The Vaccine Site: An Initial Interface for Antigen Presentation
3. Adjuvant-Mediated Recruitment and Activation of Immune Cells
| Adjuvant | Mechanism | Advantages | Disadvantages |
|---|---|---|---|
| Mineral salts |
|
| |
| Water-in-oil emulsions |
|
|
|
| TLR agonists |
| ||
| CpG oligodeoxynucleotides |
|
| |
| Poly-ICLC | |||
| TLR2 agonist |
|
| |
| TLR1/2 agonist | |||
| Saponins |
| ||
| QS-21 (AS15) |
| ||
| IL-2/anti-IL-2 complex |
| Assessed in combination with mesoporous silica rod vaccine [43] | |
| GM-CSF |
| Some local inflammatory and systemic adverse effects [60] Randomized trials show decreased T cell responses or no impact, when added to other effective adjuvants [60,62,63] | |
| Stabilizing gel matrices |
|
| Early phase development [44] |
4. Antigen-Specific T Cell Infiltration, Retention, and Potential Dysfunction at the Vaccine Site
5. Evidence of Tertiary Lymphoid Architecture at the Vaccine Site
6. Influence of Vaccine Strategies on Local Immune Signatures and Activity
7. Vaccine Strategies: Immunologic Consequences of Vaccine Site Selection
8. Translational Impact and Challenges in Peptide Vaccine Site Analysis
9. Future Directions
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VSME | Vaccine site microenvironment |
| TME | Tumor microenvironment |
| mRNA | Messenger RNA |
| MUC1 | Mucin 1 |
| TAA | Tumor-associated antigen |
| TSA | Tumor-specific antigen |
| APC | Antigen-presenting cell |
| DC | Dendritic cell |
| IFA | Incomplete Freund’s adjuvant |
| IL | Interleukin |
| PBMC | Peripheral blood mononuclear cell |
| TCR TLS FNA | T cell receptor Tertiary lymphoid structures Fine needle aspiration |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Tufail, M.; Jiang, C.-H.; Li, N. Immune Evasion in Cancer: Mechanisms and Cutting-Edge Therapeutic Approaches. Signal Transduct. Target. Ther. 2025, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Goswami, S.; Raychaudhuri, D.; Siddiqui, B.A.; Singh, P.; Nagarajan, A.; Liu, J.; Subudhi, S.K.; Poon, C.; Gant, K.L.; et al. Immune Checkpoint Therapy—Current Perspectives and Future Directions. Cell 2023, 186, 1652–1669. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.Y. Clinical Characteristics and Treatment of Immune-Related Adverse Events of Immune Checkpoint Inhibitors. Immune Netw. 2020, 20, e9. [Google Scholar] [CrossRef]
- Kamel, G.A.M.; Attia, R.A.; Al-Noman, H.G.; Salama, L.A. Advancement Insights in Cancer Vaccines: Mechanisms, Types, and Clinical Applications. Mol. Biol. Rep. 2025, 52, 290. [Google Scholar] [CrossRef]
- Sayour, E.J.; Boczkowski, D.; Mitchell, D.A.; Nair, S.K. Cancer mRNA Vaccines: Clinical Advances and Future Opportunities. Nat. Rev. Clin. Oncol. 2024, 21, 489–500. [Google Scholar] [CrossRef]
- Lorentzen, C.L.; Haanen, J.B.; Met, Ö.; Svane, I.M. Clinical Advances and Ongoing Trials of mRNA Vaccines for Cancer Treatment. Lancet Oncol. 2022, 23, e450–e458. [Google Scholar] [CrossRef]
- Pardi, N.; Weissman, D. Measuring the Adjuvant Activity of RNA Vaccines. In RNA Vaccines: Methods and Protocols; Kramps, T., Elbers, K., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1499, pp. 143–153. ISBN 978-1-4939-6481-9. [Google Scholar]
- Zhang, H.; Xia, X. RNA Cancer Vaccines: Developing mRNA Nanovaccine with Self-Adjuvant Property for Cancer Immunotherapy. Hum. Vaccines Immunother. 2021, 17, 2995–2998. [Google Scholar] [CrossRef] [PubMed]
- Ashkani, E.G.; McKenna, B.D.; Bryant, J.L.; Trevisan-Silva, D.; Sherman, N.E.; Chianese-Bullock, K.A.; Slingluff, C.L. Stability of Multi-Peptide Vaccines in Conditions Enabling Accessibility in Limited Resource Settings. Int. J. Pept. Res. Ther. 2024, 30, 42. [Google Scholar] [CrossRef]
- Sobhani, N.; Scaggiante, B.; Morris, R.; Chai, D.; Catalano, M.; Tardiel-Cyril, D.R.; Neeli, P.; Roviello, G.; Mondani, G.; Li, Y. Therapeutic Cancer Vaccines: From Biological Mechanisms and Engineering to Ongoing Clinical Trials. Cancer Treat. Rev. 2022, 109, 102429. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, I.; Padler-Karavani, V. Current Landscape of Therapeutic Cancer Vaccines. In Cancer Vaccines: Methods and Protocols; Padler-Karavani, V., Ed.; Springer US: New York, NY, USA, 2025; pp. 1–14. ISBN 978-1-07-164542-0. [Google Scholar]
- Larocca, C.; Schlom, J. Viral Vector –Based Therapeutic Cancer Vaccines. Cancer J. Sudbury Mass. 2011, 17, 359–371. [Google Scholar] [CrossRef]
- Zaidi, N.; Jaffee, E.M.; Yarchoan, M. Recent Advances in Therapeutic Cancer Vaccines. Nat. Rev. Cancer 2025, 25, 517–533. [Google Scholar] [CrossRef]
- Marchand, M.; Weynants, P.; Rankin, E.; Arienti, F.; Belli, F.; Parmiani, G.; Cascinelli, N.; Bourlond, A.; Vanwijck, R.; Humblet, Y. Tumor Regression Responses in Melanoma Patients Treated with a Peptide Encoded by Gene MAGE-3. Int. J. Cancer 1995, 63, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, L.H. Cancer Vaccines. BMJ 2015, 350, h988. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; McKenzie, I.F.C. Cellular Mucins: Targets for Immunotherapy. Crit. Rev. Immunol. 2017, 37, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Singer, C.F.; Hlauschek, D.; Pfeiler, G.; Egle, D.; Bartsch, R.; Suppan, C.; Pichler, A.; Petru, E.; Greil, R.; Rudas, M.; et al. Addition of the MUC-1 Vaccine Tecemotide to Neoadjuvant Systemic Therapy for Patients with Early Breast Cancer: Survival Results from the Prospective Randomized ABCSG 34 Trial. J. Clin. Oncol. 2024, 42, 587. [Google Scholar] [CrossRef]
- Capietto, A.-H.; Hoshyar, R.; Delamarre, L. Sources of Cancer Neoantigens beyond Single-Nucleotide Variants. Int. J. Mol. Sci. 2022, 23, 10131. [Google Scholar] [CrossRef]
- Ritter, A.; Koirala, N.; Wieland, A.; Kaumaya, P.T.P.; Mitchell, D.L. Therapeutic Cancer Vaccines for the Management of Recurrent and Metastatic Head and Neck Cancer: A Review. JAMA Otolaryngol. Neck Surg. 2023, 149, 168–176. [Google Scholar] [CrossRef]
- Wang, F.; Bade, E.; Kuniyoshi, C.; Spears, L.; Jeffery, G.; Marty, V.; Groshen, S.; Weber, J. Phase I Trial of a MART-1 Peptide Vaccine with Incomplete Freund’s Adjuvant for Resected High-Risk Melanoma. Clin. Cancer Res. 1999, 5, 2756–2765. [Google Scholar]
- Urbani, F.; Ferraresi, V.; Capone, I.; Macchia, I.; Palermo, B.; Nuzzo, C.; Torsello, A.; Pezzotti, P.; Giannarelli, D.; Pozzi, A.F.; et al. Clinical and Immunological Outcomes in High-Risk Resected Melanoma Patients Receiving Peptide-Based Vaccination and Interferon Alpha, With or Without Dacarbazine Preconditioning: A Phase II Study. Front. Oncol. 2020, 10, 202. [Google Scholar] [CrossRef]
- Jäeger, E.; Bernhard, H.; Romero, P.; Ringhoffer, M.; Arand, M.; Karbach, J.; Ilsemann, C.; Hagedorn, M.; Knuth, A. Generation of Cytotoxic T-Cell Responses with Synthetic Melanoma-Associated Peptides in Vivo: Implications for Tumor Vaccines with Melanoma-Associated Antigens. Int. J. Cancer 1996, 66, 162–169. [Google Scholar] [CrossRef]
- Bezu, L.; Kepp, O.; Cerrato, G.; Pol, J.; Fucikova, J.; Spisek, R.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial Watch: Peptide-Based Vaccines in Anticancer Therapy. Oncoimmunology 2018, 7, e1511506. [Google Scholar] [CrossRef]
- Parmiani, G.; Castelli, C.; Dalerba, P.; Mortarini, R.; Rivoltini, L.; Marincola, F.M.; Anichini, A. Cancer Immunotherapy With Peptide-Based Vaccines: What Have We Achieved? Where Are We Going? J. Natl. Cancer Inst. 2002, 94, 805–818. [Google Scholar] [CrossRef]
- Liang, F.; Lindgren, G.; Sandgren, K.J.; Thompson, E.A.; Francica, J.R.; Seubert, A.; De Gregorio, E.; Barnett, S.; O’Hagan, D.T.; Sullivan, N.J.; et al. Vaccine Priming Is Restricted to Draining Lymph Nodes and Controlled by Adjuvant-Mediated Antigen Uptake. Sci. Transl. Med. 2017, 9, eaal2094. [Google Scholar] [CrossRef] [PubMed]
- Clancy-Thompson, E.; King, L.K.; Nunnley, L.D.; Mullins, I.M.; Slingluff, C.L.; Mullins, D.W. Peptide Vaccination in Montanide Adjuvant Induces and GM-CSF Increases CXCR3 and Cutaneous Lymphocyte Antigen Expression by Tumor Antigen-Specific CD8 T Cells. Cancer Immunol. Res. 2013, 1, 332–339. [Google Scholar] [CrossRef]
- Liang, F.; Lindgren, G.; Lin, A.; Thompson, E.A.; Ols, S.; Röhss, J.; John, S.; Hassett, K.; Yuzhakov, O.; Bahl, K.; et al. Efficient Targeting and Activation of Antigen-Presenting Cells In Vivo after Modified mRNA Vaccine Administration in Rhesus Macaques. Mol. Ther. 2017, 25, 2635–2647. [Google Scholar] [CrossRef] [PubMed]
- Hassett, K.J.; Rajlic, I.L.; Bahl, K.; White, R.; Cowens, K.; Jacquinet, E.; Burke, K.E. mRNA Vaccine Trafficking and Resulting Protein Expression after Intramuscular Administration. Mol. Ther. Nucleic Acids 2023, 35, 102083. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Smolkin, M.E.; White, E.J.; Petroni, G.R.; Neese, P.Y.; Slingluff, C.L. Inflammatory Adverse Events Are Associated with Disease-Free Survival after Vaccine Therapy among Patients with Melanoma. Ann. Surg. Oncol. 2014, 21, 3978–3984. [Google Scholar] [CrossRef]
- Lindblad, E.B. Aluminium Compounds for Use in Vaccines. Immunol. Cell Biol. 2004, 82, 497–505. [Google Scholar] [CrossRef]
- Flach, T.L.; Ng, G.; Hari, A.; Desrosiers, M.D.; Zhang, P.; Ward, S.M.; Seamone, M.E.; Vilaysane, A.; Mucsi, A.D.; Fong, Y.; et al. Alum Interaction with Dendritic Cell Membrane Lipids Is Essential for Its Adjuvanticity. Nat. Med. 2011, 17, 479–487. [Google Scholar] [CrossRef]
- Eisenbarth, S.C.; Colegio, O.R.; O’Connor, W.; Sutterwala, F.S.; Flavell, R.A. Crucial Role for the Nalp3 Inflammasome in the Immunostimulatory Properties of Aluminium Adjuvants. Nature 2008, 453, 1122–1126. [Google Scholar] [CrossRef]
- Hamid, O.; Solomon, J.C.; Scotland, R.; Garcia, M.; Sian, S.; Ye, W.; Groshen, S.L.; Weber, J.S. Alum with Interleukin-12 Augments Immunity to a Melanoma Peptide Vaccine: Correlation with Time to Relapse in Patients with Resected High-Risk Disease. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 215–222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, X.; Shenashen, M.A.; Wang, X.; Ito, A.; Taniguchi, A.; EI-Safty, S.A. Hierarchically Porous, and Cu- and Zn-Containing γ-AlOOH Mesostrands as Adjuvants for Cancer Immunotherapy. Sci. Rep. 2017, 7, 16749. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Q.; Li, L.; Zeng, Q.; Li, H.; Gong, T.; Zhang, Z.; Sun, X. Turning the Old Adjuvant from Gel to Nanoparticles to Amplify CD8+ T Cell Responses. Adv. Sci. 2017, 5, 1700426. [Google Scholar] [CrossRef]
- Oreskovic, Z.; Nechvatalova, K.; Krejci, J.; Kummer, V.; Faldyna, M. Aspects of Intradermal Immunization with Different Adjuvants: The Role of Dendritic Cells and Th1/Th2 Response. PLoS ONE 2019, 14, e0211896. [Google Scholar] [CrossRef]
- Herbert, W.J. The Mode of Action of Mineral-Oil Emulsion Adjuvants on Antibody Production in Mice. Immunology 1968, 14, 301–318. [Google Scholar] [PubMed]
- Müssener, Å.; Klareskog, L.; Lorentzen, J.C.; Kleinau, S. TNF-α Dominates Cytokine mRNA Expression in Lymphoid Tissues of Rats Developing Collagen- and Oil-Induced Arthritis. Scand. J. Immunol. 1995, 42, 128–134. [Google Scholar] [CrossRef]
- Victoratos, P.; Yiangou, M.; Avramidis, N.; Hadjipetrou, L. Regulation of Cytokine Gene Expression by Adjuvants in Vivo. Clin. Exp. Immunol. 1997, 109, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Di Gianni, P.; Minnucci, F.S.; Alves Rosa, M.F.; Vulcano, M.; Isturiz, M.A. Anti-Oxidants Inhibit the Enhancement of the Immune Response Caused by Oil Adjuvants. Scand. J. Immunol. 1996, 43, 413–420. [Google Scholar] [CrossRef]
- Haining, W.N.; Davies, J.; Kanzler, H.; Drury, L.; Brenn, T.; Evans, J.; Angelosanto, J.; Rivoli, S.; Russell, K.; George, S.; et al. CpG Oligodeoxynucleotides Alter Lymphocyte and Dendritic Cell Trafficking in Humans. Clin. Cancer Res. 2008, 14, 5626–5634. [Google Scholar] [CrossRef]
- Sobral, M.C.; Cabizzosu, L.; Kang, S.J.; Ruark, K.; Najibi, A.J.; Lane, R.S.; Vitner, E.; Ijaz, H.; Dellacherie, M.O.; Dacus, M.T.; et al. IL-2/Anti-IL-2 Antibody Complexes Augment Immune Responses to Therapeutic Cancer Vaccines. Proc. Natl. Acad. Sci. USA 2024, 121, e2322356121. [Google Scholar] [CrossRef]
- Nguyen, C.L.; Bui, J.T.; Demcheva, M.; Vournakis, J.N.; Cole, D.J.; Gillanders, W.E. Sustained Release of Granulocyte–Macrophage Colony-Stimulating Factor From a Modular Peptide-Based Cancer Vaccine Alters Vaccine Microenvironment and Enhances the Antigen-Specific T-Cell Response. J. Immunother. 2001, 24, 420–429. [Google Scholar] [CrossRef]
- Melssen, M.M.; Pollack, K.E.; Meneveau, M.O.; Smolkin, M.E.; Pinczewski, J.; Koeppel, A.F.; Turner, S.D.; Sol-Church, K.; Hickman, A.; Deacon, D.H.; et al. Characterization and Comparison of Innate and Adaptive Immune Responses at Vaccine Sites in Melanoma Vaccine Clinical Trials. Cancer Immunol. Immunother. 2021, 70, 2151–2164. [Google Scholar] [CrossRef]
- Salerno, E.P.; Shea, S.M.; Olson, W.C.; Petroni, G.R.; Smolkin, M.E.; McSkimming, C.; Chianese-Bullock, K.A.; Slingluff, C.L. Activation, Dysfunction and Retention of T Cells in Vaccine Sites after Injection of Incomplete Freund’s Adjuvant, with or without Peptide. Cancer Immunol. Immunother. CII 2013, 62, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Pollack, K.E.; Meneveau, M.O.; Melssen, M.M.; Lynch, K.T.; Koeppel, A.F.; Young, S.J.; Turner, S.; Kumar, P.; Sol-Church, K.; Mauldin, I.S.; et al. Incomplete Freund’s Adjuvant Reduces Arginase and Enhances Th1 Dominance, TLR Signaling and CD40 Ligand Expression in the Vaccine Site Microenvironment. J. Immunother. Cancer 2020, 8, e000544. [Google Scholar] [CrossRef]
- Hailemichael, Y.; Dai, Z.; Jaffarzad, N.; Ye, Y.; Medina, M.A.; Huang, X.-F.; Dorta-Estremera, S.M.; Greeley, N.R.; Nitti, G.; Peng, W.; et al. Persistent Antigen at Vaccination Sites Induces Tumor-Specific CD8+ T Cell Sequestration, Dysfunction and Deletion. Nat. Med. 2013, 19, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Meneveau, M.O.; Kumar, P.; Lynch, K.T.; Patel, S.P.; Slingluff, C.L. The Vaccine-Site Microenvironment: Impacts of Antigen, Adjuvant, and Same-Site Vaccination on Antigen Presentation and Immune Signaling. J. Immunother. Cancer 2022, 10, e003533. [Google Scholar] [CrossRef] [PubMed]
- Sultan, H.; Wu, J.; Fesenkova, V.I.; Fan, A.E.; Addis, D.; Salazar, A.M.; Celis, E. Poly-IC Enhances the Effectiveness of Cancer Immunotherapy by Promoting T Cell Tumor Infiltration. J. Immunother. Cancer 2020, 8, e001224. [Google Scholar] [CrossRef]
- Zom, G.G.; Welters, M.J.P.; Loof, N.M.; Goedemans, R.; Lougheed, S.; Valentijn, R.R.P.M.; Zandvliet, M.L.; Meeuwenoord, N.J.; Melief, C.J.M.; de Gruijl, T.D.; et al. TLR2 Ligand-Synthetic Long Peptide Conjugates Effectively Stimulate Tumor-Draining Lymph Node T Cells of Cervical Cancer Patients. Oncotarget 2016, 7, 67087–67100. [Google Scholar] [CrossRef]
- Zom, G.G.; Willems, M.M.J.H.P.; Khan, S.; van der Sluis, T.C.; Kleinovink, J.W.; Camps, M.G.M.; van der Marel, G.A.; Filippov, D.V.; Melief, C.J.M.; Ossendorp, F. Novel TLR2-Binding Adjuvant Induces Enhanced T Cell Responses and Tumor Eradication. J. Immunother. Cancer 2018, 6, 146. [Google Scholar] [CrossRef]
- Speetjens, F.M.; Welters, M.J.P.; Slingerland, M.; van Poelgeest, M.I.E.; de Vos van Steenwijk, P.J.; Roozen, I.; Boekestijn, S.; Loof, N.M.; Zom, G.G.; Valentijn, A.R.P.M.; et al. Intradermal Vaccination of HPV-16 E6 Synthetic Peptides Conjugated to an Optimized Toll-like Receptor 2 Ligand Shows Safety and Potent T Cell Immunogenicity in Patients with HPV-16 Positive (Pre-)Malignant Lesions. J. Immunother. Cancer 2022, 10, e005016. [Google Scholar] [CrossRef]
- Rammensee, H.-G.; Wiesmüller, K.-H.; Chandran, P.A.; Zelba, H.; Rusch, E.; Gouttefangeas, C.; Kowalewski, D.J.; Di Marco, M.; Haen, S.P.; Walz, J.S.; et al. A New Synthetic Toll-like Receptor 1/2 Ligand Is an Efficient Adjuvant for Peptide Vaccination in a Human Volunteer. J. Immunother. Cancer 2019, 7, 307. [Google Scholar] [CrossRef] [PubMed]
- Englisch, A.; Hayn, C.; Jung, S.; Heitmann, J.S.; Hackenbruch, C.; Maringer, Y.; Nelde, A.; Wacker, M.; Denk, M.; Zieschang, L.; et al. iVAC-XS15-CLL01: Personalized Multi-Peptide Vaccination in Combination with the TLR1/2 Ligand XS15 in CLL Patients Undergoing BTK-Inhibitor-Based Regimens. Front. Oncol. 2024, 14, 1441625. [Google Scholar] [CrossRef]
- Kensil, C.R.; Wu, J.Y.; Anderson, C.A.; Wheeler, D.A.; Amsden, J. QS-21 and QS-7: Purified Saponin Adjuvants. Dev. Biol. Stand. 1998, 92, 41–47. [Google Scholar]
- Kruit, W.H.J.; Suciu, S.; Dreno, B.; Mortier, L.; Robert, C.; Chiarion-Sileni, V.; Maio, M.; Testori, A.; Dorval, T.; Grob, J.-J.; et al. Selection of Immunostimulant AS15 for Active Immunization With MAGE-A3 Protein: Results of a Randomized Phase II Study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J. Clin. Oncol. 2013, 31, 2413–2420. [Google Scholar] [CrossRef]
- Slingluff, C.L.; Petroni, G.R.; Olson, W.C.; Smolkin, M.E.; Chianese-Bullock, K.A.; Mauldin, I.S.; Smith, K.T.; Deacon, D.H.; Varhegyi, N.E.; Donnelly, S.B.; et al. A Randomized Pilot Trial Testing the Safety and Immunologic Effects of a MAGE-A3 Protein plus AS15 Immunostimulant Administered into Muscle or into Dermal/Subcutaneous Sites. Cancer Immunol. Immunother. CII 2016, 65, 25–36. [Google Scholar] [CrossRef]
- Vansteenkiste, J.F.; Cho, B.C.; Vanakesa, T.; De Pas, T.; Zielinski, M.; Kim, M.S.; Jassem, J.; Yoshimura, M.; Dahabreh, J.; Nakayama, H.; et al. Efficacy of the MAGE-A3 Cancer Immunotherapeutic as Adjuvant Therapy in Patients with Resected MAGE-A3-Positive Non-Small-Cell Lung Cancer (MAGRIT): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2016, 17, 822–835. [Google Scholar] [CrossRef]
- Slingluff, C.L.; Petroni, G.R.; Olson, W.C.; Smolkin, M.E.; Ross, M.I.; Haas, N.B.; Grosh, W.W.; Boisvert, M.E.; Kirkwood, J.M.; Chianese-Bullock, K.A. Effect of Granulocyte/Macrophage Colony-Stimulating Factor on Circulating CD8+ and CD4+ T-Cell Responses to a Multipeptide Melanoma Vaccine: Outcome of a Multicenter Randomized Trial. Clin. Cancer Res. 2009, 15, 7036–7044. [Google Scholar] [CrossRef] [PubMed]
- Jäger, E.; Ringhoffer, M.; Dienes, H.P.; Arand, M.; Karbach, J.; Jäger, D.; Ilsemann, C.; Hagedorn, M.; Oesch, F.; Knuth, A. Granulocyte-Macrophage-Colony-Stimulating Factor Enhances Immune Responses to Melanoma-Associated Peptides in Vivo. Int. J. Cancer 1996, 67, 54–62. [Google Scholar] [CrossRef]
- Scheibenbogen, C.; Schadendorf, D.; Bechrakis, N.E.; Nagorsen, D.; Hofmann, U.; Servetopoulou, F.; Letsch, A.; Philipp, A.; Foerster, M.H.; Schmittel, A.; et al. Effects of Granulocyte-Macrophage Colony-Stimulating Factor and Foreign Helper Protein as Immunologic Adjuvants on the T-Cell Response to Vaccination with Tyrosinase Peptides. Int. J. Cancer 2003, 104, 188–194. [Google Scholar] [CrossRef]
- Kirkwood, J.M.; Lee, S.; Moschos, S.J.; Albertini, M.R.; Michalak, J.C.; Sander, C.; Whiteside, T.; Butterfield, L.H.; Weiner, L. Immunogenicity and Antitumor Effects of Vaccination with Peptide Vaccine+/-Granulocyte-Monocyte Colony-Stimulating Factor and/or IFN-Alpha2b in Advanced Metastatic Melanoma: Eastern Cooperative Oncology Group Phase II Trial E1696. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Roberti, M.P.; Charoentong, P.; Lyu, Y.; Meyer, M.; Eichmüller, S.B.; Schmidt, P.; Momburg, F.; Cetin, M.; Hartmann, F.; Valous, N.A.; et al. Isolation of a Tumor Neoantigen Specific CD8+ TCR from a Skin Biopsy of a Vaccination Site. Oncoimmunology 2025, 14, 2457793. [Google Scholar] [CrossRef]
- Hailemichael, Y.; Woods, A.; Fu, T.; He, Q.; Nielsen, M.C.; Hasan, F.; Roszik, J.; Xiao, Z.; Vianden, C.; Khong, H.; et al. Cancer Vaccine Formulation Dictates Synergy with CTLA-4 and PD-L1 Checkpoint Blockade Therapy. J. Clin. Investig. 2018, 128, 1338–1354. [Google Scholar] [CrossRef] [PubMed]
- Melssen, M.M.; Olson, W.; Wages, N.A.; Capaldo, B.J.; Mauldin, I.S.; Mahmutovic, A.; Hutchison, C.; Melief, C.J.M.; Bullock, T.N.; Engelhard, V.H.; et al. Formation and Phenotypic Characterization of CD49a, CD49b and CD103 Expressing CD8 T Cell Populations in Human Metastatic Melanoma. Oncoimmunology 2018, 7, e1490855. [Google Scholar] [CrossRef]
- Salerno, E.P.; Olson, W.C.; McSkimming, C.; Shea, S.; Slingluff, C.L. T Cells in the Human Metastatic Melanoma Microenvironment Express Site-Specific Homing Receptors and Retention Integrins. Int. J. Cancer J. Int. Cancer 2014, 134, 563–574. [Google Scholar] [CrossRef]
- Melssen, M.M.; Lindsay, R.S.; Stasiak, K.; Rodriguez, A.B.; Briegel, A.M.; Cyranowski, S.; Rutkowski, M.R.; Conaway, M.R.; Melief, C.J.M.; van der Burg, S.H.; et al. Differential Expression of CD49a and CD49b Determines Localization and Function of Tumor-Infiltrating CD8+ T Cells. Cancer Immunol. Res. 2021, 9, 583–597. [Google Scholar] [CrossRef]
- Melssen, M.M.; Fisher, C.T.; Slingluff, C.L.; Melief, C.J.M. Peptide Emulsions in Incomplete Freund’s Adjuvant Create Effective Nurseries Promoting Egress of Systemic CD4+ and CD8+ T Cells for Immunotherapy of Cancer. J. Immunother. Cancer 2022, 10, e004709. [Google Scholar] [CrossRef] [PubMed]
- Melssen, M.M.; Petroni, G.R.; Chianese-Bullock, K.A.; Wages, N.A.; Grosh, W.W.; Varhegyi, N.; Smolkin, M.E.; Smith, K.T.; Galeassi, N.V.; Deacon, D.H.; et al. A Multipeptide Vaccine plus Toll-like Receptor Agonists LPS or polyICLC in Combination with Incomplete Freund’s Adjuvant in Melanoma Patients. J. Immunother. Cancer 2019, 7, 163. [Google Scholar] [CrossRef]
- Patel, S.P.; Petroni, G.R.; Roszik, J.; Olson, W.C.; Wages, N.A.; Chianese-Bullock, K.A.; Smolkin, M.; Varhegyi, N.; Gaughan, E.; Smith, K.T.; et al. Phase I/II Trial of a Long Peptide Vaccine (LPV7) plus Toll-like Receptor (TLR) Agonists with or without Incomplete Freund’s Adjuvant (IFA) for Resected High-Risk Melanoma. J. Immunother. Cancer 2021, 9, e003220. [Google Scholar] [CrossRef]
- Scheiermann, J.; Klinman, D.M. Clinical Evaluation of CpG Oligonucleotides as Adjuvants for Vaccines Targeting Infectious Diseases and Cancer. Vaccine 2014, 32, 6377–6389. [Google Scholar] [CrossRef]
- Harris, R.C.; Chianese-Bullock, K.A.; Petroni, G.R.; Schaefer, J.T.; Brill, L.B.; Molhoek, K.R.; Deacon, D.H.; Patterson, J.W.; Slingluff, C.L. The Vaccine-Site Microenvironment Induced by Injection of Incomplete Freund’s Adjuvant, with or without Melanoma Peptides. J. Immunother. 2012, 35, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Gu, X.; Li, D.; Wu, P.; Sun, N.; Zhang, C.; He, J. Tertiary Lymphoid Structures as a Biomarker in Immunotherapy and beyond: Advancing towards Clinical Application. Cancer Lett. 2025, 613, 217491. [Google Scholar] [CrossRef] [PubMed]
- Sautès-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary Lymphoid Structures in the Era of Cancer Immunotherapy. Nat. Rev. Cancer 2019, 19, 307–325. [Google Scholar] [CrossRef]
- Trüb, M.; Zippelius, A. Tertiary Lymphoid Structures as a Predictive Biomarker of Response to Cancer Immunotherapies. Front. Immunol. 2021, 12, 674565. [Google Scholar] [CrossRef]
- Li, L.; Guo, Y.; Gong, B.; Wang, S.; Wang, M.M.; Sun, P.; Jiang, S.; Yang, L. Association between Tertiary Lymphoid Structures and Clinical Outcomes in Cancer Patients Treated with Immune Checkpoint Inhibitors: An Updated Meta-Analysis. Front. Immunol. 2024, 15, 1385802. [Google Scholar] [CrossRef]
- Jiang, B.; Wu, Z.; Zhang, Y.; Yang, X. Associations between Tertiary Lymphoid Structure Density and Immune Checkpoint Inhibitor Efficacy in Solid Tumors: Systematic Review and Meta-Analysis. Front. Immunol. 2024, 15, 1414884. [Google Scholar] [CrossRef] [PubMed]
- Knief, J.; Reddemann, K.; Petrova, E.; Herhahn, T.; Wellner, U.; Thorns, C. High Density of Tumor-Infiltrating B-Lymphocytes and Plasma Cells Signifies Prolonged Overall Survival in Adenocarcinoma of the Esophagogastric Junction. Anticancer Res. 2016, 36, 5339–5345. [Google Scholar] [CrossRef]
- Garnelo, M.; Tan, A.; Her, Z.; Yeong, J.; Lim, C.J.; Chen, J.; Lim, K.H.; Weber, A.; Chow, P.; Chung, A.; et al. Interaction between Tumour-Infiltrating B Cells and T Cells Controls the Progression of Hepatocellular Carcinoma. Gut 2017, 66, 342–351. [Google Scholar] [CrossRef]
- Wada, Y.; Nakashima, O.; Kutami, R.; Yamamoto, O.; Kojiro, M. Clinicopathological Study on Hepatocellular Carcinoma with Lymphocytic Infiltration. Hepatology 1998, 27, 407–414. [Google Scholar] [CrossRef]
- Hiraoka, N.; Ino, Y.; Yamazaki-Itoh, R. Tertiary Lymphoid Organs in Cancer Tissues. Front. Immunol. 2016, 7, 244. [Google Scholar] [CrossRef]
- Espinosa-Carrasco, G.; Chiu, E.; Scrivo, A.; Zumbo, P.; Dave, A.; Betel, D.; Kang, S.W.; Jang, H.-J.; Hellmann, M.D.; Burt, B.M.; et al. Intratumoral Immune Triads Are Required for Immunotherapy-Mediated Elimination of Solid Tumors. Cancer Cell 2024, 42, 1202–1216.e8. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, J.T.; Patterson, J.W.; Deacon, D.H.; Smolkin, M.E.; Petroni, G.R.; Jackson, E.M.; Slingluff, C.L. Dynamic Changes in Cellular Infiltrates with Repeated Cutaneous Vaccination: A Histologic and Immunophenotypic Analysis. J. Transl. Med. 2010, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Johansen, P.; Mohanan, D.; Martínez-Gómez, J.M.; Kündig, T.M.; Gander, B. Lympho-Geographical Concepts in Vaccine Delivery. J. Control. Release 2010, 148, 56–62. [Google Scholar] [CrossRef]
- Mohanan, D.; Slütter, B.; Henriksen-Lacey, M.; Jiskoot, W.; Bouwstra, J.A.; Perrie, Y.; Kündig, T.M.; Gander, B.; Johansen, P. Administration Routes Affect the Quality of Immune Responses: A Cross-Sectional Evaluation of Particulate Antigen-Delivery Systems. J. Control. Release 2010, 147, 342–349. [Google Scholar] [CrossRef]
- Ohlfest, J.R.; Andersen, B.M.; Litterman, A.J.; Junzhe, X.; Pennell, C.A.; Swier, L.E.; Salazar, A.M.; Olin, M.R. Vaccine Injection Site Matters: Qualitative and Quantitative Defects in CD8 T Cells Primed as a Function of Proximity to the Tumor in a Murine Glioma Model. J. Immunol. 2013, 190, 613–620. [Google Scholar] [CrossRef]
- Walker, E.B.; Haley, D.; Petrausch, U.; Floyd, K.; Miller, W.; Sanjuan, N.; Alvord, G.; Fox, B.A.; Urba, W.J. Phenotype and Functional Characterization of Long-Term Gp100-Specific Memory CD8+ T Cells in Disease-Free Melanoma Patients before and after Boosting Immunization. Clin. Cancer Res. 2008, 14, 5270–5283. [Google Scholar] [CrossRef]
- Schwartzentruber, D.J.; Lawson, D.H.; Richards, J.M.; Conry, R.M.; Miller, D.M.; Treisman, J.; Gailani, F.; Riley, L.; Conlon, K.; Pockaj, B.; et al. Gp100 Peptide Vaccine and Interleukin-2 in Patients with Advanced Melanoma. N. Engl. J. Med. 2011, 364, 2119–2127. [Google Scholar] [CrossRef]
- Melanoma Study Group of the Mayo Clinic Cancer Center; Celis, E. Overlapping Human Leukocyte Antigen Class I/II Binding Peptide Vaccine for the Treatment of Patients with Stage IV Melanoma: Evidence of Systemic Immune Dysfunction. Cancer 2007, 110, 203–214. [Google Scholar] [CrossRef]
- Braun, D.A.; Moranzoni, G.; Chea, V.; McGregor, B.A.; Blass, E.; Tu, C.R.; Vanasse, A.P.; Forman, C.; Forman, J.; Afeyan, A.B.; et al. A Neoantigen Vaccine Generates Antitumour Immunity in Renal Cell Carcinoma. Nature 2025, 639, 474–482. [Google Scholar] [CrossRef]
- Barr, K.M.; Jing, W.; Hallett, W.H.D.; Gershan, J.A.; Johnson, B.D. Examining T Cells at Vaccine Sites of Tumor-Bearing Hosts Provides Insights to Dysfunctional T Cell Immunity. J. Immunother. 2013, 36, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Kasraeian, S.; Allison, D.C.; Ahlmann, E.R.; Fedenko, A.N.; Menendez, L.R. A Comparison of Fine-Needle Aspiration, Core Biopsy, and Surgical Biopsy in the Diagnosis of Extremity Soft Tissue Masses. Clin. Orthop. 2010, 468, 2992–3002. [Google Scholar] [CrossRef]
- Domanski, H.A. Fine-Needle Aspiration Cytology of Soft Tissue Lesions: Diagnostic Challenges. Diagn. Cytopathol. 2007, 35, 768–773. [Google Scholar] [CrossRef]
- VanderLaan, P.A. Fine-Needle Aspiration and Core Needle Biopsy: An Update on 2 Common Minimally Invasive Tissue Sampling Modalities. Cancer Cytopathol. 2016, 124, 862–870. [Google Scholar] [CrossRef]
- Nakayama, T.; Kashiwagi, Y.; Kawashima, H. Long-Term Regulation of Local Cytokine Production Following Immunization in Mice. Microbiol. Immunol. 2018, 62, 124–131. [Google Scholar] [CrossRef]
- Schaefer, C.; Butterfield, L.H.; Lee, S.; Kim, G.G.; Visus, C.; Albers, A.; Kirkwood, J.M.; Whiteside, T.L. Function but Not Phenotype of Melanoma Peptide-Specific CD8+ T Cells Correlate with Survival in a Multiepitope Peptide Vaccine Trial (ECOG 1696). Int. J. Cancer 2012, 131, 874–884. [Google Scholar] [CrossRef]
- Molla Desta, G.; Birhanu, A.G. Advancements in Single-Cell RNA Sequencing and Spatial Transcriptomics: Transforming Biomedical Research. Acta Biochim. Pol. 2025, 72, 13922. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Vanderzee, I.O.; Wen, F. The Application of Single-Cell Technologies for Vaccine Development Against Viral Infections. Vaccines 2025, 13, 687. [Google Scholar] [CrossRef]
- Wimmers, F.; Pulendran, B. Emerging Technologies for Systems Vaccinology–Multi-Omics Integration and Single-Cell (Epi)Genomic Profiling. Curr. Opin. Immunol. 2020, 65, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Meldgaard, T.S.; Blengio, F.; Maffione, D.; Sammicheli, C.; Tavarini, S.; Nuti, S.; Kratzer, R.; Medini, D.; Siena, E.; Bertholet, S. Single-Cell Analysis of Antigen-Specific CD8+ T-Cell Transcripts Reveals Profiles Specific to mRNA or Adjuvanted Protein Vaccines. Front. Immunol. 2021, 12, 757151. [Google Scholar] [CrossRef] [PubMed]
- Robles-Remacho, A.; Sanchez-Martin, R.M.; Diaz-Mochon, J.J. Spatial Transcriptomics: Emerging Technologies in Tissue Gene Expression Profiling. Anal. Chem. 2023, 95, 15450–15460. [Google Scholar] [CrossRef]
- Denisenko, E.; de Kock, L.; Tan, A.; Beasley, A.B.; Beilin, M.; Jones, M.E.; Hou, R.; Muirí, D.Ó.; Bilic, S.; Mohan, G.R.K.A.; et al. Spatial Transcriptomics Reveals Discrete Tumour Microenvironments and Autocrine Loops within Ovarian Cancer Subclones. Nat. Commun. 2024, 15, 2860. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, Z.; Slieker, R.C.; McGuire, D.; Welters, M.J.P.; van Poelgeest, M.I.E.; van der Burg, S.H. Single-Cell Spatial Transcriptomics Unravels Cell States and Ecosystems Associated with Clinical Response to Immunotherapy. J. Immunother. Cancer 2025, 13, e011308. [Google Scholar] [CrossRef] [PubMed]
- Longo, S.K.; Guo, M.G.; Ji, A.L.; Khavari, P.A. Integrating Single-Cell and Spatial Transcriptomics to Elucidate Intercellular Tissue Dynamics. Nat. Rev. Genet. 2021, 22, 627–644. [Google Scholar] [CrossRef] [PubMed]

| VSME Sampling Method | Advantages | Disadvantages |
|---|---|---|
| Excisional or incisional biopsy |
|
|
| Fine needle aspiration (FNA) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkar, A.; Rabinovich, E.P.; Slingluff, C.L., Jr. Shaping Antitumor Immunity with Peptide Vaccines: Implications of Immune Modulation at the Vaccine Site. Vaccines 2025, 13, 1150. https://doi.org/10.3390/vaccines13111150
Sarkar A, Rabinovich EP, Slingluff CL Jr. Shaping Antitumor Immunity with Peptide Vaccines: Implications of Immune Modulation at the Vaccine Site. Vaccines. 2025; 13(11):1150. https://doi.org/10.3390/vaccines13111150
Chicago/Turabian StyleSarkar, Amrita, Emily Pauline Rabinovich, and Craig Lee Slingluff, Jr. 2025. "Shaping Antitumor Immunity with Peptide Vaccines: Implications of Immune Modulation at the Vaccine Site" Vaccines 13, no. 11: 1150. https://doi.org/10.3390/vaccines13111150
APA StyleSarkar, A., Rabinovich, E. P., & Slingluff, C. L., Jr. (2025). Shaping Antitumor Immunity with Peptide Vaccines: Implications of Immune Modulation at the Vaccine Site. Vaccines, 13(11), 1150. https://doi.org/10.3390/vaccines13111150






