Vaccination of Sheep with Bovine Viral Diarrhea Vaccines Does Not Protect against Fetal Infection after Challenge of Pregnant Ewes with Border Disease Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus and Inoculum
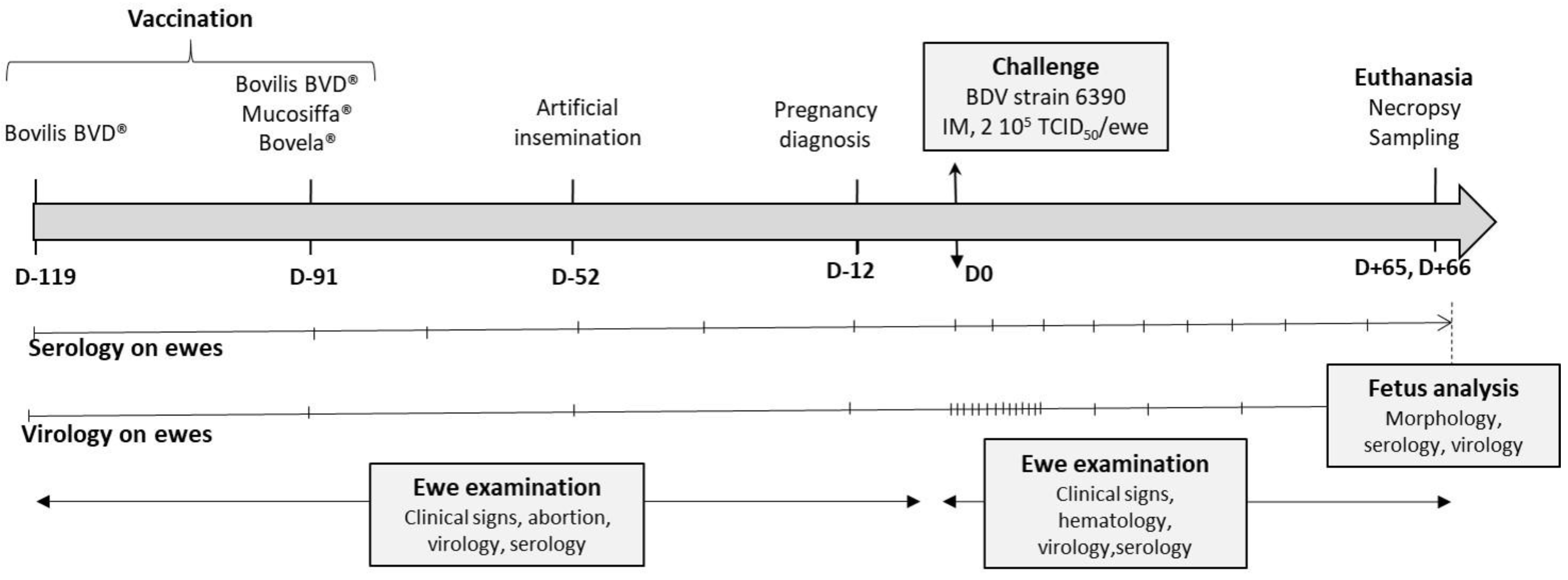
2.2. Vaccination of Ewes and Artificial Insemination
2.3. Challenge and Sampling
2.4. Antibody Response
2.5. Virus Detection
2.6. Statistical Analyses
3. Results
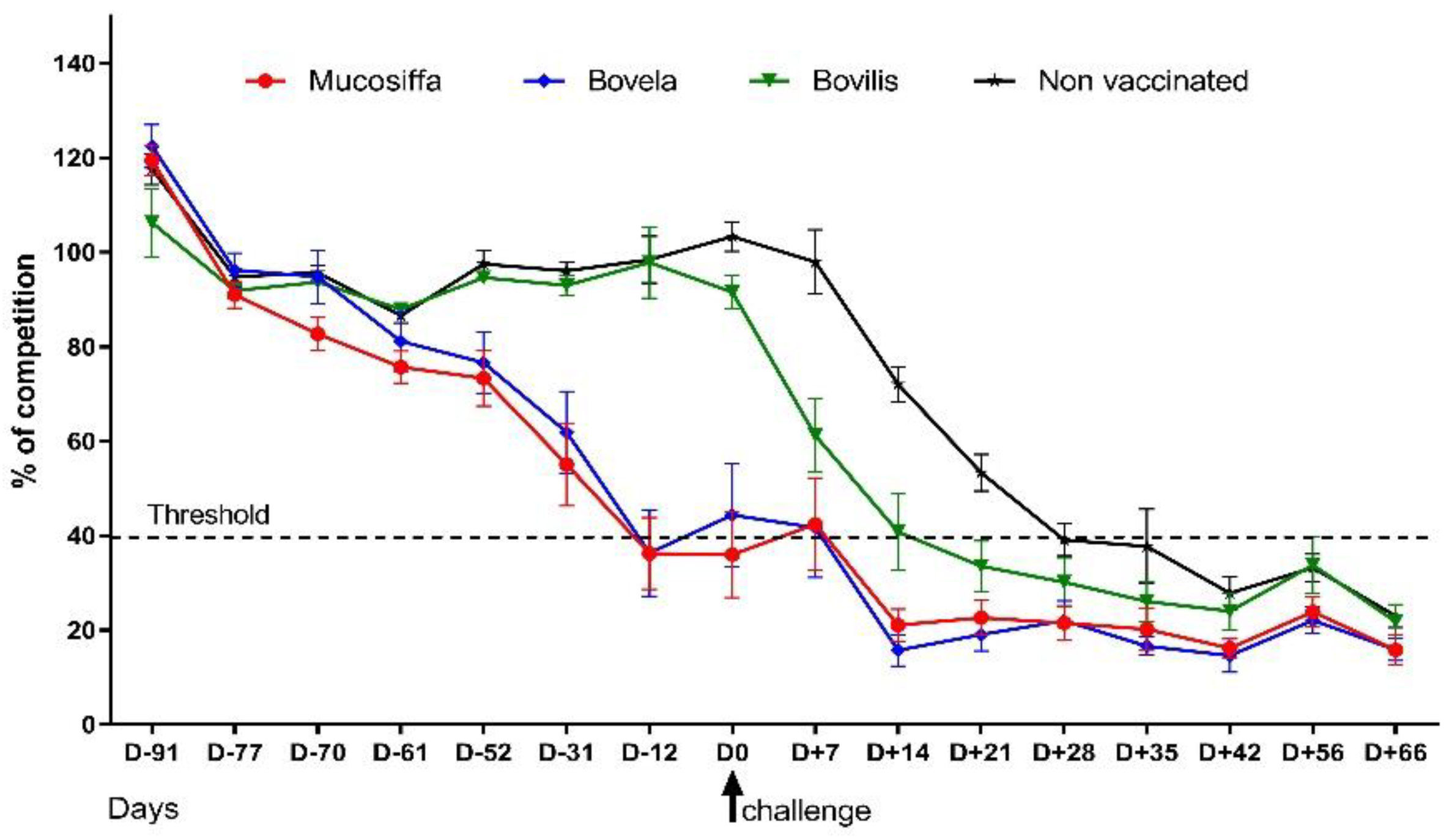
3.1. The Two Attenuated BVDV Vaccines But Not the Inactivated One, Induced Seroconversion against NS3 in Sheep
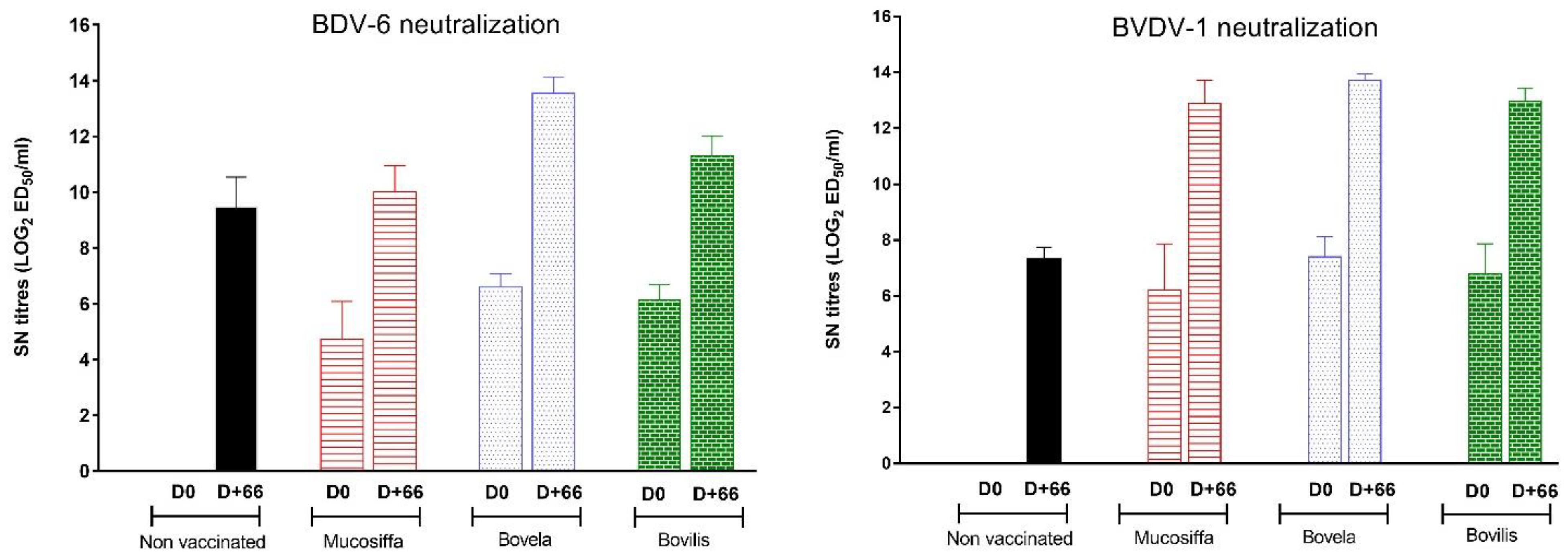
3.2. The Three Vaccines Induce a Neutralizing Antibody Response against BVDV-1 and BDV-6
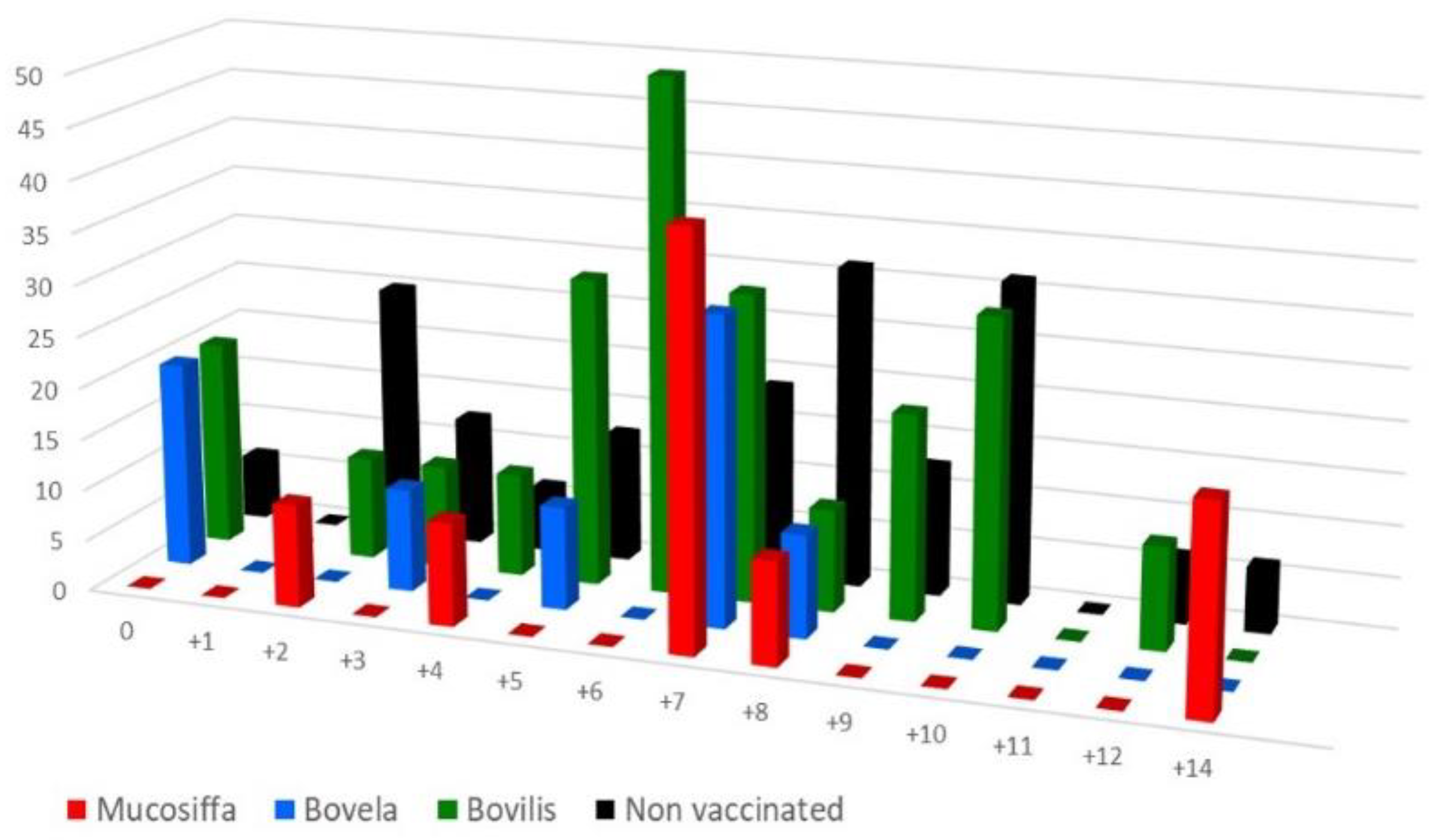
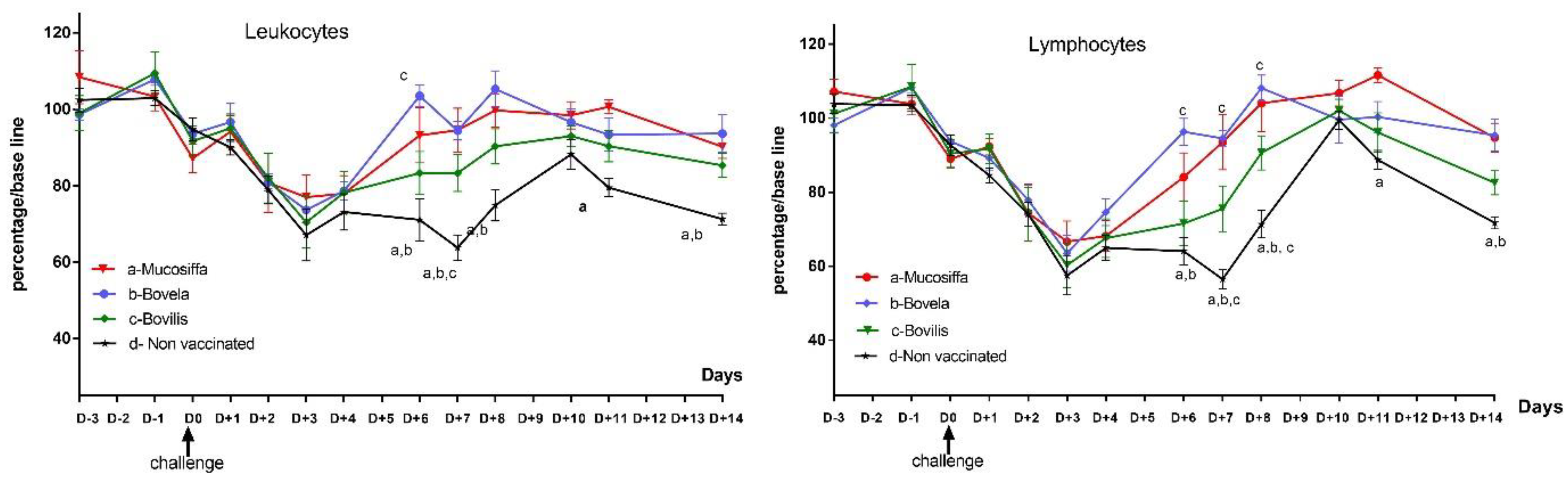
3.3. Vaccination Partially Protects Ewes against BDV-6 Challenge-Induced Leukopenia
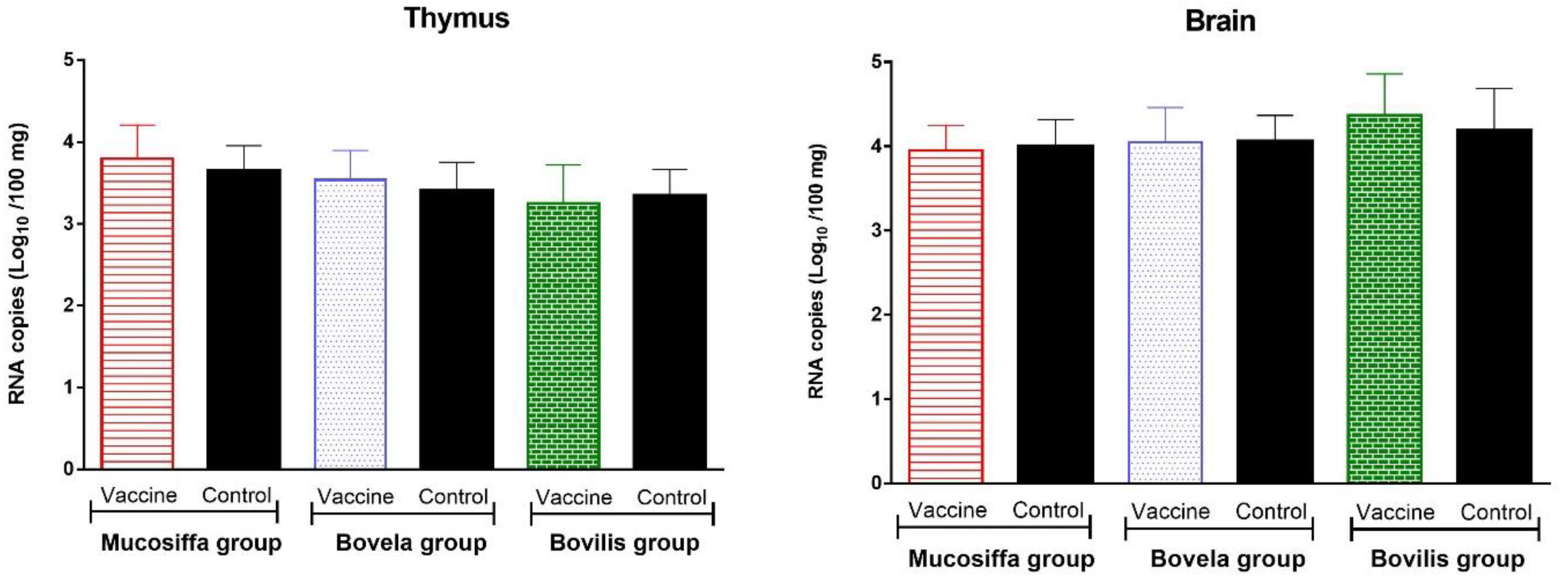
3.4. Vaccination with Half Dose of BVDV Vaccines Does Not Protect against Fetal Infection by BDV
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, D.B.; Meyers, G.; Bukh, J.; Gould, E.A.; Monath, T.; Scott Muerhoff, A.; Pletnev, A.; Rico-Hesse, R.; Stapleton, J.T.; Simmonds, P. Proposed revision to the taxonomy of the genus Pestivirus, family Flaviviridae. J. Gen. Virol. 2017, 98, 2106–2112. [Google Scholar] [CrossRef]
- Nettleton, P.F.; Gilray, J.A.; Russo, P.; Dlissi, E. Border disease of sheep and goats. Vet. Res. 1998, 29, 327–340. [Google Scholar] [PubMed]
- Righi, C.; Petrini, S.; Pierini, I.; Giammarioli, M.; Mia, G.M.D. Global Distribution and Genetic Heterogeneity of Border Disease Virus. Viruses 2021, 13, 950. [Google Scholar] [CrossRef]
- Chappuis, G.; Brun, A.; Kato, F.; Dauvergne, M.; Reynaud, G.; Duret, C. Études Sérologiques et Immunologiques Réalisées à la Suite de l’Isolement d’un Pestivirus dans un Foyer Ovin chez des Moutons de l’Aveyron. In Pestivirose des Ovins et des Bovins:Nouvelles Connaissances, Utilisation pour une Stratégie de Contrôle, Journées Nationales de la Société Française de Buiatrie et de son Grouped’Étude sur la Pathologie des Ovins et des Caprins (GEPOC); Espinasse, J., Savey, M., Eds.; Societe Francaise de Buiatrie: Paris, France, 1986; pp. 55–65. [Google Scholar]
- Vilcek, S.; Leskova, V.; Meyer, D.; Postel, A.; Becher, P. Molecular characterization of Border Disease Virus strain Aveyron. Vet. Microbiol. 2014, 171, 87–92. [Google Scholar] [CrossRef]
- Pouget, C.; Brugidou, P.R.; Blancard, P.; Corbière, F. Border Disease (Maladies des Frontières): Vers un Dépistage Sérologique sur lait de Tank; Journées Nationales des GTV: Nantes, France, 2010. [Google Scholar]
- Vantsis, J.T.; Barlow, R.M.; Gardiner, A.C. The effects of challenge with homologous and heterologous strains of Border Disease Virus on ewes with previous experience of the disease. J. Comp. Pathol. 1980, 90, 39–45. [Google Scholar] [CrossRef]
- Barlow, R.M. Experiments in Border disease. IV. Pathological changes in ewes. J. Comp. Pathol. 1972, 82, 151–157. [Google Scholar] [CrossRef]
- Becher, P.; Ramirez, R.A.; Orlich, M.; Rosales, S.C.; König, M.; Schweizer, M.; Stalder, H.; Schirrmeier, H.; Thiel, H.-J. Genetic and antigenic characterization of novel pestivirus genotypes: Implications for classification. Virology 2003, 311, 96–104. [Google Scholar] [CrossRef]
- Fernandez, F.; Constantini, V.; Barrandeguy, M.; Parrenoi, G.; Schiappacassi, G.; Maliandi, F.; Leunda, M.; Odeon, A. Evaluation of experimental vaccines for bovine viral diarrhea in bovines, ovines and guinea pigs. Rev. Argent. Microbiol. 2009, 41, 86–91. [Google Scholar] [PubMed]
- Anne, S. Etude de L’efficacité Croisée d’un Vaccin Inactivé de la Diarrhée Virale Bovine (Virus BVDV) Contre des Souches Récentes de Virus Border Disease (BDV). Ph.D. Thesis, Paul-Sabatier University of Toulouse, Toulouse, France, 2012. [Google Scholar]
- Bethune, M.-A. Etude Comparative du Pouvoir Pathogène de Différents Génotypes du Virus Border: Infection Fœtale, Avortements et Production D’agneaux Infectés Permanents Immunotolérants. Ph.D. Thesis, Paul-Sabatier University of Toulouse, Toulouse, France, 2015. [Google Scholar]
- Hamers, C.; Di Valentin, E.; Lecomte, C.; Lambot, M.; Joris, E.; Genicot, B.; Pastoret, P.-P. Virus neutralising antibodies against 22 Bovine Viral Diarrhoea Virus isolates in vaccinated calves. Vet. J. 2002, 163, 61–67. [Google Scholar] [CrossRef]
- Vilček, Š.; Herring, A.J.; Herring, J.A.; Nettleton, P.F.; Lowings, J.P.; Paton, D.J. Pestiviruses isolated from pigs, cattle and sheep can be allocated into at least three genogroups using polymerase chain reaction and restriction endonuclease analysis. Arch. Virol. 1994, 136, 309–323. [Google Scholar] [CrossRef]
- Anderson, C.A.; Sawyer, M.; Higgins, R.J.; East, N.; Osburn, B.I. Experimentally induced ovine border disease: Extensive hypomyelination with minimal viral antigen in neonatal spinal cord. Am. J. Vet. Res. 1987, 48, 499–503. [Google Scholar] [PubMed]
- Garcıa-Perez, A.L.; Minguijon, E.; Barandika, J.F.; Juste, R.A.; Hurtado, A. Detection of Border disease virus in fetuses, stillbirths lambs from natural and experimental. J. Vet. Diagn. Investig. 2009, 21, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Hussin, A.A.; Woldehiwet, Z. Effects of experimental infection with Border Disease Virus on lymphocyte subpopulations in the peripheral blood of lambs. Res. Vet. Sci. 1994, 56, 201–207. [Google Scholar] [CrossRef]
- Thabti, F.; Fronzaroli, L.; Dlissi, E.; Guibert, J.-M.; Hammami, S.; Pepin, M.; Russo, P. Experimental model of Border Disease Virus infection in lambs: Comparative pathogenicity of pestiviruses isolated in France and Tunisia. Vet. Res. 2002, 33, 35–457. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meyer, G.; Deplanche, M.; Roux, D.; Moulignie, M.; Picard-Hagen, N.; Lyazrhi, F.; Raboisson, D.; Mathevet, P.; Schelcher, F. Fetal protection against bovine viral diarrhoea type 1 virus infection after one administration of a live-attenuated vaccine. Vet. J. 2012, 192, 242–245. [Google Scholar] [CrossRef]
- Fulton, R.W.; Cook, B.J.; Payton, M.E.; Burge, L.J.; Step, D.L. Immune response to Bovine Viral Diarrhea Virus (BVDV) vaccines detecting antibodies to BVDV subtypes 1a, 1b, 2a, and 2c. Vaccine 2020, 38, 4032–4037. [Google Scholar] [CrossRef]
- Platt, R.; Burdett, W.; Roth, J.A. Induction of antigen-specific T-cell subset activation to bovine respiratory disease viruses by a modified-live virus vaccine. Am. J. Vet. Res. 2006, 67, 1179–1184. [Google Scholar] [CrossRef]
- Platt, R.; Kesl, L.; Guidarini, C.; Wang, C.; Roth, J.A. Comparison of humoral and T-cell-mediated immune responses to a single dose of Bovela live double deleted BVDV vaccine or to a field BVDV strain. Vet. Immunol. Immunopathol. 2017, 187, 20–27. [Google Scholar] [CrossRef]
- Theurer, M.E.; Larson, R.L.; White, B.J. Systematic review and meta-analysis of the effectiveness of commercially available vaccines against bovine herpesvirus, Bovine Viral Diarrhea Virus, bovine respiratory syncytial virus, and parainfluenza type 3 virus for mitigation of bovine respiratory disease complex in cattle. J. Am. Vet. Med. Assoc. 2015, 246, 126–142. [Google Scholar] [CrossRef]
- Downey-Slinker, E.D.; Ridpath, J.F.; Sawyer, J.E.; Skow, L.C.; Herring, A.D. Antibody titers to vaccination are not predictive of level of protection against a BVDV type 1b challenge in Bos indicus—Bos taurus steers. Vaccine 2016, 34, 5053–5059. [Google Scholar] [CrossRef]
- Platt, R.; Coutu, C.; Meinert, T.; Roth, J.A. Humoral and T cell-mediated immune responses to bivalent killed Bovine Viral Diarrhea Virus vaccine in beef cattle. Vet. Immunol. Immunopathol. 2008, 122, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ridpath, J.F. Immunology of BVDV vaccines. Biologicals 2013, 41, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, B.W.; Walz, P.H.; Givens, M.D.; Wilson, A.E. Efficacy of Bovine Viral Diarrhea Virus vaccination to prevent reproductive disease: A meta-analysis. Theriogenology 2015, 83, 360–365. [Google Scholar] [CrossRef]
- Rodning, S.P.; Marley, M.S.D.; Zhang, Y.; Eason, A.B.; Nunley, C.; Walz, P.H.; Riddell, K.P.; Galik, P.K.; Brodersen, B.W.; Givens, M.D. Comparison of three commercial vaccines for preventing infection with Bovine Viral Diarrhea Virus. Theriogenology 2010, 73, 1154–1163. [Google Scholar] [CrossRef]
- Zimmer, G.M.; Wentink, G.H.; Bruschke, C.; Westenbrink, F.J.; Brinkhof, J.; de Goey, I. Failure of foetal protection after vaccination against an experimental infection with bovine virus diarrhea virus. Vet. Microbiol. 2002, 89, 255–265. [Google Scholar] [CrossRef]
- Meyers, G.; Ege, A.; Fetzer, C.; von Freyburg, M.; Elbers, K.; Carr, V.; Prentice, H.; Charleston, B.; Schürmann, E.-M. Bovine Viral Diarrhea Virus: Prevention of persistent fetal infection by a combination of two mutations affecting Erns RNase and Npro protease. J. Virol. 2007, 81, 3327–3338. [Google Scholar] [CrossRef]
- Wernike, K.; Michelitsch, A.; Aebischer, A.; Schaarschmidt, U.; Konrath, A.; Nieper, H.; Sehl, J.; Teifke, J.P.; Beer, M. The Occurrence of a Commercial N(pro) and E(rns) Double Mutant BVDV-1 Live-Vaccine Strain in Newborn Calves. Viruses 2018, 10, 274. [Google Scholar] [CrossRef]
- Arias, P.; Orlich, M.; Prieto, M.; Cedillo Rosales, S.; Thiel, H.J.; Alvarez, M.; Becher, P. Genetic heterogeneity of bovine viral diarrhoea viruses from Spain. Vet. Microbiol. 2003, 96, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.A.; German, A.; Mawhinney, K.; Goodall, E.A. Antibody responses of naive cattle to two inactivated bovine viral diarrhoea virus vaccines, measured by indirect and blocking ELISAs and virus neutralisation. Vet. Rec. 2003, 152, 795–800. [Google Scholar] [CrossRef]
- Makoschey, B.; Sonnemans, D.; Bielsa, J.M.; Franken, P.; Mars, M.; Santos, L.; Alvarez, M. Evaluation of the induction of NS3 specific BVDV antibodies using a commercial inactivated BVDV vaccine in immunization and challenge trials. Vaccine 2007, 25, 6140–6145. [Google Scholar] [CrossRef] [PubMed]
- Raue, R.; Harmeyer, S.S.; Nanjiani, I.A. Antibody responses to inactivated vaccines and natural infection in cattle using bovine viral diarrhoea virus ELISA kits: Assessment of potential to differentiate infected and vaccinated animals. Vet. J. 2011, 187, 330–334. [Google Scholar] [CrossRef]
- Álvarez, M.; Donate, J.; Makoschey, B. Antibody responses against non-structural protein 3 of bovine viral diarrhoea virus in milk and serum samples from animals immunised with an inactivated vaccine. Vet. J. 2012, 191, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Riitho, V.; Strong, R.; Larska, M.; Graham, S.P.; Steinbach, F. Bovine pestivirus heterogeneity and its potential impact on vaccination and diagnosis. Viruses 2020, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, E.; Righi, C.; Boldini, M.; Bazzucchi, M.; Pezzoni, G.; Gradassi, M.; Petrini, S.; Lelli, D.; Ventura, G.; Pierini, I.; et al. Cross-reactivity antibody response after vaccination with modified live and killed Bovine Viral Diarrhoea Virus (BVD) vaccines. Vaccines 2020, 8, 374. [Google Scholar] [CrossRef] [PubMed]
- Kelling, C.L.; Hunsaker, B.D.; Steffen, D.J.; Topliff, C.L.; Eskridge, K.M. Characterization of protection against systemic infection and disease from experimental Bovine Viral Diarrhea Virus type 2 infection by use of a modified-live noncytopathic type 1 vaccine in calves. Am. J. Vet. Res. 2007, 68, 788–796. [Google Scholar] [CrossRef]
- Bolin, S.R.; Grooms, D.L. Origination and consequences of Bovine Viral Diarrhea Virus diversity. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, B.W.; Chamorro, M.F.; Walz, P.H. Vaccination of cattle against Bovine Viral Diarrhea Virus. Vet. Microbiol. 2017, 206, 78–83. [Google Scholar] [CrossRef]
- Fulton, R.W. Impact of species and subgenotypes of Bovine Viral Diarrhea Virus on control by vaccination. Anim. Health Res. Rev. 2015, 16, 40–54. [Google Scholar] [CrossRef]
- Lambot, M.; Letesson, J.-J.; Douart, A.; Pastoret, P.P.; Joris, E. Characterization of the immune response of cattle against non-cytopathic and cytopathic biotypes of bovine viral diarrhoea virus. J. Gen. Virol. 1997, 78 Pt 5, 1041–1047. [Google Scholar] [CrossRef][Green Version]
- Collen, T.; Morrison, W.I. CD4+ T-cell responses to bovine viral diarrhoea virus in cattle. Virus Res. 2000, 67, 67–80. [Google Scholar] [CrossRef]
- Collen, T.; Carr, V.; Parsons, K.; Charleston, B.; Morrison, W.I. Analysis of the repertoire of cattle CD4(+) T cells reactive with bovine viral diarrhoea virus. Vet. Immunol. Immunopathol. 2002, 87, 235–238. [Google Scholar] [CrossRef]
- Bolin, S.R. Immunogens of Bovine Viral Diarrhea Virus. Vet. Microbiol. 1993, 37, 263–271. [Google Scholar] [CrossRef]
- Beer, M.; Hehnen, H.R.; Wolfmeyer, A.; Poll, G.; Kaaden, O.R.; Wolf, G. A new inactivated BVDV genotype I and II vaccine. An immunization and challenge study with BVDV genotype I. Vet. Microbiol. 2000, 77, 195–208. [Google Scholar] [CrossRef]
- Bolin, S.R.; Ridpath, J.F. Assessment of protection from systemic infection or disease afforded by low to intermediate titers of passively acquired neutralizing antibody against Bovine Viral Diarrhea Virus in calves. Am. J. Vet. Res. 1995, 56, 755–759. [Google Scholar] [PubMed]
| Groups/Days | D0 | D+7 | D+14 | D+21 | D+28 | D+35 | D+42 | D+66 |
|---|---|---|---|---|---|---|---|---|
| Mucosiffa (n = 11) | 73% | 73% | 91% | 91% | 91% | 91% | 100% | 100% |
| Bovela (n = 11) | 64% | 64% | 100% | 91% | 82% | 100% | 91% | 100% |
| Bovilis (n = 11) | 0% | 18% | 64% | 73% | 82% | 91% | 82% | 100% |
| Non vaccinated (n = 18) | 0% | 0% | 0% | 17% | 67% | 72% | 94% | 94% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, G.; Combes, M.; Teillaud, A.; Pouget, C.; Bethune, M.-A.; Cassard, H. Vaccination of Sheep with Bovine Viral Diarrhea Vaccines Does Not Protect against Fetal Infection after Challenge of Pregnant Ewes with Border Disease Virus. Vaccines 2021, 9, 805. https://doi.org/10.3390/vaccines9080805
Meyer G, Combes M, Teillaud A, Pouget C, Bethune M-A, Cassard H. Vaccination of Sheep with Bovine Viral Diarrhea Vaccines Does Not Protect against Fetal Infection after Challenge of Pregnant Ewes with Border Disease Virus. Vaccines. 2021; 9(8):805. https://doi.org/10.3390/vaccines9080805
Chicago/Turabian StyleMeyer, Gilles, Mickael Combes, Angelique Teillaud, Celine Pouget, Marie-Anne Bethune, and Herve Cassard. 2021. "Vaccination of Sheep with Bovine Viral Diarrhea Vaccines Does Not Protect against Fetal Infection after Challenge of Pregnant Ewes with Border Disease Virus" Vaccines 9, no. 8: 805. https://doi.org/10.3390/vaccines9080805
APA StyleMeyer, G., Combes, M., Teillaud, A., Pouget, C., Bethune, M.-A., & Cassard, H. (2021). Vaccination of Sheep with Bovine Viral Diarrhea Vaccines Does Not Protect against Fetal Infection after Challenge of Pregnant Ewes with Border Disease Virus. Vaccines, 9(8), 805. https://doi.org/10.3390/vaccines9080805






