Specific Treatment Exists for SARS-CoV-2 ARDS
Abstract
1. Introduction
2. Evidence from Brain Diseases
3. Projected Neurological Findings for COVID-19-Associated ARDS
4. The Preliminary Clinical Features of Neurological Disease Associated with COVID-19 ARDS
4.1. Statistics 1: Chi-Square Statistics
4.2. Statistics 2: Fisher’s Exact Test
4.3. Statistics 3: Chi-Squared Test for ARDS Onset and Mortality
Significance Level
5. Treatment Mechanisms
6. Investigation of SARS-CoV-2 Vaccination and Nanobody Drugs
7. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riley, S.; Ainslie, K.E.C.; Eales, O.; Walters, C.E.; Wang, H.; Atchison, C.; Fronterre, C.; Diggle, P.J.; Ashby, D.; Donnelly, C.A.; et al. Resurgence of SARS-CoV-2: Detection by community viral surveillance. Science 2021, 372, 990–995. [Google Scholar] [CrossRef]
- Johns Hopkins University, C.R.C. Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/ (accessed on 24 April 2021).
- Cabinet Office. Coronavirus (COVID-19): What Has Changed—9 September. Available online: https://www.gov.uk/government/news/coronavirus-covid-19-what-has-changed-9-september (accessed on 24 April 2021).
- Ellul, M.A.; Benjamin, L.; Singh, B.; Lant, S.; Michael, B.D.; Easton, A.; Kneen, R.; Defres, S.; Sejvar, J.; Solomon, T. Neurological associations of COVID-19. Lancet Neurol. 2020, 19, 767–783. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Lee, J.-H.; An, H.K.; Sohn, M.-G.; Kivela, P.; Oh, S. 4,4′-Diaminodiphenyl Sulfone (DDS) as an Inflammasome Competitor. Int. J. Mol. Sci. 2020, 21, 5953. [Google Scholar] [CrossRef]
- Lee, M.-H.; Perl, D.P.; Nair, G.; Li, W.; Maric, D.; Murray, H.; Dodd, S.J.; Koretsky, A.P.; Watts, J.A.; Cheung, V.; et al. Microvascular injury in the brains of patients with COVID-19. N. Engl. J. Med. 2020, 384, 481–483. [Google Scholar] [CrossRef]
- Yachou, Y.; El Idrissi, A.; Belapasov, V.; Ait Benali, S. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: Understanding the neurological manifestations in COVID-19 patients. Neurol. Sci. 2020, 41, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, S.H.; Lee, C.J.; Oh, S.S. Recovery of Dementia Syndrome following Treatment of Brain Inflammation. Dement. Geriatr. Cogn. Disord. Extra 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Namba, Y.; Kawatsu, K.; Izumi, S.; Ueki, A.; Ikeda, K. Neurofibrillary tangles and senile plaques in brain of elderly leprosy patients. Lancet 1992, 340, 978. [Google Scholar] [CrossRef]
- Kimura, T.; Goto, M. Existence of senile plaques in the brains of elderly leprosy patients. Lancet 1993, 342, 1364. [Google Scholar] [CrossRef]
- Endoh, M.; Kunishita, T.; Tabira, T. No effect of anti-leprosy drugs in the prevention of Alzheimer’s disease and β-amyloid neurotoxicity. J. Neurol. Sci. 1999, 165, 28–30. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, C.J.; Park, J.; Lee, S.J.; Su-Hee, C. The neuro-inflammasome in Alzheimer’s disease and cerebral stroke. Dement. Geriatr. Cogn. Disord. Extra 2021. [Google Scholar] [CrossRef]
- Jong-Hoon, L.; Chul Joong, L.; Jungwuk, P.; So Jeong, L.; Su-Hee, C.; Sang-Suk, O. The Preventive and Treatment of the Neuroinflammasome in Sorokdo National Hospital. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Tirado-Sánchez, A.; Díaz-Molina, V.; Ponce-Olivera, R. Efficacy and safety of azathioprine and dapsone as an adjuvant in the treatment of bullous pemphigoid. Allergol. Immunopathol. 2012, 40, 152–155. [Google Scholar] [CrossRef]
- Salehzadeh, F.; Jahangiri, S.; Mohammadi, E. Dapsone as an alternative therapy in children with familial Mediterranean fever. Iran. J. Pediatr. 2012, 22, 23. [Google Scholar] [PubMed]
- Tamarkin, D.; Friedman, D.; Eini, M. Anti-Infection Augmentation Foamable Compositions and Kit and Uses Thereof. U.S. Patent Application US11/732,547, 20 December 2007. [Google Scholar]
- Kast, R.E. Erlotinib augmentation with dapsone for rash mitigation and increased anti-cancer effectiveness. SpringerPlus 2015, 4, 638. [Google Scholar] [CrossRef]
- Park, S.C.; Lee, J.; Cho, S.C.; Park, M.C.; Cho, Y.J. Composition for Control of Aging and/or Extension of Life, Containing Dapsone as Active Ingredient. U.S. Patent Application US12/737,553, 23 June 2011. [Google Scholar]
- McGeer, P.L.; Harada, N.; Kimura, H.; McGeer, E.G.; Schulzer, M. Dapsone and Promin for the Treatment of Dementia. U.S. Patent Application US5532219A, 2 July 1996. [Google Scholar]
- Yang, N.; Li, L.; Li, Z.; Ni, C.; Cao, Y.; Liu, T.; Tian, M.; Chui, D.; Guo, X. Protective effect of dapsone on cognitive impairment induced by propofol involves hippocampal autophagy. Neurosci. Lett. 2017, 649, 85–92. [Google Scholar] [CrossRef]
- Sulzer, D.; Antonini, A.; Leta, V.; Nordvig, A.; Smeyne, R.J.; Goldman, J.E.; Al-Dalahmah, O.; Zecca, L.; Sette, A.; Bubacco, L.; et al. COVID-19 and possible links with Parkinson’s disease and parkinsonism: From bench to bedside. NPJ Parkinson’s Dis. 2020, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Lim, Y.J.; Liu, Z.; Long, H.; Sun, Y.; Hu, J.J.; Zhao, C.; Tao, Y.; Zhang, X.; Li, D.; et al. Parkinson’s disease-related phosphorylation at Tyr39 rearranges alpha-synuclein amyloid fibril structure revealed by cryo-EM. Proc. Natl. Acad. Sci. USA 2020, 117, 20305–20315. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E.; Lefranc, F.; Karpel-Massler, G.; Halatsch, M.E. Why dapsone stops seizures and may stop neutrophils’ delivery of VEGF to glioblastoma. Br. J. Neurosurg. 2012, 26, 813–817. [Google Scholar] [CrossRef]
- Nader-Kawachi, J.; Góngora-Rivera, F.; Santos-Zambrano, J.; Calzada, P.; Ríos, C. Neuroprotective effect of dapsone in patients with acute ischemic stroke: A pilot study. Neurol. Res. 2007, 29, 331–334. [Google Scholar] [CrossRef]
- Cruz-Cruz, C.; Kravzov-Jinich, J.; Martínez-Núñez, J.M.; Ríos-Castañeda, C.; Perez, M.E.; Altagracia-Martínez, M. Cost–utility analysis in acute ischemic stroke survivors treated with dapsone in a public hospital in Mexico City. J. Pharm. Health Serv. Res. 2014, 5, 95–102. [Google Scholar] [CrossRef]
- Jong-Hoon, L.; Badar, K.; Chul Joong, L.; Asif, K.; Jenny, B.; Richard, E.K.; Sang-Suk, O.; Consolato, S. Randomized Controlled Trial of Dapsone for Targeting SARS-CoV-2-Activated Inflammasomes. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.R.; Liu, H.; Irwanto, A.; Fu, X.A.; Li, Y.; Yu, G.Q.; Yu, Y.X.; Chen, M.F.; Low, H.Q.; Li, J.H.; et al. HLA-B*13:01 and the Dapsone Hypersensitivity Syndrome. N. Engl. J. Med. 2013, 369, 1620–1628. [Google Scholar] [CrossRef]
- Tian, S.; Hu, W.; Niu, L.; Liu, H.; Xu, H.; Xiao, S.-Y. Pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020, 15, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Frater, J.L.; Zini, G.; D’Onofrio, G.; Rogers, H.J. COVID-19 and the clinical hematology laboratory. Int. J. Lab. Hematol. 2020, 42, 11–18. [Google Scholar] [CrossRef]
- Schmitt, J.; Lorenz, M.; Wozel, G. Hypersensitivity Reactions to Dapsone: A Systematic Review. Acta Derm. Venereol. 2012, 92, 194–199. [Google Scholar] [CrossRef]
- Alves-Rodrigues, E.N.; Ribeiro, L.C.; Silva, M.D.; Takiuchi, A.; Fontes, C.J.F. Dapsone syndrome with acute renal failure during leprosy treatment: Case report. Braz. J. Infect. Dis. 2005, 9, 84–86. [Google Scholar] [CrossRef]
- Wozel, V.G. Innovative use of dapsone. Dermatol. Clin. 2010, 28, 599–610. [Google Scholar] [CrossRef]
- Bangash, M.N.; Patel, J.; Parekh, D. COVID-19 and the liver: Little cause for concern. Lancet Gastroenterol. Hepatol. 2020, 5, 529–530. [Google Scholar] [CrossRef]
- Liu, F.; Long, X.; Zou, W.; Fang, M.; Wu, W.; Li, W.; Zhang, B.; Zhang, W.; Chen, X.; Zhang, Z. Highly ACE2 Expression in Pancreas May Cause Pancreas Damage After SARS-CoV-2 Infection. medRxiv 2020. [Google Scholar] [CrossRef]
- Zhu, K.; He, F.; Jin, N.; Lou, J.; Cheng, H. Complete atrioventricular block associated with dapsone therapy: A rare complication of dapsone-induced hypersensitivity syndrome. J. Clin. Pharm. Ther. 2009, 34, 489–492. [Google Scholar] [CrossRef]
- Ghishan, F.K. The sulfone syndrome complicated by pancreatitis and pleural effusion in an adolescent receiving dapsone for treatment of acne vulgaris. J. Pediatr. Gastroenterol. Nutr. 1998, 26, 103–105. [Google Scholar]
- Kissling, S.; Rotman, S.; Gerber, C.; Halfon, M.; Lamoth, F.; Comte, D.; Lhopitallier, L.; Sadallah, S.; Fakhouri, F. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020, 98, 228–231. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Ma, Y.-T.; Zhang, J.-Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef]
- Kang, K.S.; Kim, H.I.; Kim, O.H.; Cha, K.C.; Kim, H.; Lee, K.H.; Hwang, S.O.; Cha, Y.S. Clinical outcomes of adverse cardiovascular events in patients with acute dapsone poisoning. Clin. Exp. Emerg. Med. 2016, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Oxley, T.J.; Mocco, J.; Majidi, S.; Kellner, C.P.; Shoirah, H.; Singh, I.P.; De Leacy, R.A.; Shigematsu, T.; Ladner, T.R.; Yaeger, K.A.; et al. Large-Vessel Stroke as a Presenting Feature of COVID-19 in the Young. N. Engl. J. Med. 2020, 382, e60. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef]
- Kast, R.E. Dapsone as treatment adjunct in ARDS. Exp. Lung Res. 2020, 46, 157–161. [Google Scholar] [CrossRef]
- Miller, D.S. Regulation of P-glycoprotein and other ABC drug transporters at the blood–brain barrier. Trends Pharmacol Sci. 2010, 31, 246–254. [Google Scholar] [CrossRef]
- Nau, R.; Sorgel, F.; Eiffert, H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin. Microbiol. Rev. 2010, 23, 858–883. [Google Scholar] [CrossRef]
- Kanwar, B.; Sergi, C.; Lee, J.-H. Hunt Regional Medical Center Policy for Dapsone Administration of ARDS by SARS-CoV-2; Kanwar, B., Ed.; OSF (Open Science Foundation): Charlotteville, VA, USA, 2020. [Google Scholar] [CrossRef]
- Chakraborty, A.; Panda, A.K.; Ghosh, R.; Biswas, A. DNA minor groove binding of a well known anti-mycobacterial drug dapsone: A spectroscopic, viscometric and molecular docking study. Arch. Biochem. Biophys. 2019, 665, 107–113. [Google Scholar] [CrossRef]
- Rajendran, N.D.; Mookan, N.; Samuel, I.; Mookan, S.B.; Munusamy, G.; Gurudeeban, S.; Kaliamurthi, S. A theoretical study of chemical bonding and topological and electrostatic properties of the anti-leprosy drug dapsone. J. Mol. Model. 2020, 26, 138. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Lee, S.-B.; Kang, T.-J.; Chae, G.-T. Detection of gene mutations related with drug resistance inMycobacterium lepraefrom leprosy patients using Touch-Down (TD) PCR. FEMS Immunol. Med. Microbiol. 2003, 36, 27–32. [Google Scholar] [CrossRef]
- Mendes, A.P.; Schalcher, T.R.; Barros, T.G.; Almeida, E.D.; Maia, C.S.; Barros, C.A.; Monteiro, M.C.; Borges, R.S. A Geometric and Electronic Study of Dapsone. J. Comput. Theor. Nanosci. 2011, 8, 1428–1431. [Google Scholar] [CrossRef]
- Schrodinger, R.; Schrödinger, E. What Is life? With Mind and Matter and Autobiographical Sketches; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Gatti, G.; Hossein, J.; Malena, M.; Cruciani, M.; Bassetti, M. Penetration of dapsone into cerebrospinal fluid of patients with AIDS. J. Antimicrob. Chemother. 1997, 40, 113–115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rich, J.D.; Mirochnick, M. Dapsone penetrates cerebrospinal fluid during Pneumocystis carinii pneumonia prophylaxis. Diagn. Microbiol. Infect. Dis. 1996, 24, 77–79. [Google Scholar] [CrossRef]
- Varadarajan, S.; Yatin, S.; Kanski, J.; Jahanshahi, F.; Butterfield, D.A. Methionine residue 35 is important in amyloid β-peptide-associated free radical oxidative stress. Brain Res. Bull. 1999, 50, 133–141. [Google Scholar] [CrossRef]
- Vogt, W. Oxidation of methionyl residues in proteins: Tools, targets, and reversal. Free Radic. Biol. Med. 1995, 18, 93–105. [Google Scholar] [CrossRef]
- Enache, T.A.; Oliveira-Brett, A.M. Alzheimer’s disease amyloid beta peptides in vitro electrochemical oxidation. Bioelectrochemistry 2017, 114, 13–23. [Google Scholar] [CrossRef]
- Francioso, A.; Baseggio Conrado, A.; Blarzino, C.; Foppoli, C.; Montanari, E.; Dinarelli, S.; Giorgi, A.; Mosca, L.; Fontana, M. One- and Two-Electron Oxidations of beta-Amyloid25-35 by Carbonate Radical Anion (CO3(*-)) and Peroxymonocarbonate (HCO4(-)): Role of Sulfur in Radical Reactions and Peptide Aggregation. Molecules 2020, 25, 961. [Google Scholar] [CrossRef]
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Hacisuleyman, E.; Hale, C.; Saito, Y.; Blachere, N.E.; Bergh, M.; Conlon, E.G.; Schaefer-Babajew, D.J.; Dasilva, J.; Muecksch, F.; Gaebler, C.; et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N. Engl. J. Med. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Nambulli, S.; Xiao, Z.; Liu, H.; Sang, Z.; Duprex, W.P.; Schneidman-Duhovny, D.; Zhang, C.; Shi, Y. Versatile and multivalent nanobodies efficiently neutralize SARS-CoV-2. Science 2020, 370, 1479–1484. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Reynoso-Robles, R.; Kulesza, R.J.; Mukherjee, P.S.; Torres-Jardón, R.; Rönkkö, T.; Doty, R.L. Alzheimer’s disease and alpha-synuclein pathology in the olfactory bulbs of infants, children, teens and adults ≤ 40 years in Metropolitan Mexico City. APOE4 carriers at higher risk of suicide accelerate their olfactory bulb pathology. Environ. Res. 2018, 166, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Pal, I.; Nath, A.K.; Dey, S.G. Peroxidase activity of heme bound amyloid β peptides associated with Alzheimer’s disease. Chem. Commun. 2020, 56, 4505–4518. [Google Scholar] [CrossRef]
- Kiko, T.; Nakagawa, K.; Satoh, A.; Tsuduki, T.; Furukawa, K.; Arai, H.; Miyazawa, T. Amyloid β levels in human red blood cells. PLoS ONE 2012, 7, e49620. [Google Scholar] [CrossRef]
- Blair-Johnson, M.; Fiedler, T.; Fenna, R. Human myeloperoxidase: Structure of a cyanide complex and its interaction with bromide and thiocyanate substrates at 1.9 Å resolution. Biochemistry 2001, 40, 13990–13997. [Google Scholar] [CrossRef] [PubMed]
- Shamova, E.V.; Gorudko, I.V.; Grigorieva, D.V.; Sokolov, A.V.; Kokhan, A.U.; Melnikova, G.B.; Yafremau, N.A.; Gusev, S.A.; Sveshnikova, A.N.; Vasilyev, V.B. The effect of myeloperoxidase isoforms on biophysical properties of red blood cells. Mol. Cell. Biochem. 2020, 464, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Ruggiano, A.; Ramadan, K. DNA–protein crosslink proteases in genome stability. Commun. Biol. 2021, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Ghodke, P.P.; Gonzalez-Vasquez, G.; Wang, H.; Johnson, K.M.; Sedgeman, C.A.; Guengerich, F.P. Enzymatic bypass of an N6-deoxyadenosine DNA–ethylene dibromide–peptide cross-link by translesion DNA polymerases. J. Biol. Chem. 2021, 296, 100444. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequalae of COVID-19. Nature 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
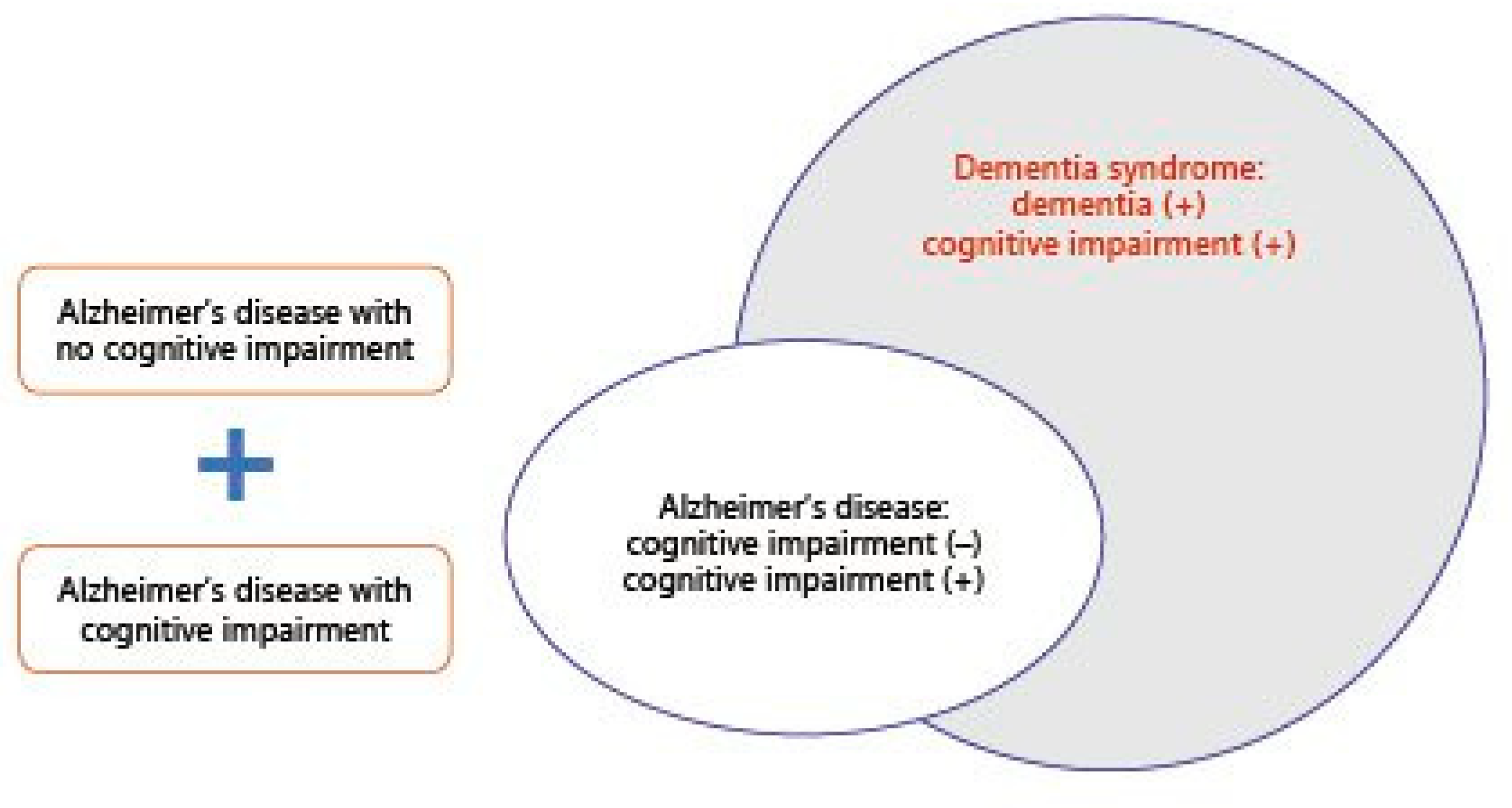
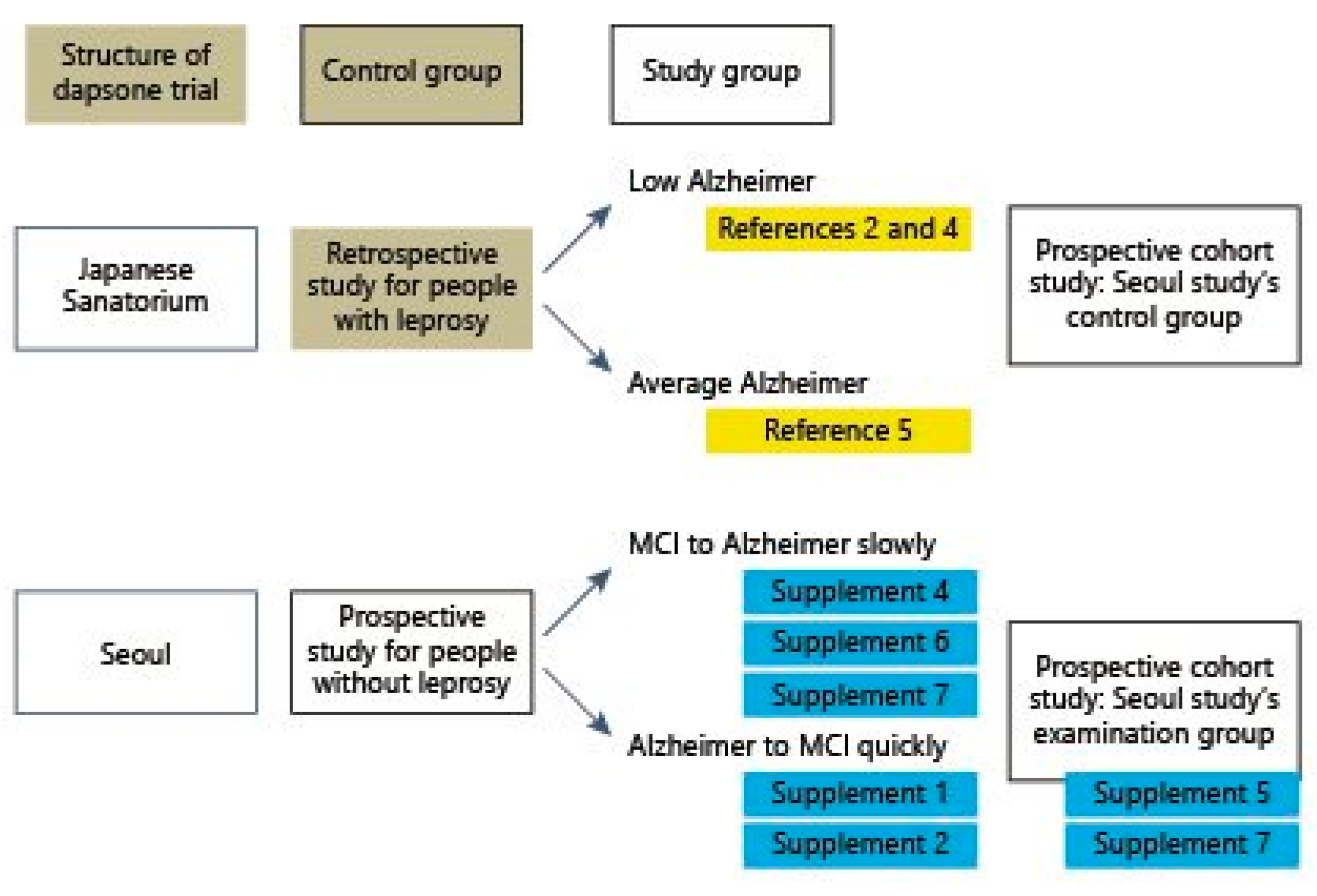
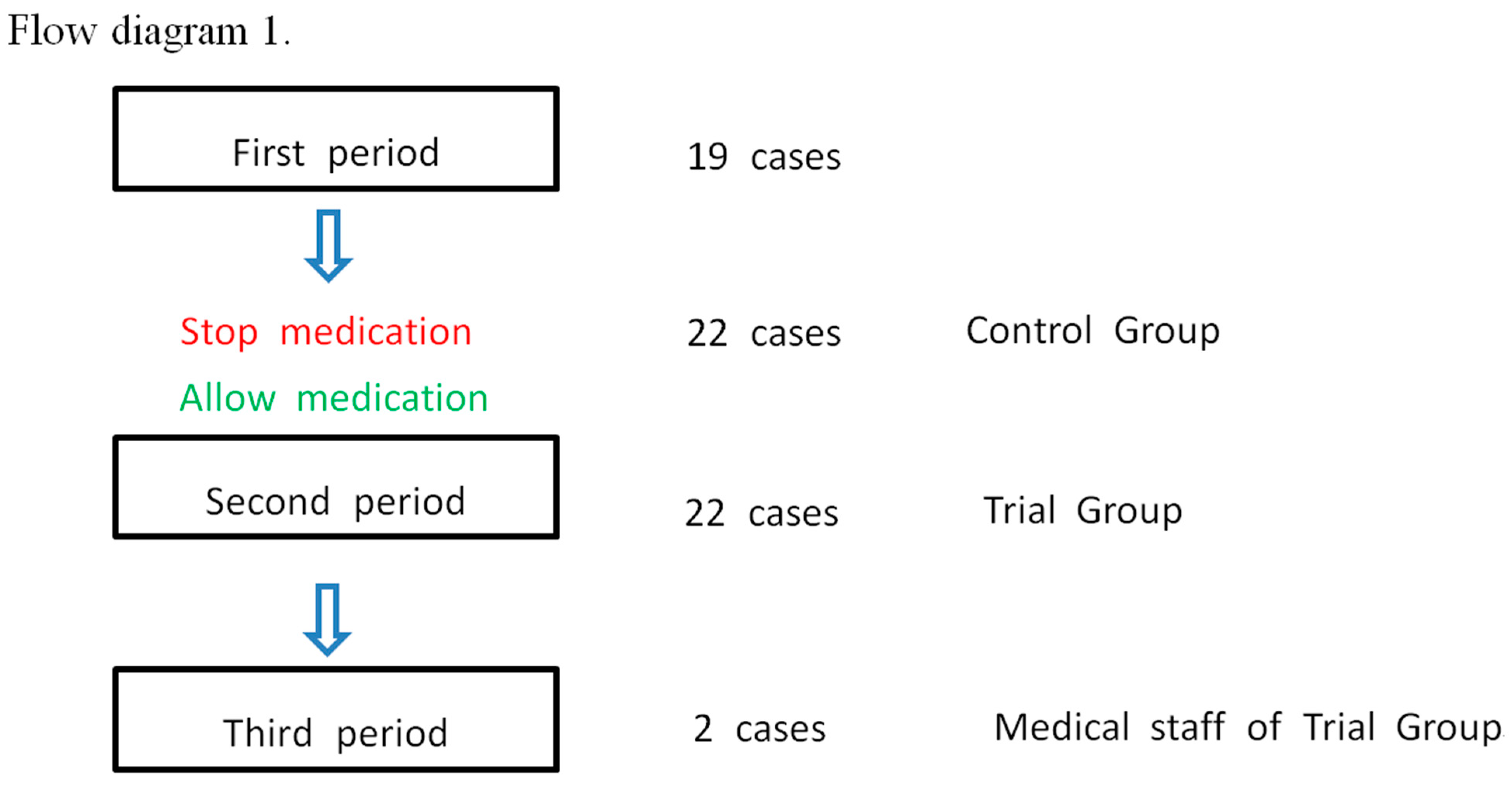
| Clinical Manifestations | SARS-CoV-2 Symptoms | Dapsone’s Effects | |
|---|---|---|---|
| Hypersensitivity reactions | SARS-CoV-2 symptoms are like a severe idiosyncratic reaction to dapsone characterized by the clinical triad of fever, rash, and systemic involvement, which can cause powerful organ (heart, kidney, lung, and brain) dysfunction [28] | The syndrome is a severe idiosyncratic reaction to dapsone characterized by the clinical triad of fever, rash, and systemic involvement (most commonly of the liver and the hematologic system), which can cause severe organ dysfunction [29] | Similar |
| Hematology laboratory | Focal fibrin clusters mixed with mononuclear inflammatory cells, decreased eosinophils, decreased lymphocytes, increased neutrophils [30], lymphopenia, leukocytosis, neutrophilia, and thrombocytopenia [31] | Leukocytosis and eosinophilia [32], resembling a mononucleosis infection [33] | Similar |
| Anemia | Thrombocytopenia and consumptive coagulopathy [31] | Hemolytic anemia and methemoglobinemia [34] | Similar |
| Liver disease, pancreatic disease | Clinically significant liver injury is uncommon [35]; pancreatic cells highly express ACE2 [36] | Hepatitis/liver toxicity [33]; cholangitis, colitis, and thyroiditis [37]; pancreatitis and pleural effusion [38] | Similar |
| Renal disease | Severe collapsing, focal segmental glomerulosclerosis, and acute tubular necrosis [39] | Acute renal failure [33] | Similar |
| Cardiac disease | Acute myocardial injury and chronic damage to the cardiovascular system [40] | Myocarditis, and dapsone-induced hypersensitivity syndrome-associated complete atrioventricular block [37]; myocardial injury [41] | Similar |
| Pulmonary disease | Coronavirus disease (COVID-19)-related pneumonia [28,40] | Pneumonitis [37]; pneumonia or multiple organ failure [41] | Similar |
| Neurologic disease | Large-vessel stroke [42] encephalopathy, prominent agitation and confusion, and corticospinal tract signs [43,44] | Recovery of dementia syndrome following treatment of brain inflammation [45]; dapsone alleviated MCI, early AD, and stroke [13] | Different |
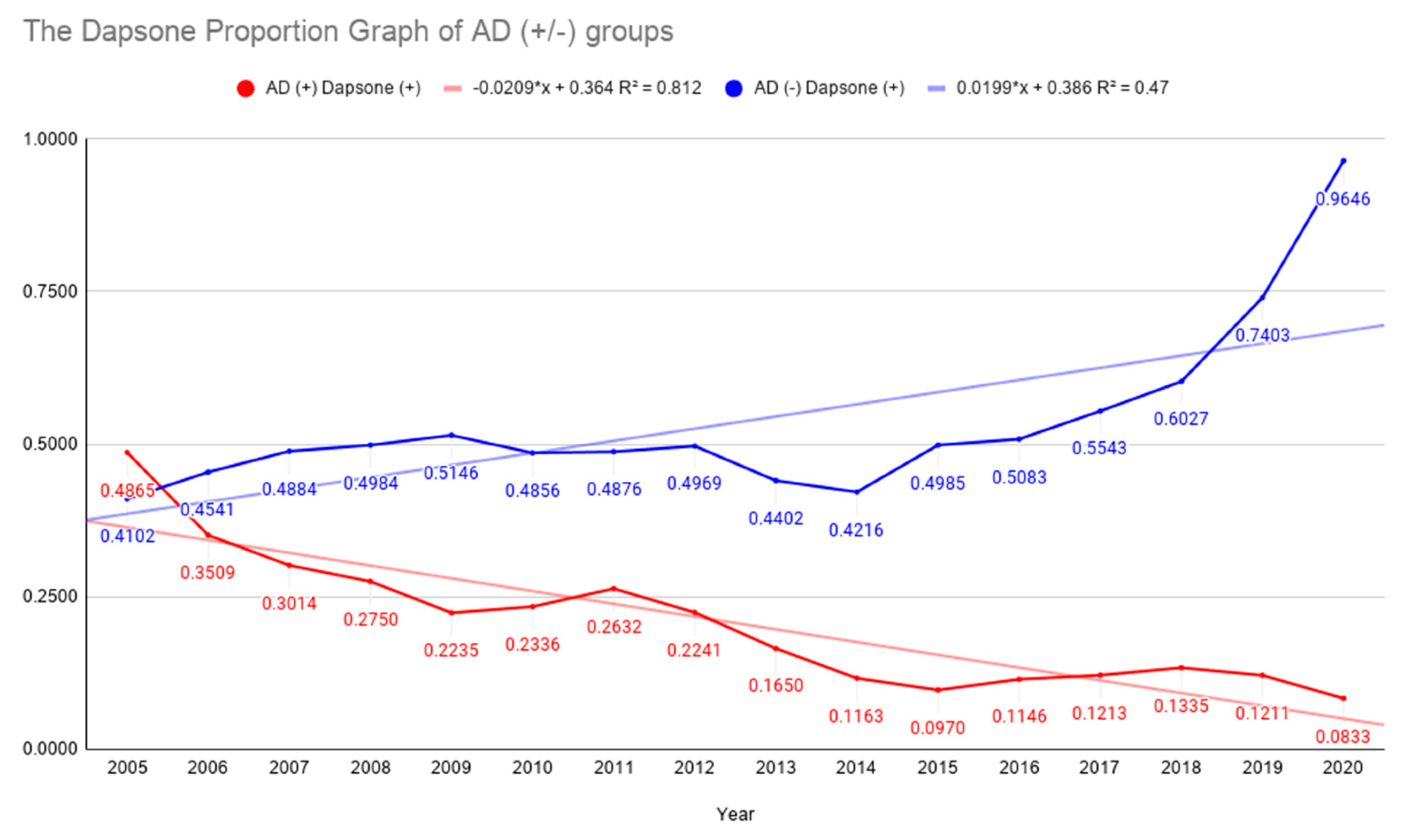
| Year | Dapsone (+) | Dapsone (−) | AD (+) Total | Dapsone (+) | Dapsone (−) | AD (−) Total | Dapsone (+)/AD (+) | Dapsone (+)/AD (−) |
|---|---|---|---|---|---|---|---|---|
| 2005 | 18 | 19 | 37 | 290 | 417 | 707 | 0.4865 | 0.4102 |
| 2006 | 20 | 37 | 57 | 302 | 363 | 665 | 0.3509 | 0.4541 |
| 2007 | 22 | 51 | 73 | 317 | 332 | 649 | 0.3014 | 0.4884 |
| 2008 | 22 | 58 | 80 | 310 | 312 | 622 | 0.2750 | 0.4984 |
| 2009 | 19 | 66 | 85 | 300 | 283 | 583 | 0.2235 | 0.5146 |
| 2010 | 25 | 82 | 107 | 270 | 286 | 556 | 0.2336 | 0.4856 |
| 2011 | 35 | 98 | 133 | 255 | 268 | 523 | 0.2632 | 0.4876 |
| 2012 | 39 | 135 | 174 | 238 | 241 | 479 | 0.2241 | 0.4969 |
| 2013 | 34 | 172 | 206 | 195 | 248 | 443 | 0.1650 | 0.4402 |
| 2014 | 25 | 190 | 215 | 172 | 236 | 408 | 0.1163 | 0.4216 |
| 2015 | 26 | 242 | 268 | 167 | 168 | 335 | 0.0970 | 0.4985 |
| 2016 | 33 | 255 | 288 | 154 | 149 | 303 | 0.1146 | 0.5083 |
| 2017 | 37 | 268 | 305 | 143 | 115 | 258 | 0.1213 | 0.5543 |
| 2018 | 45 | 292 | 337 | 132 | 87 | 219 | 0.1335 | 0.6027 |
| 2019 | 46 | 334 | 380 | 114 | 40 | 154 | 0.1211 | 0.7403 |
| 2020 | 32 | 352 | 384 | 109 | 4 | 113 | 0.0833 | 0.9646 |
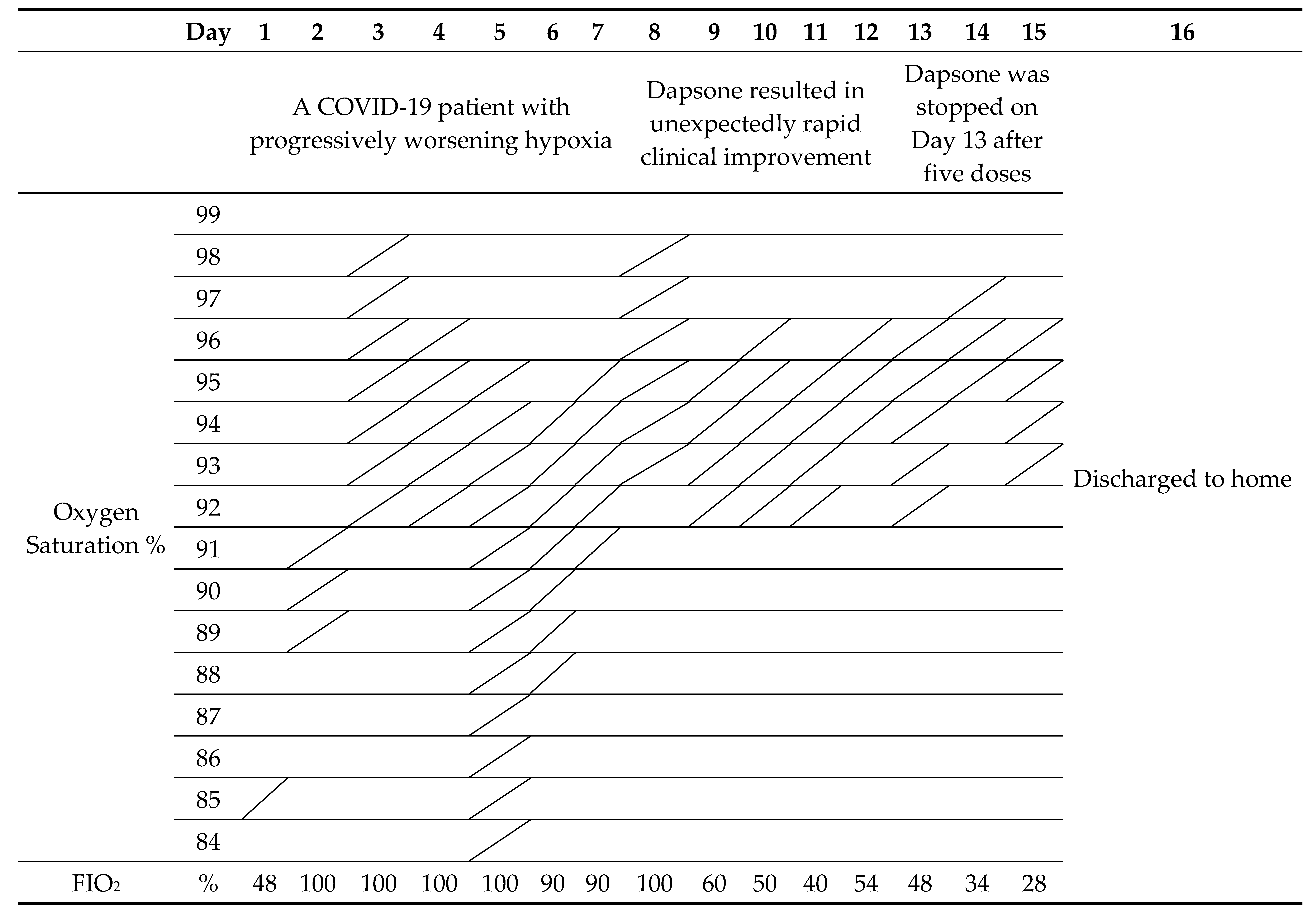
| Study 3 | Decreased FIO2 | Others | Row Totals |
|---|---|---|---|
| Dapsone (+) onset | 7 (4.29) (1.72) | 1 (3.71) (1.98) | 8 |
| Dapsone (−) onset | 8 (10.71) (0.69) | 12 (9.29) (0.79) | 20 |
| Totals | 15 | 13 | 28 |
| Study 2–4 | decFIO2 + No Progressive | Progressive | Row Totals |
|---|---|---|---|
| Dapsone (+) onset + aggravated | 17 | 3 | 20 |
| Dapsone (+) severe | 0 | 2 | 2 |
| Total | 17 | 5 | 22 |
| Death | Survival | Row Totals | |
|---|---|---|---|
| ARDS onset (with dapsone) | 1 | 16 | 17 |
| ARDS onset (without dapsone) | 8 | 12 | 20 |
| Totals | 9 | 28 | 37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanwar, B.; Lee, C.J.; Lee, J.-H. Specific Treatment Exists for SARS-CoV-2 ARDS. Vaccines 2021, 9, 635. https://doi.org/10.3390/vaccines9060635
Kanwar B, Lee CJ, Lee J-H. Specific Treatment Exists for SARS-CoV-2 ARDS. Vaccines. 2021; 9(6):635. https://doi.org/10.3390/vaccines9060635
Chicago/Turabian StyleKanwar, Badar, Chul Joong Lee, and Jong-Hoon Lee. 2021. "Specific Treatment Exists for SARS-CoV-2 ARDS" Vaccines 9, no. 6: 635. https://doi.org/10.3390/vaccines9060635
APA StyleKanwar, B., Lee, C. J., & Lee, J.-H. (2021). Specific Treatment Exists for SARS-CoV-2 ARDS. Vaccines, 9(6), 635. https://doi.org/10.3390/vaccines9060635






