Wide Spectrum Analysis of Human Papillomavirus Genotypes in External Anogenital Warts
Abstract
1. Background
2. Materials and Methods
2.1. Study Population and Sample Preparation
2.2. HPV Genotyping
2.3. Statistics
3. Results
3.1. Patients Characteristics
3.2. HPV Prevalence
3.3. Occurrence of the Specific Genotypes
4. Discussion
5. Conclusions
6. Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Patel, H.; Wagner, M.; Singhal, P.; Kothari, S. Systematic review of the incidence and prevalence of genital warts. BMC Infect. Dis. 2013, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Anic, G.; Nyitray, A.G. Epidemiology and pathology of HPV disease in males. Gynecol. Oncol. 2010, 117, S15–S19. [Google Scholar] [CrossRef]
- Pennycook, B.K.; McCready, A.T. Condyloma Acuminata; StatPearls NCBI Bookshelf 2019; StatPearls Publishing: Treasure Island, FL, USA, 2019; pp. 1–9. [Google Scholar]
- Léonard, B.; Kridelka, F.; Delbecque, K.; Goffin, F.; Demoulin, S.; Doyen, J.; Delvenne, P. A Clinical and Pathological Overview of Vulvar Condyloma Acuminatum, Intraepithelial Neoplasia, and Squamous Cell Carcinoma. BioMed Res. Int. 2014, 2014, 480573. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, L.S.; Basaras, M.; Arrese, E.; Hernáez, S.; Esteban, V.; Cisterna, R. Distribution of genital human papillomavirus genotypes in benign clinical manifestations among men from Northern Spain. BMC Public Health 2015, 16, 81. [Google Scholar] [CrossRef]
- Li, Q.; Li, W.; Li, H.; Liu, Z. A correlative analysis of cervical lesions in patients with vulva condyloma acuminatum. Chin. J. Clin. Oncol. 2006, 3, 419–422. [Google Scholar] [CrossRef]
- Tchernev, G. Sexually transmitted papillomavirus infections: Epidemiology pathogenesis, clinic, morphology, important differential diagnostic aspects, current diagnostic and treatment options. An. Bras. Dermatol. 2009, 84, 377–389. [Google Scholar] [CrossRef]
- Cubie, H.A. Diseases associated with human papillomavirus infection. Virology 2013, 445, 21–34. [Google Scholar] [CrossRef]
- Sichero, L.; Giuliano, A.R.; Villa, L.L. Human Papillomavirus and Genital Disease in Men: What We Have Learned from the HIM Study. Acta Cytol. 2019, 63, 109–117. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Kellner, J.D.; Dele Davies, H. Genital infection with human papillomavirus in adolescents. Adv. Ther. 2005, 22, 187–197. [Google Scholar] [CrossRef]
- Sycuro, L.; Xi, L.F.; Hughes, J.P.; Feng, Q.; Winer, R.L.; Lee, S.; O’Reilly, S.; Kiviat, N.B.; Koutsky, L.A. Persistence of Genital Human Papillomavirus Infection in a Long-Term Follow-Up Study of Female University Students. J. Infect. Dis. 2008, 198, 971–978. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stern, P.L.; Van Der Burg, S.H.; Hampson, I.N.; Broker, T.R.; Fiander, A.; Lacey, C.J.; Kitchener, H.C.; Einstein, M.H. Therapy of Human Papillomavirus-Related Disease. Vaccine 2012, 30, F71–F82. [Google Scholar] [CrossRef] [PubMed]
- Rosen, T. Condylomata Acuminata (Anogenital Warts): Management of External Condylomata Acuminata in Men; UpToDate Wolters Kluwer: Waltham, MA, USA, 2019; pp. 1–26. [Google Scholar]
- Carusi, A.D. Condylomata Acuminata (Anogenital Warts): Treatment of Vulvar and Vaginal Warts; UpToDate Wolters Kluwer: Waltham, MA, USA, 2019; pp. 1–36. [Google Scholar]
- Kásler, M. A humán papillomavírus okozta malignus és rekurráló megbetegedések társadalmi terhei. Orv. Hetil. 2012, 153, 3–15. [Google Scholar]
- Kásler, M. A HPV okozta malignus és rekurráló megbetegedések megelőzésére szolgáló vakcinák. Orv. Hetil. 2012, 153, 16–32. [Google Scholar]
- Kásler, M. Javaslat a HPV okozta megbetegedések megelőzésének népegészségügyi programjára. Orv. Hetil. 2012, 153, 33–38. [Google Scholar]
- Scheinfeld, N. Update on the treatment of genital warts. Dermatol. Online J. 2013, 19, 18559. [Google Scholar]
- Várkonyi, V. The role of HPV vaccination in management and prevention of condyloma. Bőrgyógyászati Venerológiai Szle. 2016, 2, 90–93. [Google Scholar]
- Sousa, H.; Tavares, A.; Campos, C.; Marinho-Dias, J.; Brito, M.; Medeiros, R.; Baldaque, I.; Lobo, C.; Leça, L.; Monteiro, P.; et al. High-Risk human papillomavirus genotype distribution in the Northern region of Portugal: Data from regional cervical cancer screening program. Papillomavirus Res. 2019, 8, 100179. [Google Scholar] [CrossRef]
- Aubin, F.; Prétet, J.-L.; Jacquard, A.-C.; Saunier, M.; Carcopino, X.; Jaroud, F.; Pradat, P.; Soubeyrand, B.; Leocmach, Y.; Mougin, C.; et al. Human Papillomavirus Genotype Distribution in External Acuminata Condylomata: A Large French National Study (EDiTH IV). Clin. Infect. Dis. 2008, 47, 610–615. [Google Scholar] [CrossRef]
- Tachezy, R.; Smahelova, J.; Saláková, M.; Arbyn, M.; Rob, L.; Skapa, P.; Jirásek, T.; Hamsikova, E. Human Papillomavirus Genotype Distribution in Czech Women and Men with Diseases Etiologically Linked to HPV. PLoS ONE 2011, 6, e21913. [Google Scholar] [CrossRef] [PubMed]
- Kraut, A.; Schink, T.; Schulze-Rath, R.; Mikolajczyk, R.T.; Garbe, E. Incidence of anogenital warts in Germany: A population-based cohort study. BMC Infect. Dis. 2010, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Man, I.; Vänskä, S.; Lehtinen, M.; Bogaards, J. Human Papillomavirus Genotype Replacement: Still Too Early to Tell? J. Infect. Dis. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Lacey, C.; Woodhall, S.; Wikstrom, A.; Ross, J. 2012 European guideline for the management of anogenital warts. J. Eur. Acad. Dermatol. Venereol. 2012, 27, e263–e270. [Google Scholar] [CrossRef]
- Castle, P.E.; Maza, M. Prophylactic HPV vaccination: Past, present, and future. Epidemiol. Infect. 2015, 144, 449–468. [Google Scholar] [CrossRef]
- Fischer, S.; Bettstetter, M.; Becher, A.; Lessel, M.; Bank, C.; Krams, M.; Becker, I.; Hartmann, A.; Jagla, W.; Gaumann, A. Shift in prevalence of HPV types in cervical cytology specimens in the era of HPV vaccination. Oncol. Lett. 2016, 12, 601–610. [Google Scholar] [CrossRef]
- Ault, K.A. Human papillomavirus vaccines and the potential for cross-protection between related HPV types. Gynecol. Oncol. 2007, 107, S31–S33. [Google Scholar] [CrossRef] [PubMed]
- Herraez-Hernandez, E.; Alvarez-Perez, M.; Navarro-Bustos, G.; Esquivias, J.; Alonso, S.; Aneiros-Fernandez, J.; Lacruz-Pelea, C.; Sanchez-Aguera, M.; Santamaria, J.S.; De Antonio, J.C.; et al. HPV Direct Flow CHIP: A new human papillomavirus genotyping method based on direct PCR from crude-cell extracts. J. Virol. Methods 2013, 193, 9–17. [Google Scholar] [CrossRef][Green Version]
- Kocjan, B.J.; Hošnjak, L.; Poljak, M. Detection of alpha human papillomaviruses in archival formalin-fixed, paraffin-embedded (FFPE) tissue specimens. J. Clin. Virol. 2016, 76, S88–S97. [Google Scholar] [CrossRef]
- Herraez-Hernandez, E.H.-H.; Preda, O.; Alonso, S.; Pardo, R.S.; Olmo, A. Detection and Genotyping of Human Papillomavirus DNA in Formalin-Fixed Paraffin-Embedded Specimens with the HPV Direct Flow CHIP System. Open Virol. J. 2013, 7, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Kovács, K.; Varnai, A.D.; Bollmann, M.; Bankfalvi, A.; Szendy, M.; Speich, N.; Schmitt, C.; Pajor, L.; Bollmann, R. Prevalence and genotype distribution of multiple human papillomavirus infection in the uterine cervix: A 7.5-year longitudinal study in a routine cytology-based screening population in West Germany. J. Med. Virol. 2008, 80, 1814–1823. [Google Scholar] [CrossRef]
- Chaturwedi, A.K. HPV and Incidence Trends for Head and Neck Cancer. National Cancer Institute 2010. Available online: http://www.thelancetconferences.com/hpv-and-cancer/presentations/chaturvedi_presentation.pdf (accessed on 1 January 2010).
- Mayeaux, M.J. Reducing the Economic Burden of HPV-Related Diseases. J. Am. Osteopath. Assoc. 2008, 108, S2–S7. [Google Scholar] [PubMed]
- Hartwig, S.; Baldauf, J.-J.; Dominiak-Felden, G.; Simondon, F.; Alemany, L.; De Sanjosé, S.; Castellsagué, X. Estimation of the epidemiological burden of HPV-related anogenital cancers, precancerous lesions, and genital warts in women and men in Europe: Potential additional benefit of a nine-valent second generation HPV vaccine compared to first generation HPV vaccines. Papillomavirus Res. 2015, 1, 90–100. [Google Scholar] [CrossRef]
- The FUTURE I/II Study Group. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: Randomised controlled trial. BMJ 2010, 341, c3493. [Google Scholar] [CrossRef] [PubMed]
- Donovan, B.; Franklin, N.; Guy, R.; Grulich, A.; Regan, D.G.; Ali, H.; Wand, H.; Fairley, C.K. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: Analysis of national sentinel surveillance data. Lancet Infect. Dis. 2011, 11, 39–44. [Google Scholar] [CrossRef]
- Leite, K.R.M.; Pimenta, R.; Canavez, J.; Canavez, F.; De Souza, F.R.; Vara, L.; Estivallet, C.; Camara-Lopes, L.H. HPV Genotype Prevalence and Success of Vaccination to Prevent Cervical Cancer. Acta Cytol. 2020, 64, 420–424. [Google Scholar] [CrossRef]
- Li, Z.; Song, S.; He, M.; Wang, D.; Shi, J.; Liu, X.; Li, Y.; Chi, X.; Wei, S.; Yang, Y.; et al. Rational design of a triple-type human papillomavirus vaccine by compromising viral-type specificity. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Balla, B.C.; Terebessy, A.; Tóth, E.; Balázs, P. Young Hungarian Students’ Knowledge about HPV and Their Attitude Toward HPV Vaccination. Vaccines 2016, 5, 1. [Google Scholar] [CrossRef] [PubMed]
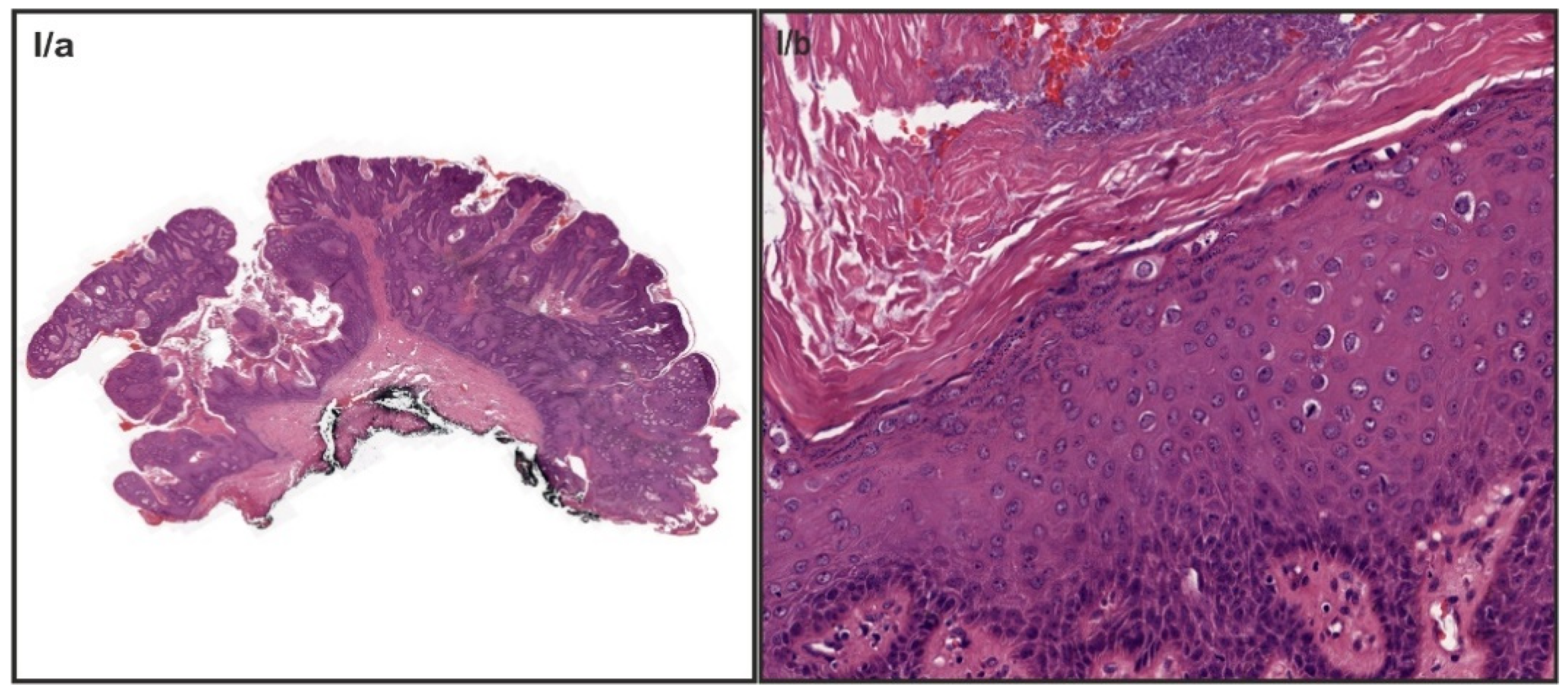
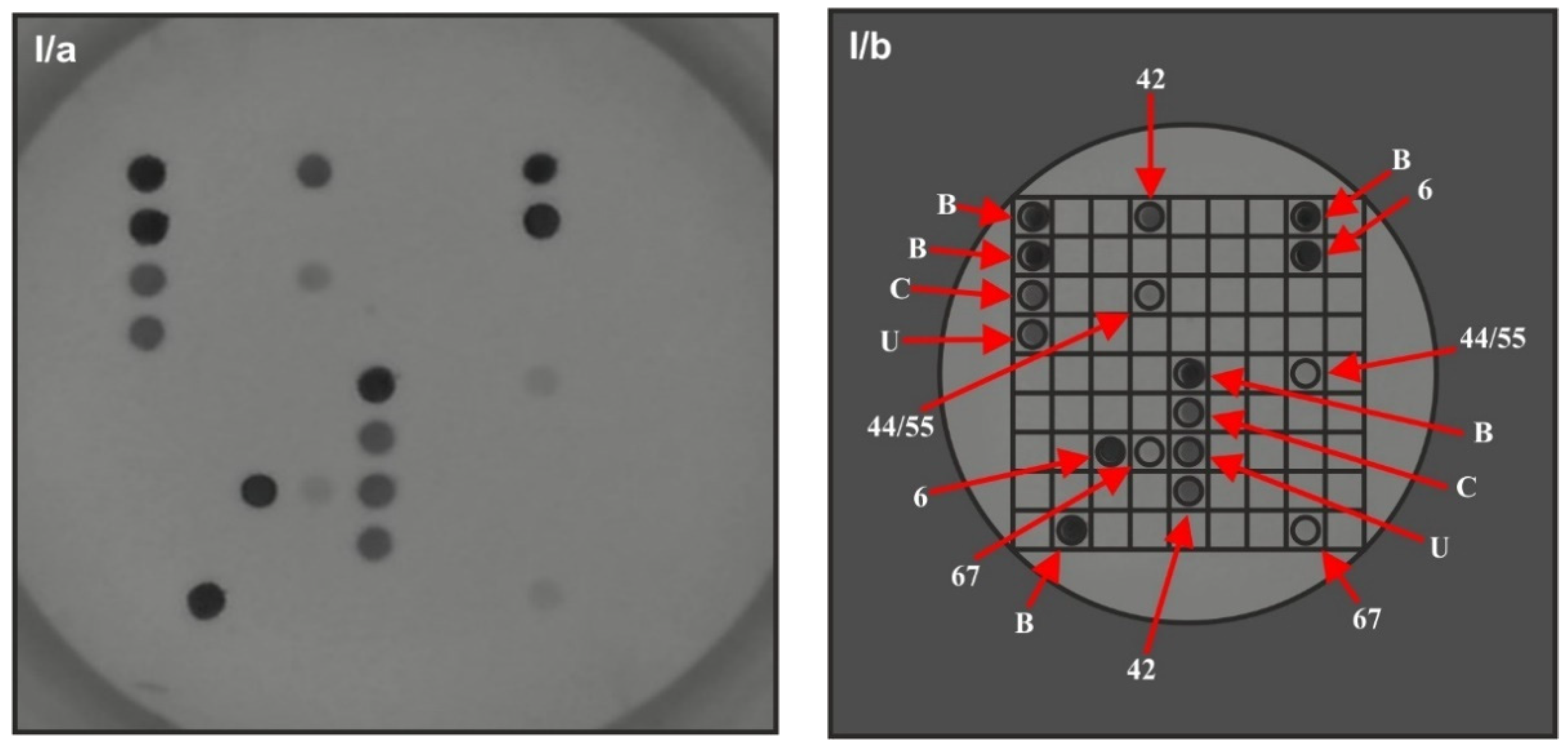
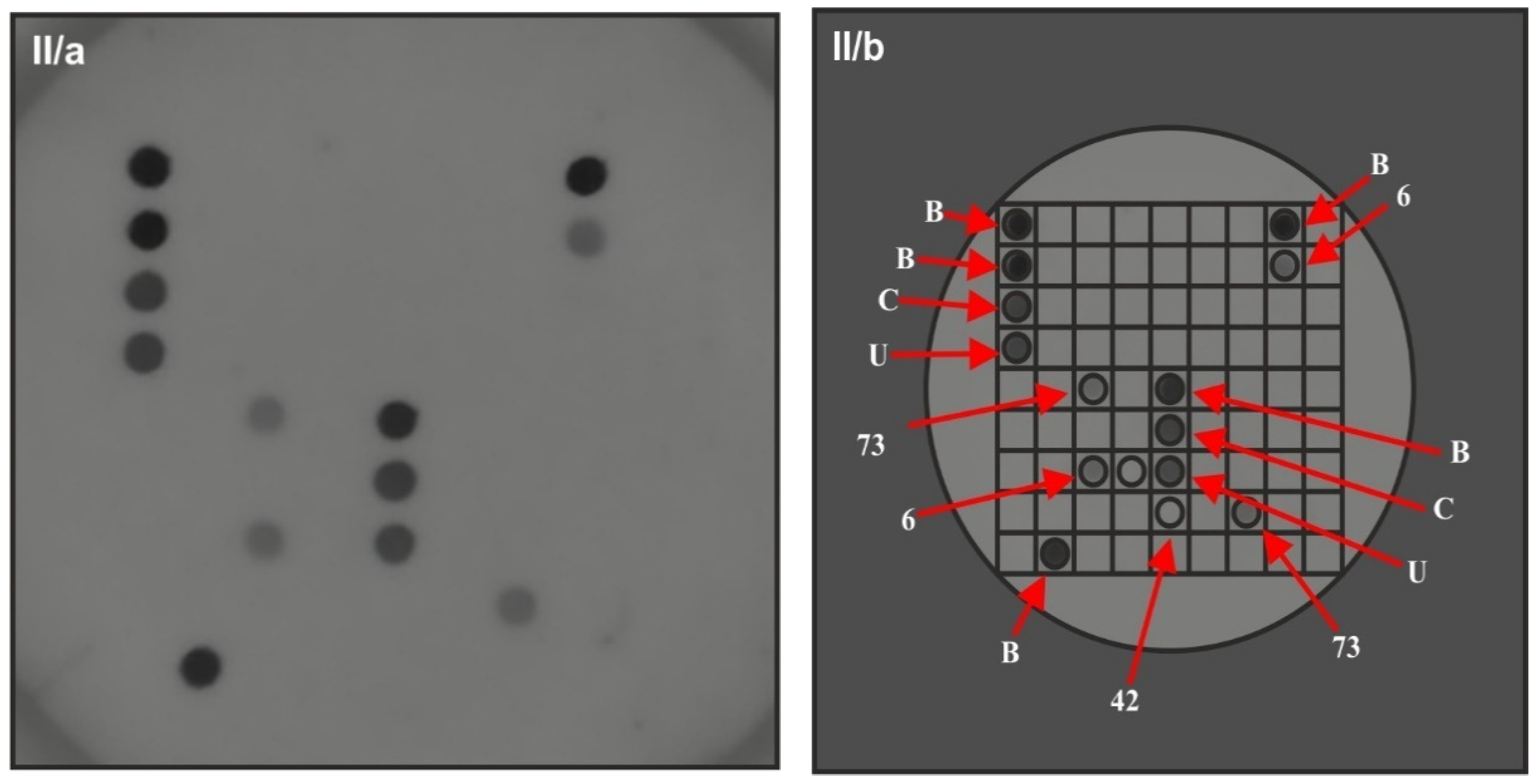
| Characteristic | Value |
|---|---|
| Sex | |
| Female | 66 (70.2%) |
| Age, median years (range) | 36 (15–68) |
| Range of age groups | |
| 15–20 | 17 (25.7%) |
| 20–24 | 9 (13.6%) |
| 24–30 | 8 (12.1%) |
| 30–40 | 4 (6.0%) |
| 40–50 | 9 (13.6%) |
| 50–60 | 13 (19.6%) |
| >60 | 6 (9.0%) |
| Pregnant at time of diagnosis (n = 66) | |
| yes | 6 (9.1%) |
| no | 60 (90.9%) |
| Type of EGW diagnosis | |
| New diagnosis | 46 (69.7%) |
| Clinically recurrent disease | 20 (30.3%) |
| Appearance of the lesions | |
| Simple | 22 (33.3%) |
| Multiple | 44 (66.6%) |
| Smoking | |
| yes | 27 (40.9%) |
| no | 39 (59.0%) |
| data not available | 5 (7.6%) |
| Sex | |
| Male | 28 (29.8%) |
| Age, median years (range) | 37.5 (21–77) |
| Range of age groups | |
| 20–24 | 6 (21.4%) |
| 24–30 | 6 (21.4%) |
| 30–40 | 7 (25.0%) |
| 40–50 | - |
| 50–60 | 3 (10.7%) |
| >60 | 6 (21.4%) |
| The appearance of the lesions | |
| Simple | 10 (35.7%) |
| Multiple | 18 (64.2%) |
| No (%) of Cases | ||||
|---|---|---|---|---|
| Variable | Male | Female | Total | p |
| (n = 28) | (n = 66) | (n = 94) | ||
| Mono-infections | 21 (75.0%) | 47 (71.2%) | 68 (72.3%) | 0.707 |
| LR HPV types | 21 (75%) | 47 (71.2%) | 68 (72.3%) | 0.707 |
| HR HPV types | 0 | 0 | 0 | N.A. |
| Multi-infections | 7 (25.0%) | 19 (28.8%) | 26 (27.6%) | |
| Only LR HPV | 2 (7.1%) | 6 (9.1%) | 8 (8.5%) | 1 |
| Only HR HPV | 0 | 0 | 0 | N.A. |
| LR and HR HPV | 5 (17.8%) | 13 (19.7%) | 18 (19.1%) | 0.836 |
| 1 HR HPV | 4 (14.3%) | 10 (15.2%) | 14 (14.9%) | 1 |
| >1 HR HPV | 1 (3.6%) | 3 (4.5%) | 4 (4.3%) | 1 |
| No (%) of Cases | |||
|---|---|---|---|
| HPV Genotype | Number of Mono-Infection (68) | Number of Multi-Infection (63) (with other LR and/or HR) | Number of Total Infections (131) |
| Low-risk (LR) | |||
| HPV genotypes | |||
| HPV 6 | 59 (86.8%) | 22 (34.9%) | 81 (61.8%) |
| HPV 11 | 9 (13.2%) | 3 (4.7%) | 12 (9.2%) |
| HPV 42 | - | 6 (9.5%) | 6 (4.6%) |
| HPV 40 | - | 3 (4.7%) | 3 (2.3%) |
| HPV 44/55 | - | 2 (3.2%) | 2 (1.5%) |
| HPV 54 | - | 2 (3.2%) | 2 (1.5%) |
| HPV 67 | - | 2 (3.2%) | 2 (1.5%) |
| HPV 62/81 | - | 1 (1.6%) | 1 (0.8%) |
| High-risk (HR) | |||
| HPV genotypes | |||
| HPV 56 | - | 3 (4.7%) | 3 (2.3%) |
| HPV59 | - | 3 (4.7%) | 3 (2.3%) |
| HPV 66 | - | 3 (4.7%) | 3 (2.3%) |
| HPV 73 | - | 3 (4.7%) | 3 (2.3%) |
| HPV 16 | - | 2 (3.2%) | 2 (1.5%) |
| HPV 18 | - | 2 (3.2%) | 2 (1.5%) |
| HPV 51 | - | 2 (3.2%) | 2 (1.5%) |
| HPV 39 | - | 1 (1.6%) | 1 (0.8%) |
| HPV 52 | - | 1 (1.6%) | 1 (0.8%) |
| HPV 53 | - | 1 (1.6%) | 1 (0.8%) |
| HPV 68 | - | 1 (1.6%) | 1 (0.8%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rideg, O.; Oszter, A.; Makk, E.; Kálmán, E.; Farkas, K.; Tornóczky, T.; Kovács, K. Wide Spectrum Analysis of Human Papillomavirus Genotypes in External Anogenital Warts. Vaccines 2021, 9, 604. https://doi.org/10.3390/vaccines9060604
Rideg O, Oszter A, Makk E, Kálmán E, Farkas K, Tornóczky T, Kovács K. Wide Spectrum Analysis of Human Papillomavirus Genotypes in External Anogenital Warts. Vaccines. 2021; 9(6):604. https://doi.org/10.3390/vaccines9060604
Chicago/Turabian StyleRideg, Orsolya, Angéla Oszter, Evelin Makk, Endre Kálmán, Kornélia Farkas, Tamás Tornóczky, and Krisztina Kovács. 2021. "Wide Spectrum Analysis of Human Papillomavirus Genotypes in External Anogenital Warts" Vaccines 9, no. 6: 604. https://doi.org/10.3390/vaccines9060604
APA StyleRideg, O., Oszter, A., Makk, E., Kálmán, E., Farkas, K., Tornóczky, T., & Kovács, K. (2021). Wide Spectrum Analysis of Human Papillomavirus Genotypes in External Anogenital Warts. Vaccines, 9(6), 604. https://doi.org/10.3390/vaccines9060604





