RETRACTED: The Safety of COVID-19 Vaccinations—We Should Rethink the Policy
Abstract
:1. Introduction
2. Methods
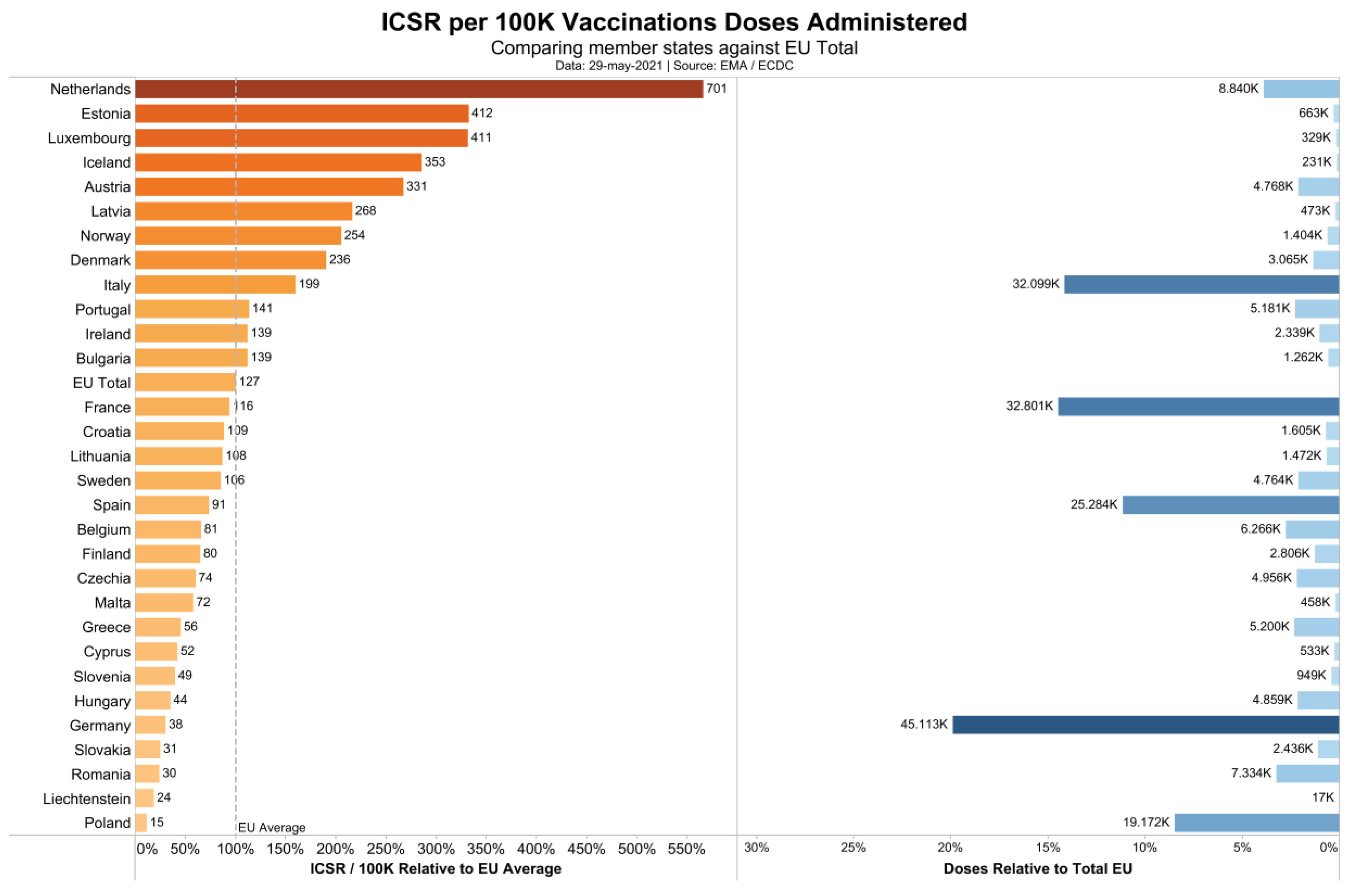
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arvay, C.G. Genetische Impfstoffe gegen COVID-19: Hoffnung oder Risiko. Schweiz. Ärztezeitung 2020, 101, 862–864. [Google Scholar]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2020, 396, 1979–1993. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Cunningham, A.S. Rapid response: COVID-19 vaccine candidate is unimpressive: NNTV is around 256. BMJ 2020, 371, m4347. [Google Scholar]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Klement, R.J.; Bandyopadhyay, P.S. The Epistemology of a Positive SARS-CoV-2 Test. Acta Biotheor. 2020. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A.; Axfors, C.; Contopoulos-Ioannidis, D.G. Population-level COVID-19 mortality risk for non-elderly individuals overall and for non-elderly individuals without underlying diseases in pandemic epicenters. Environ. Res. 2020, 188, 109890. [Google Scholar] [CrossRef] [PubMed]
- Rose, J. A report on the U.S. vaccine adverse events reporting system (VAERS) on the COVID-19 messenger ribonucleic acid (mRNA) biologicals. Sci. Public Health Policy Law 2021, 2, 59–80. [Google Scholar]
- Edridge, A.W.; Kaczorowska, J.M.; Hoste, A.C.; Bakker, M.; Klein, M.; Jebbink, M.F.; Matser, A.; Kinsella, C.; Rueda, P.; Prins, M.; et al. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 2020, 26, 1691–1693. [Google Scholar] [CrossRef] [PubMed]
- Havers, F.P.; Reed, C.; Lim, T.; Montgomery, J.M.; Klena, J.D.; Hall, A.J.; Fry, A.M.; Cannon, D.L.; Chiang, C.F.; Gibbons, A.; et al. Seroprevalence of Antibodies to SARS-CoV-2 in 10 Sites in the United States, March 23–May 12, 2020. JAMA Intern. Med. 2020, 180, 1576–1586. [Google Scholar] [CrossRef]
- Mateus, J.; Grifoni, A.; Tarke, A.; Sidney, J.; Ramirez, S.I.; Dan, J.M.; Burger, Z.C.; Rawlings, S.A.; Smith, D.M.; Phillips, E.; et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 2020, 370, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Doshi, P. COVID-19: Do many people have pre-existing immunity? BMJ 2020, 370, m3563. [Google Scholar] [CrossRef]
- Lavine, J.S.; Bjornstad, O.N.; Antia, R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science 2021, 371, 741–745. [Google Scholar] [CrossRef]
- Brandal, L.T.; Ofitserova, T.S.; Meijerink, H.; Rykkvin, R.; Lund, H.M.; Hungnes, O.; Greve-Isdahl, M.; Bragstad, K.; Nygård, K.; Winje, B.A. Minimal transmission of SARS-CoV-2 from paediatric COVID-19 cases in primary schools, Norway, August to November 2020. Eurosurveillance 2021, 26, 2002011. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Engerström, L.; Nordenhäll, C.; Larsson, E. Open Schools, COVID-19, and Child and Teacher Morbidity in Sweden. N. Engl. J. Med. 2021, 384, 669–671. [Google Scholar] [CrossRef]
- Lorent, D.; Nowak, R.; Roxo, C.; Lenartowicz, E.; Makarewicz, A.; Zaremba, B.; Nowak, S.; Kuszel, L.; Stefaniak, J.; Kierzek, R.; et al. Prevalence of Anti-SARS-CoV-2 Antibodies in Poznań, Poland, after the First Wave of the COVID-19 Pandemic. Vaccines 2021, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J. The infection fatality rate of COVID-19 inferred from seroprevalence data. Bull. World Health Organ. 2021, 99, 19F–33F. [Google Scholar] [CrossRef] [PubMed]
- Bendavid, E.; Mulaney, B.; Sood, N.; Shah, S.; Ling, E.; Bromley-Dulfano, R.; Lai, C.; Weissberg, Z.; Saavedra-Walker, R.; Tedrow, J.; et al. COVID-19 Antibody Seroprevalence in Santa Clara County, California. Int. J. Epidemiol. 2021, 50, 410–419. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, J.; Schiavon Cara, R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L.; et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ. Res. 2021, 128, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Kowarz, E.; Krutzke, L.; Reis, J.; Bracharz, S.; Kochanek, S.; Marschalek, R. “Vaccine-Induced COVID-19 Mimicry” Syndrome: Splice reactions within the SARS-CoV-2 Spike open reading frame result in Spike protein variants that may cause thromboembolic events in patients immunized with vector-based vaccines (non-peer reviewed preprint). Res. Sq. 2021. [Google Scholar] [CrossRef]
- Farsalinos, K.; Eliopoulos, E.; Leonidas, D.D.; Papadopoulos, G.E.; Tzartos, S.; Poulas, K. Nicotinic Cholinergic System and COVID-19: In Silico Identification of an Interaction between SARS-CoV-2 and Nicotinic Receptors with Potential Therapeutic Targeting Implications. Int. J. Mol. Sci. 2020, 21, 5807. [Google Scholar] [CrossRef] [PubMed]
- Seneff, S.; Nigh, G. Worse than the disease? Reviewing some possible unintended consequences of the mRNA vaccines against COVID-19. Int. J. Vaccine Theory Pract. Res. 2021, 2, 38–79. [Google Scholar]
- Alatawi, Y.M.; Hansen, R.A. Empirical estimation of under-reporting in the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS). Expert Opin. Drug Saf. 2017, 16, 761–767. [Google Scholar] [CrossRef]
- Moore, T.J.; Bennett, C.L. Underreporting of Hemorrhagic and Thrombotic Complications of Pharmaceuticals to the U.S. Food and Drug Administration: Empirical Findings for Warfarin, Clopidogrel, Ticlopidine, and Thalidomide from the Southern Network on Adverse Reactions (SONAR). Semin. Thromb. Hemost. 2012, 38, 905–907. [Google Scholar] [CrossRef]
- Hazell, L.; Shakri, S.A.W. Under-reporting of adverse drug reactions. A systematic review. Drug Saf. 2006, 29, 385–396. [Google Scholar] [CrossRef]
| Documented Infection | Symptomatic Illness | Death from COVID-19 | ||||
|---|---|---|---|---|---|---|
| Period | Risk Difference (No./1000 Persons) (95% CI) | NNTV (95% CI) | Risk Difference (No./1000 Persons) (95% CI) | NNTV (95% CI) | Risk Difference (No./1000 Persons) (95% CI) | NNTV (95% CI) |
| 14–20 days after first dose | 2.06 (1.70–2.40) | 486 (417–589) | 1.54 (1.28–1.80) | 650 (556–782) | 0.03 (0.01–0.07) | 33,334 (14,286–100,000) |
| 21–27 days after first dose | 2.31 (1.96–2.69) | 433 (372–511) | 1.34 (1.09–1.62) | 747 (618–918) | 0.06 (0.02–0.11) | 16,667 (9091–50,000) |
| 7 days after second dose to end of follow-up | 8.58 (6.22–11.18) | 117 (90–161) | 4.61 (3.29–6.53) | 217 (154–304) | NA | NA |
| Vaccine | N Participants Vaccine Group | N Participants Placebo Group | CoV2 Positive End of Trial Vaccine Group | CoV2 Positive End of Trial Placebo Group | Absolute Risk Difference (ARD) | Number Needed to Vaccinate 1/ARR |
|---|---|---|---|---|---|---|
| Moderna [5] $ | 15,181(14,550 *) | 15,170 (14,598 *) | 19 (0.13%) 1 | 269 (1.77%) 1 | 0.0165 | 61 |
| Comirnaty (BioNTech/Pfizer) [4] $ | 18,860 | 18,846 | 8 (0.042%) 2 | 162 (0.86%) 2 | 0.00817 | 123 |
| Sputnik V [7] § | 14,964 | 4902 | 13 (0.087%) **,3 | 47 (1%) **,3 | 0.0091 | 110 |
| General Number of Reports (1) | Serious Side Effects (1) | Deaths (2) | Number of Vaccinations According to (3) | Number of Vaccinations According to ECDC (4) | |
|---|---|---|---|---|---|
| Comirnaty (Pfizer) | 21,321 | 864 | 280 | 5,946,031 | 6,004,808 |
| Moderna | 6390 | 114 | 35 | 531,449 | 540,862 |
| Vaxzevria (AstraZeneca) | 29,865 | 411 | 31 | 1,837,407 | 1,852,996 |
| Janssen | 2596 | 7 | - | 142,069 | 143,525 |
| Unknown | 129 | 15 | 5 | - | 540 |
| Total | 60,301 | 1.411 | 351 | 8,456,956 | 8,542,731 |
| Per 100,000 vaccinations according to Dutch data | 713.03 | 16.68 | 4.15 | ||
| Per 100,000 vaccinations according to ECDC | 705.87 | 16.52 | 4.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walach, H.; Klement, R.J.; Aukema, W. RETRACTED: The Safety of COVID-19 Vaccinations—We Should Rethink the Policy. Vaccines 2021, 9, 693. https://doi.org/10.3390/vaccines9070693
Walach H, Klement RJ, Aukema W. RETRACTED: The Safety of COVID-19 Vaccinations—We Should Rethink the Policy. Vaccines. 2021; 9(7):693. https://doi.org/10.3390/vaccines9070693
Chicago/Turabian StyleWalach, Harald, Rainer J. Klement, and Wouter Aukema. 2021. "RETRACTED: The Safety of COVID-19 Vaccinations—We Should Rethink the Policy" Vaccines 9, no. 7: 693. https://doi.org/10.3390/vaccines9070693







