Bone Marrow Transfer in Relapsing-Remitting EAE Ameliorates Disease at First Remission, with No Synergistic Effect upon Co-Transplantation with Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. RR-EAE Induction
2.2. Generation of Bone Marrow-Derived MSCs
2.3. Intense Immunosuppression and Cell Therapy
2.4. Flow Cytometry
2.5. ELISA
2.6. Statistical Analysis
3. Results
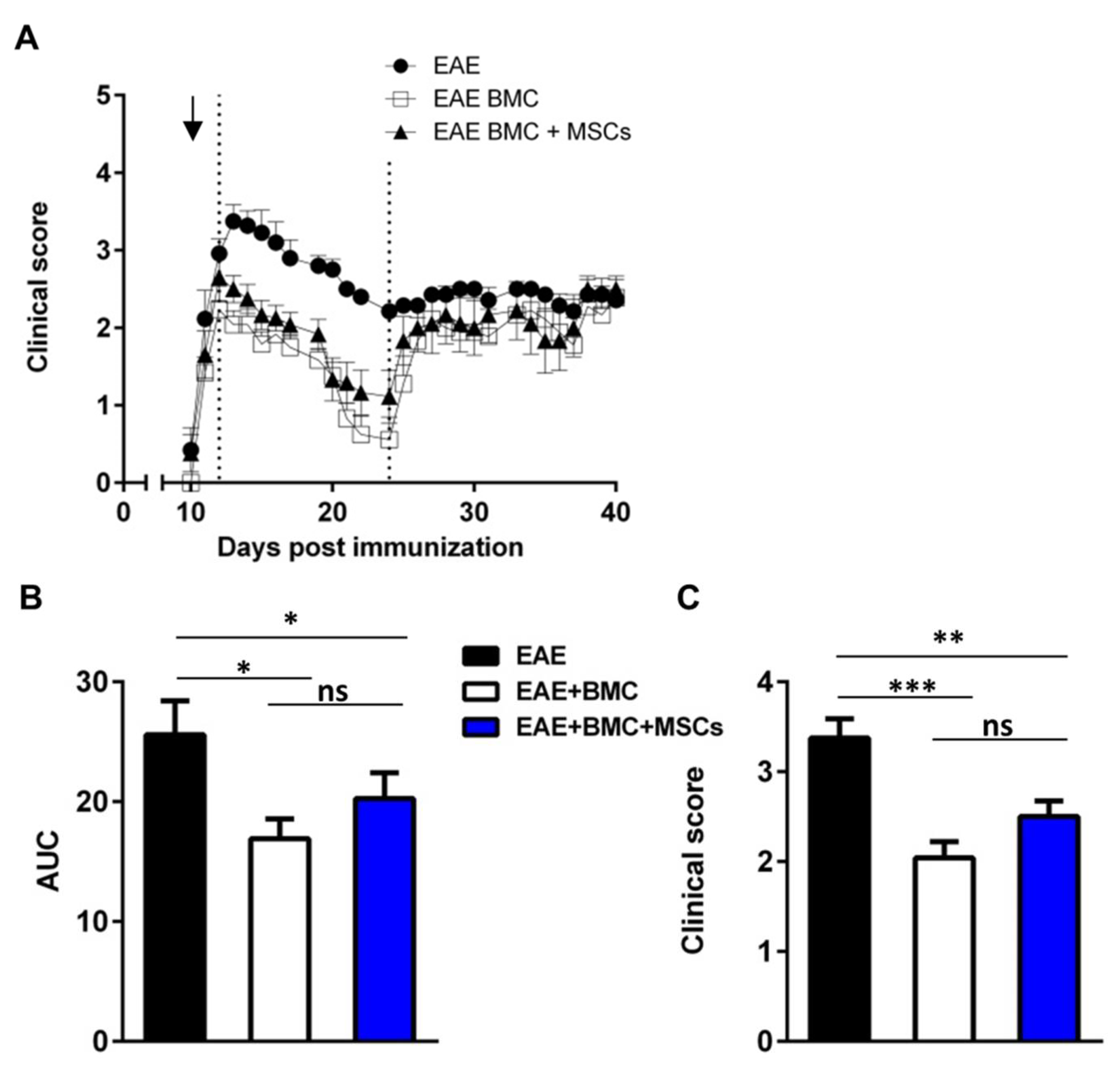
3.1. Immunosuppressed, BMC-Transplanted Mice Develop Milder RR-EAE Than Their Untreated Counterparts
3.2. Co-Transplantation of MSCs with BMC Has No Synergistic Beneficial Effect on RR-EAE
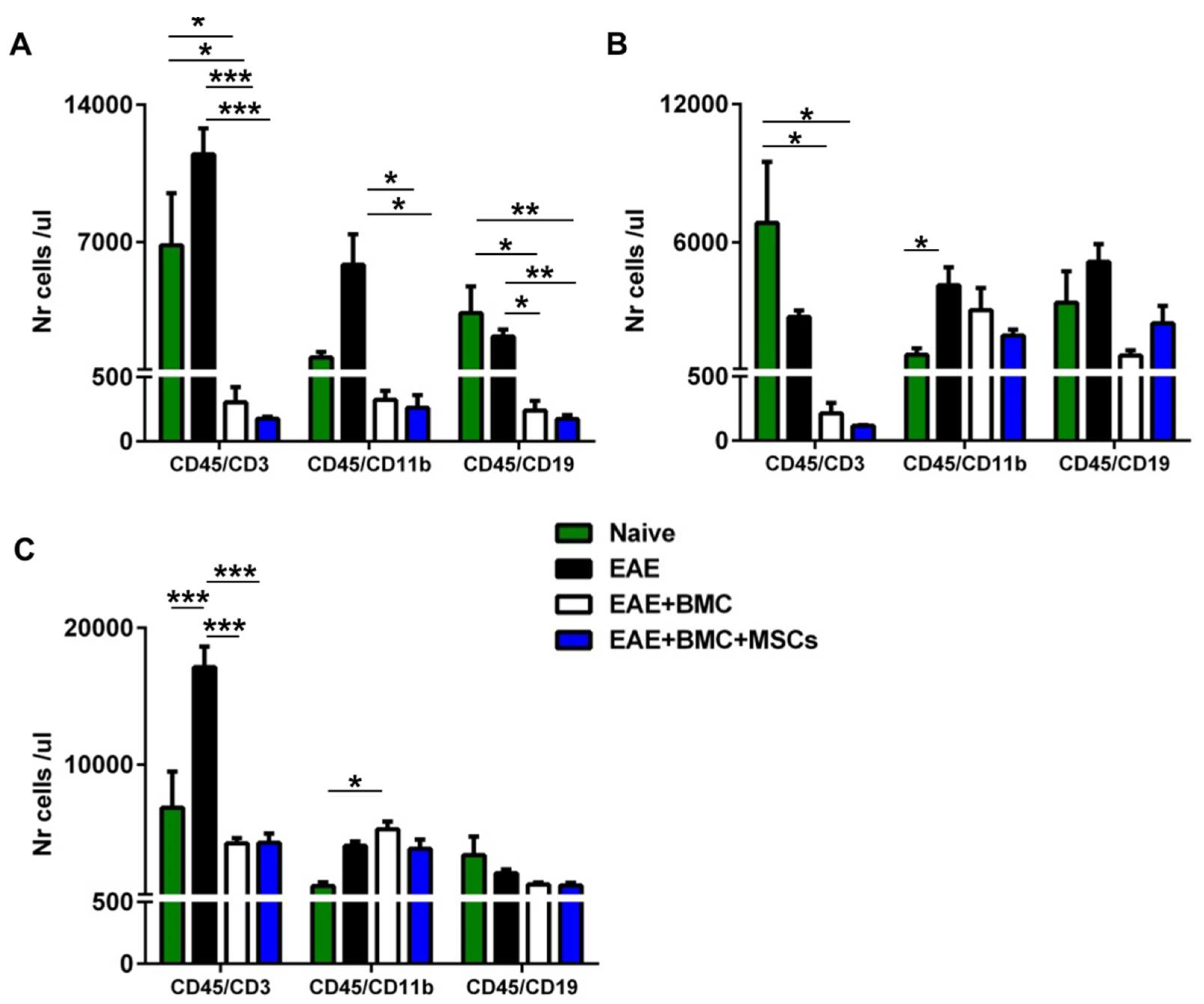
3.3. TBI Induces an Aplastic Phase in RR-EAE-Affected Mice That Is Reverted as a Result of BMC Administration; Co-Transplantation with MSCs Does Not Increase Immune Cell Engraftment
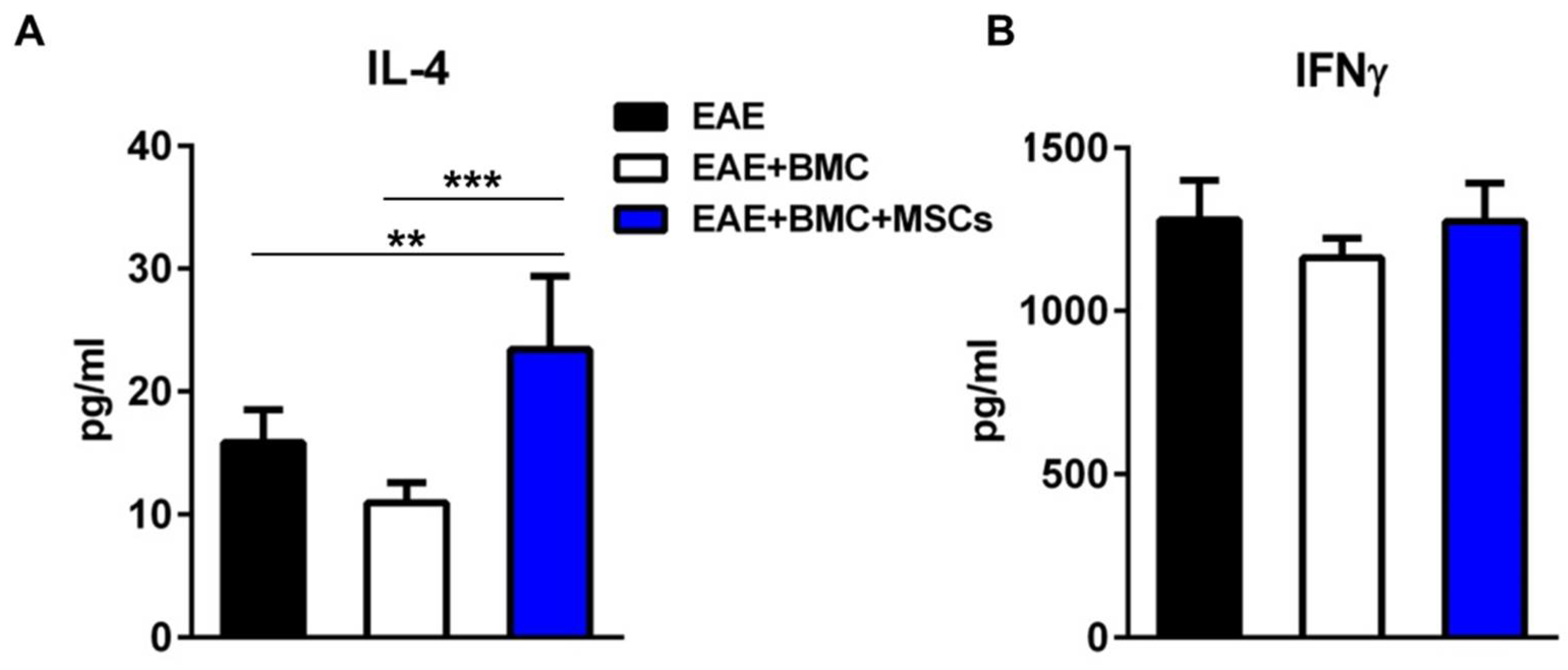
3.4. The Plasma Level of IL4 Is Significantly Elevated in Co-Transplanted Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Dendrou, C.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef]
- Muraro, P.A.; Pasquini, M.; Atkins, H.L.; Bowen, J.D.; Farge, D.; Fassas, A.; Freedman, M.S.; Georges, G.E.; Gualandi, F.; Hamerschlak, N.; et al. Long-term Outcomes After Autologous Hematopoietic Stem Cell Transplantation for Multiple Sclerosis. JAMA Neurol. 2017, 74, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Sormani, M.P.; Muraro, P.A.; Schiavetti, I.; Signori, A.; Laroni, A.; Saccardi, R.; Mancardi, G.L. Autologous hematopoietic stem cell transplantation in multiple sclerosis: A meta-analysis. Neurology 2017, 88, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.D.; Karpus, W.J. Experimental autoimmune encephalomyelitis in the mouse. Curr. Protoc. Immunol. 2007. [Google Scholar] [CrossRef] [Green Version]
- McRae, B.L.; Kennedy, M.K.; Tan, L.; Canto, M.C.D.; Picha, K.S.; Miller, S.D. Induction of active and adoptive relapsing experimental autoimmune encephalomyelitis (EAE) using an encephalitogenic epitope of proteolipid protein. J. Neuroimmunol. 1992, 38, 229–240. [Google Scholar] [CrossRef]
- Zappia, E.; Casazza, S.; Pedemonte, E.; Benvenuto, F.; Bonanni, I.; Gerdoni, E.; Giunti, D.; Ceravolo, A.; Cazzanti, F.; Frassoni, F.; et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 2005, 106, 1755–1761. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Lennon, D.P.; Caplan, A.; DeChant, A.; Hecker, J.; Kranso, J.; Zaremba, A.; Miller, R.H. Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat. Neurosci. 2012, 15, 862–870. [Google Scholar] [CrossRef] [Green Version]
- Karussis, D.M.; Vourka-Karussis, U.; Lehmann, D.; Ovadia, H.; Mizrachi-Koll, R.; Ben-Nun, A.; Abramsky, O.; Slavin, S. Prevention and reversal of adoptively transferred, chronic relapsing experimental autoimmune encephalomyelitis with a single high dose cytoreductive treatment followed by syngeneic bone marrow transplantation. J. Clin. Investig. 1993, 92, 765–772. [Google Scholar] [CrossRef] [Green Version]
- Van Gelder, M.; Kinwel-Bohre, E.P.; van Bekkum, D.W. Treatment of experimental allergic encephalomyelitis in rats with total body irradiation and syngeneic BMT. Bone Marrow Transpl. 1993, 11, 233–241. [Google Scholar]
- Karussis, D.M.; Slavin, S.; Lehmann, D.; Mizrachi-Koll, R.; Abramsky, O.; Ben-Nun, A. Prevention of experimental autoimmune encephalomyelitis and induction of tolerance with acute immunosuppression followed by syngeneic bone marrow transplantation. J. Immunol. 1992, 148, 1693–1698. [Google Scholar] [PubMed]
- Gerdoni, E.; Gallo, B.; Casazza, S.; Musio, S.; Bonanni, I.; Pedemonte, E.; Mantegazza, R.; Frassoni, F.; Mancardi, G.; Pedotti, R.; et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann. Neurol. 2007, 61, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Frenette, P.S. Niches for Hematopoietic Stem Cells and Their Progeny. Immunity 2018, 48, 632–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jitschin, R.; Mougiakakos, D.; Von Bahr, L.; Völkl, S.; Moll, G.; Ringdén, O.; Kiessling, R.; Linder, S.; Le Blanc, K. Alterations in the Cellular Immune Compartment of Patients Treated with Third-Party Mesenchymal Stromal Cells Following Allogeneic Hematopoietic Stem Cell Transplantation. Stem Cells 2013, 31, 1715–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarus, H.M.; Koc, O.N.; Devine, S.M.; Curtin, P.; Maziarz, R.T.; Holland, H.K.; Shpall, E.J.; McCarthy, P.; Atkinson, K.; Cooper, B.W.; et al. Cotransplantation of HLA-Identical Sibling Culture-Expanded Mesenchymal Stem Cells and Hematopoietic Stem Cells in Hematologic Malignancy Patients. Biol. Blood Marrow Transplant. 2005, 11, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Schulte, B.A.; LaRue, A.C.; Ogawa, M.; Zhou, D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood 2006, 107, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Al Jumah, M.A.; Abumaree, M.H. The immunomodulatory and neuroprotective effects of mesenchymal stem cells (MSCs) in experimental autoimmune encephalomyelitis (EAE): A model of multiple sclerosis (MS). Int. J. Mol. Sci. 2012, 13, 9298–9331. [Google Scholar] [CrossRef] [Green Version]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef] [Green Version]
- Tintore, M.; Vidal-Jordana, A.; Garriga, J.S. Treatment of multiple sclerosis—success from bench to bedside. Nat. Rev. Neurol. 2019, 15, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A. Challenge of progressive multiple sclerosis therapy. Curr. Opin. Neurol. 2017, 30, 237–240. [Google Scholar] [CrossRef]
- Saccardi, R.; Freedman, M.S.; Sormani, M.P.; Atkins, H.; Farge, D.; Griffith, L.M.; Kraft, G.; Mancardi, G.L.; Nash, R.; Pasquini, M.; et al. A prospective, randomized, controlled trial of autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: A position paper. Mult. Scler 2012, 18, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, M.; van Bekkum, D.W. Effective treatment of relapsing experimental autoimmune encephalomyelitis with pseudoautologous bone marrow transplantation. Bone Marrow Transpl. 1996, 18, 1029–1034. [Google Scholar]
- Van Bekkum, D.W. Stem cell transplantation for autoimmune disorders. Preclinical experiments. Best Pract. Res. Clin. Haematol. 2004, 17, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Karussis, D.; Vourka-Karussis, U.; Mizrachi-Koll, R.; Abramsky, O. Acute/relapsing experimental autoimmune encephalomyelitis: Induction of long lasting, antigen-specific tolerance by syngeneic bone marrow transplantation. Mult. Scler. 1999, 5, 17–21. [Google Scholar] [CrossRef]
- Karussis, D.; Slavin, S. Hematopoietic stem cell transplantation in multiple sclerosis: Experimental evidence to rethink the procedures. J. Neurol. Sci. 2004, 223, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, B.; Kerschensteiner, M.; Korn, T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol. 2015, 14, 406–419. [Google Scholar] [CrossRef]
- Steinman, L.; Zamvil, S.S. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann. Neurol. 2006, 60, 12–21. [Google Scholar] [CrossRef]
- Tuohy, V.K.; Yu, M.; Yin, L.; Kawczak, J.A.; Johnson, J.M.; Mathisen, P.M.; Weinstock-Guttnnan, B.; Kinkel, R.P. The epitope spreading cascade during progression of experimental autoimmune encephalomyelitis and multiple sclerosis. Immunol. Rev. 1998, 164, 93–100. [Google Scholar] [CrossRef]
- McRae, B.L.; VanderLugt, C.L.; Canto, M.C.D.; Miller, S.D. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J. Exp. Med. 1995, 182, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Derdelinckx, J.; Mansilla, M.J.; De Laere, M.; Lee, W.-P.; Navarro-Barriuso, J.; Wens, I.; Nkansah, I.; Daans, J.; De Reu, H.; Keliris, A.J.; et al. Clinical and immunological control of experimental autoimmune encephalomyelitis by tolerogenic dendritic cells loaded with MOG-encoding mRNA. J. Neuroinflamm. 2019, 16, 167. [Google Scholar] [CrossRef] [Green Version]
- Muraro, P.A.; Douek, D.C.; Packer, A.; Chung, K.; Guenaga, F.J.; Cassiani-Ingoni, R.; Campbell, C.; Memon, S.; Nagle, J.W.; Hakim, F.T.; et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J. Exp. Med. 2005, 201, 805–816. [Google Scholar] [CrossRef]
- Miura, N.; Okada, S.; Zsebo, K.M.; Miura, Y.; Suda, T. Rat stem cell factor and IL-6 preferentially support the proliferation of c-kit-positive murine hemopoietic cells rather than their differentiation. Exp. Hematol. 1993, 21, 143–149. [Google Scholar]
- Zhao, K.; Liu, Q. The clinical application of mesenchymal stromal cells in hematopoietic stem cell transplantation. J. Hematol. Oncol. 2016, 9, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Blanc, K.; Samuelsson, H.; Gustafsson, B.; Remberger, M.; Sundberg, B.; Arvidson, J.; Ljungman, P.; Lonnies, H.; Nava, S.; Ringden, O. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia 2007, 21, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Koç, O.N.; Gerson, S.L.; Cooper, B.W.; Dyhouse, S.M.; Haynesworth, S.E.; Caplan, A.; Lazarus, H.M. Rapid Hematopoietic Recovery After Coinfusion of Autologous-Blood Stem Cells and Culture-Expanded Marrow Mesenchymal Stem Cells in Advanced Breast Cancer Patients Receiving High-Dose Chemotherapy. J. Clin. Oncol. 2000, 18, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Morando, S.; Vigo, T.; Esposito, M.; Casazza, S.; Novi, G.; Principato, M.C.; Furlan, R.; Uccelli, A. The therapeutic effect of mesenchymal stem cell transplantation in experimental autoimmune encephalomyelitis is mediated by peripheral and central mechanisms. Stem Cell Res. Ther. 2012, 3, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiesa, S.; Morbelli, S.; Morando, S.; Massollo, M.; Marini, C.; Bertoni, A.; Frassoni, F.; Bartolome, S.T.; Sambuceti, G.; Traggiai, E.; et al. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc. Natl. Acad. Sci. USA 2011, 108, 17384–17389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guenova, E.; Skabytska, Y.; Hoetzenecker, W.; Weindl, G.; Sauer, K.; Tham, M.; Kim, K.W.; Park, J.H.; Seo, J.H.; Ignatova, D.; et al. IL-4 abrogates T(H)17 cell-mediated inflammation by selective silencing of IL-23 in antigen-presenting cells. Proc. Natl. Acad. Sci. USA 2015, 112, 2163–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutcher, I.; Becher, B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J. Clin. Investig. 2007, 117, 1119–1127. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, R.E.; Hassan, M.; Burton, B.R.; Britton, G.; Hill, E.V.; Verhagen, J.; Wraith, D.C. IL-4 enhances IL-10 production in Th1 cells: Implications for Th1 and Th2 regulation. Sci. Rep. 2017, 7, 11315. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrara, G.; Ivaldi, F.; Mancardi, G.; Kerlero de Rosbo, N.; Uccelli, A. Bone Marrow Transfer in Relapsing-Remitting EAE Ameliorates Disease at First Remission, with No Synergistic Effect upon Co-Transplantation with Mesenchymal Stem Cells. Vaccines 2021, 9, 736. https://doi.org/10.3390/vaccines9070736
Ferrara G, Ivaldi F, Mancardi G, Kerlero de Rosbo N, Uccelli A. Bone Marrow Transfer in Relapsing-Remitting EAE Ameliorates Disease at First Remission, with No Synergistic Effect upon Co-Transplantation with Mesenchymal Stem Cells. Vaccines. 2021; 9(7):736. https://doi.org/10.3390/vaccines9070736
Chicago/Turabian StyleFerrara, Giovanni, Federico Ivaldi, Gianluigi Mancardi, Nicole Kerlero de Rosbo, and Antonio Uccelli. 2021. "Bone Marrow Transfer in Relapsing-Remitting EAE Ameliorates Disease at First Remission, with No Synergistic Effect upon Co-Transplantation with Mesenchymal Stem Cells" Vaccines 9, no. 7: 736. https://doi.org/10.3390/vaccines9070736
APA StyleFerrara, G., Ivaldi, F., Mancardi, G., Kerlero de Rosbo, N., & Uccelli, A. (2021). Bone Marrow Transfer in Relapsing-Remitting EAE Ameliorates Disease at First Remission, with No Synergistic Effect upon Co-Transplantation with Mesenchymal Stem Cells. Vaccines, 9(7), 736. https://doi.org/10.3390/vaccines9070736






