Immune Responses in Laying Hens after an Infectious Bronchitis Vaccination of Pullets: A Comparison of Two Vaccination Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Vaccines
2.3. Experimental Design
2.4. Techniques
2.4.1. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4.2. Virus Neutralization (VN) Assay
2.4.3. Spleen Mononuclear Cell Isolation
2.4.4. Flow Cytometry Technique
2.4.5. Immunofluorescent Assay for CD4+ and CD8+ T Cells
2.4.6. RNA Extraction and Complementary (c) DNA Synthesis
2.4.7. Real-Time Quantitative Polymerase Chain Reaction (qPCR) Assay
2.5. Data Analyses
3. Results
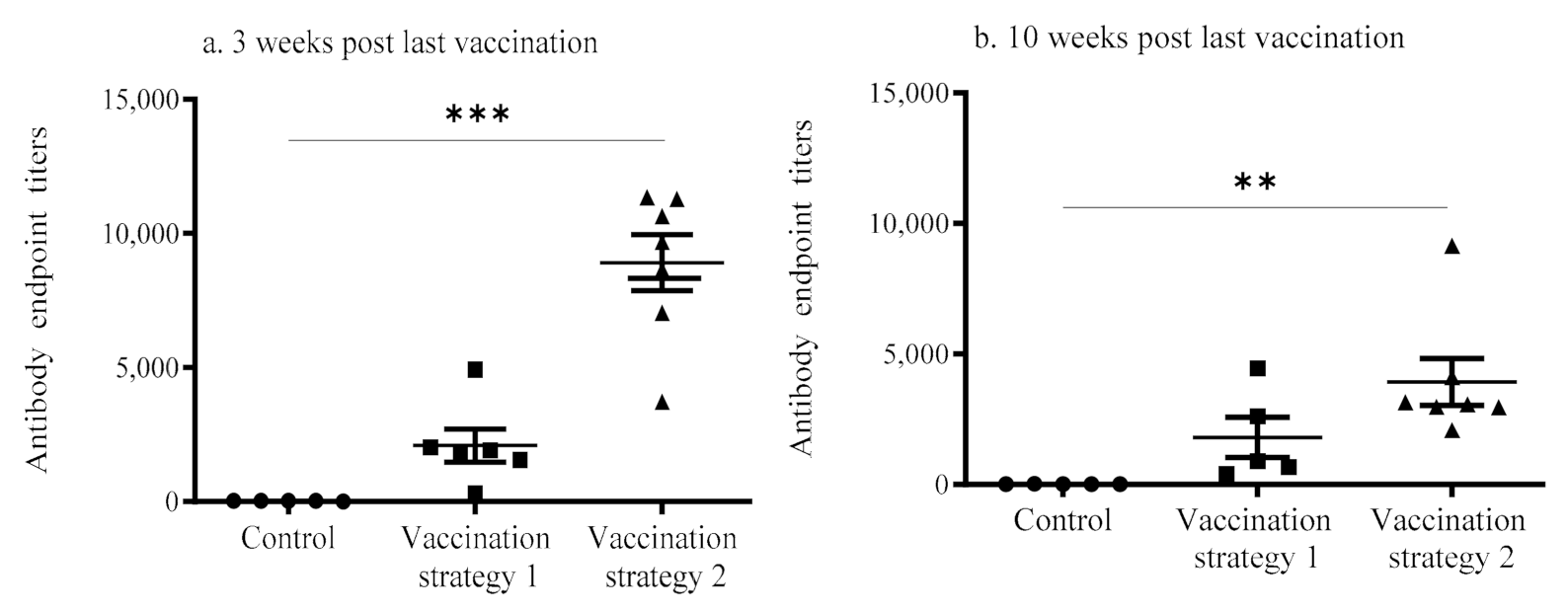
3.1. Serum Anti-IBV Antibody Response
3.2. Virus Neutralization
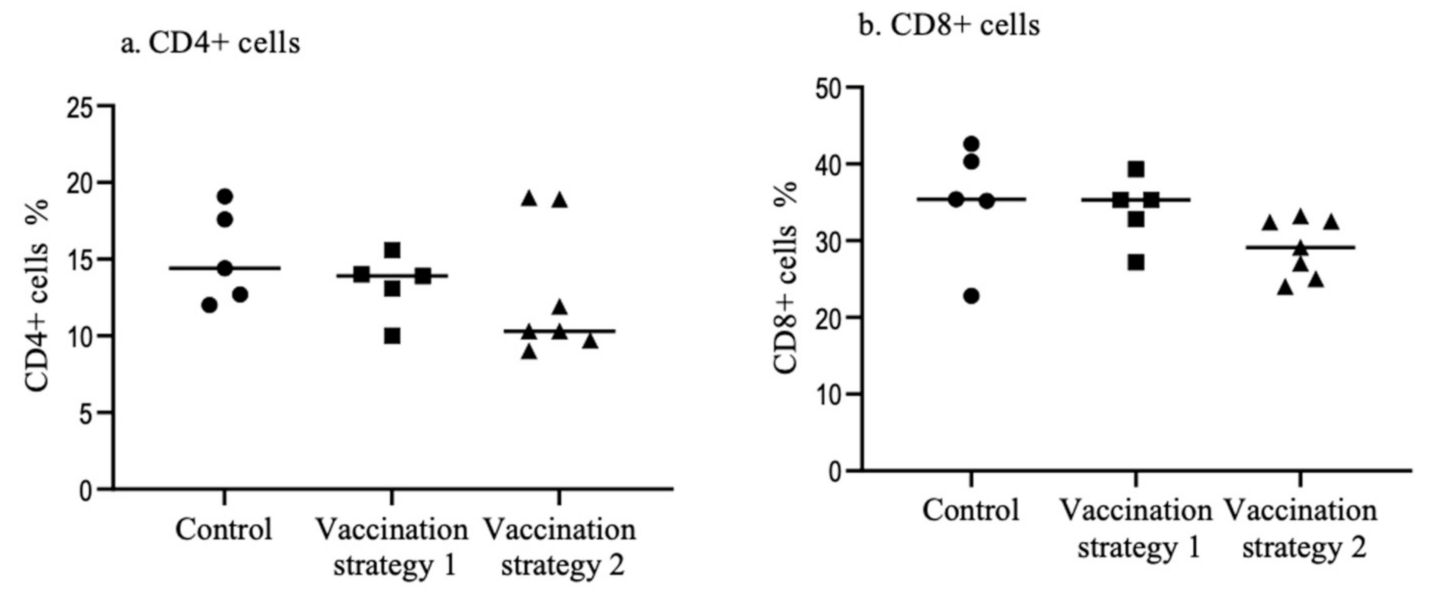
3.3. Spleen CD4+ and CD8+ T Cells
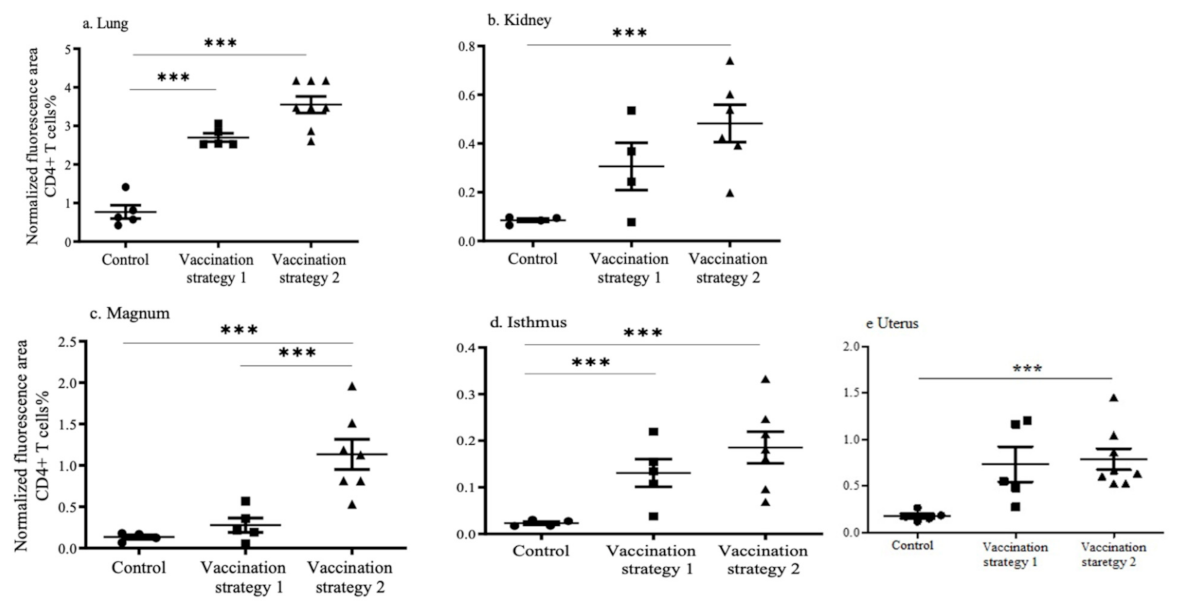
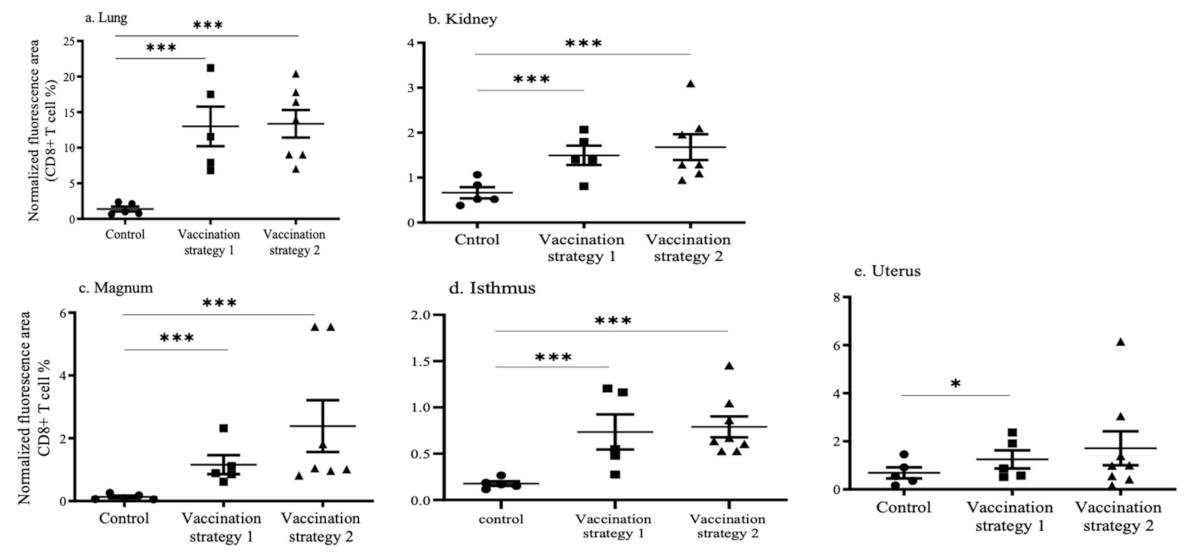
3.4. CD4+ and CD8+ T Cell Recruitments in the Lungs, Kidneys, and Reproductive Tract Tissues
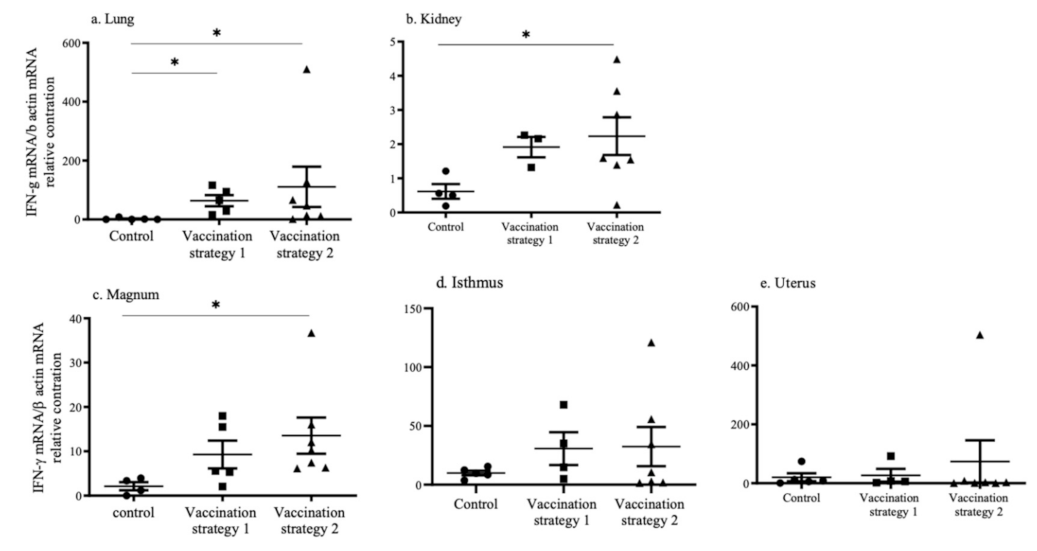
3.5. INF-γ mRNA Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackwood, M.W. Review of Infectious Bronchitis Virus around the World. Avian Dis. 2012, 56, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007, 38, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Miłek, J.; Blicharz-Domańska, K. Coronaviruses in avian species—Review with focus on epidemiology and diagnosis in wild birds. J. Vet. Res. 2018, 62, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.S. Coronaviridae: Infectious Bronchitis Virus. In Emerging and Re-emerging Infectious Diseases of Livestock; Bayry, J., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 133–166. [Google Scholar]
- Schalk, A.F. An Apparently New Respiratory Disease of Baby Chicks. Vet. Med. Assoc. 1931, 73, 413–423. [Google Scholar]
- Van Roekel, H.; Clarke, M.K.; Bullis, K.L.; Olesiuk, O.M.; Sperling, F.G. Infectious bronchitis. Am. J. Vet. Res. 1951, 12, 140–146. [Google Scholar] [PubMed]
- Cumming, R.B. The Etiology of “Uraemia” of Chickens. Aust. Vet. J. 1962, 38, 554. [Google Scholar] [CrossRef]
- Najimudeen, S.M.; Hassan, M.S.H.; Cork, S.C.; Abdul-Careem, M.F. Infectious Bronchitis Coronavirus Infection in Chickens: Multiple System Disease with Immune Suppression. Pathogens 2020, 9, 17. [Google Scholar] [CrossRef]
- Ambali, A.G.; Jones, R.C. Early Pathogenesis in Chicks of Infection with an Enterotropic Strain of Infectious Bronchitis Virus. Avian Dis. 1990, 34, 809–817. [Google Scholar] [CrossRef]
- Ignjatovic, J.; Ashton, D.; Reece, R.; Scott, P.; Hooper, P. Pathogenicity of Australian Strains of Avian Infectious Bronchitis Virus. J. Comp. Pathol. 2002, 126, 115–123. [Google Scholar] [CrossRef]
- Chong, K.; Apostolov, K. The pathogenesis of nephritis in chickens induced by infectious bronchitis virus. J. Comp. Pathol. 1982, 92, 199–211. [Google Scholar] [CrossRef]
- Sevoian, M.; Levine, P.P. Effects of Infectious Bronchitis on the Reproductive Tracts, Egg Production, and Egg Quality of Laying Chickens. Avian Dis. 1957, 1, 136–164. [Google Scholar] [CrossRef]
- Amarasinghe, A.; Popowich, S.; De Silva Senapathi, U.; Abdul-Cader, M.S.; Marshall, F.; Van der Meer, F.; Cork, S.C.; Gomis, S.; Abdul-Careem, M.F. Shell-Less Egg Syndrome (SES) Widespread in Western Canadian Layer Operations Is Linked to a Massachusetts (Mass) Type Infectious Bronchitis Virus (IBV) Isolate. Viruses 2018, 10, 437. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.S.H.; Ojkic, D.; Coffin, C.S.; Cork, S.C.; Van Der Meer, F.; Abdul-Careem, M.F. Delmarva (DMV/1639) Infectious Bronchitis Virus (IBV) Variants Isolated in Eastern Canada Show Evidence of Recombination. Viruses 2019, 11, 1054. [Google Scholar] [CrossRef]
- Martin, E.A.; Brash, M.L.; Hoyland, S.K.; Coventry, J.M.; Sandrock, C.; Guerin, M.T.; Ojkic, D. Genotyping of infectious bronchitis viruses identified in Canada between 2000 and 2013. Avian Pathol. 2014, 43, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B. Vaccination against infectious bronchitis virus: A continuous challenge. Vet. Microbiol. 2017, 206, 137–143. [Google Scholar] [CrossRef]
- Cavanagh, D. Severe acute respiratory syndrome vaccine development: Experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003, 32, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.K.A.; Jackwood, M.; Jones, R.C. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012, 41, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.K.; Orbell, S.J.; Woods, M.A.; Huggins, M.B. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathol. 1999, 28, 477–485. [Google Scholar] [CrossRef]
- Roh, H.-J.; Hilt, D.A.; Williams, S.M.; Jackwood, M.W. Evaluation of Infectious Bronchitis Virus Arkansas-Type Vaccine Failure in Commercial Broilers. Avian Dis. 2013, 57, 248–259. [Google Scholar] [CrossRef]
- De Wit, J.J.; Swart, W.A.J.M.; Fabri, T.H.F. Efficacy of infectious bronchitis virus vaccinations in the field: Association between the α-IBV IgM response, protection and vaccine application parameters. Avian Pathol. 2010, 39, 123–131. [Google Scholar] [CrossRef]
- Lee, H.J.; Youn, H.N.; Kwon, J.S.; Kim, J.H.; Lee, J.B.; Park, S.Y.; Choi, I.S.; Song, C.S. Characterization of a novel live attenuated infectious bronchitis virus vaccine candidate derived from a Korean nephropathogenic strain. Vaccine 2010, 28, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.D.; Okino, C.H.; Fernando, F.S.; Pavani, C.; Casagrande, V.M.; Lopez, R.F.; Montassier, M.d.F.S.; Montassier, H.J. Inactivated infectious bronchitis virus vaccine encapsulated in chitosan nanoparticles induces mucosal immune responses and effective protection against challenge. Vaccine 2018, 36, 2630–2636. [Google Scholar] [CrossRef]
- Ladman, B.S.; Pope, C.R.; Ziegler, A.F.; Swieczkowski, T.; Callahan, J.M.; Davison, S.; Gelb, J., Jr. Protection of Chickens After Live and Inactivated Virus Vaccination Against Challenge with Nephropathogenic Infectious Bronchitis Virus PA/Wolgemuth/98. Avian Dis. 2002, 46, 938–944. [Google Scholar] [CrossRef]
- Kusters, J.; Jager, E.; Niesters, H.; Van Der Zeijst, B.; Van Der Zeijst, B.A. Sequence evidence for RNA recombination in field isolates of avian coronavirus infectious bronchitis virus. Vaccine 1990, 8, 605–608. [Google Scholar] [CrossRef]
- Sasipreeyajan, J.; Pohuang, T.; Sirikobkul, N. Efficacy of Different Vaccination Programs against Thai QX-like Infectious Bronchitis Virus. Thai J. Vet. Med. 2012, 42, 73–79. [Google Scholar]
- Ismail, M.I.; Tan, S.W.; Hair-Bejo, M.; Omar, A.R. Evaluation of the antigen relatedness and efficacy of a single vaccination with different infectious bronchitis virus strains against a challenge with Malaysian variant and QX-like IBV strains. J. Vet. Sci. 2020, 21, e76. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.K.A.; Chesher, J.; Baxendale, W.; Greenwood, N.; Huggins, M.B.; Orbell, S.J. Protection of chickens against renal damage caused by a nephropathogenic infectious bronchitis virus. Avian Pathol. 2001, 30, 423–426. [Google Scholar] [CrossRef]
- Finney, P.M.; Box, P.G.; Holmes, H.C. Studies with a bivalent infectious bronchitis killed virus vaccine. Avian Pathol. 1990, 19, 435–450. [Google Scholar] [CrossRef]
- de Wit, J.J.S.; Malo, A.; Cook, J.K.A. Induction of IBV strain-specific neutralizing antibodies and broad spectrum protection in layer pullets primed with IBV Massachusetts (Mass) and 793B vaccines prior to injection of inactivated vaccine containing Mass antigen. Avian Pathol. 2019, 48, 135–147. [Google Scholar] [CrossRef]
- Sjaak de Wit, J.J.; Ter Veen, C.; Koopman, H.C.R. Effect of IBV D1466 on egg production and egg quality and the effect of heterologous priming to increase the efficacy of an inactivated IBV vaccine. Avian Pathol. 2020, 49, 185–192. [Google Scholar] [CrossRef]
- Box, P.; Ellis, K.R. Infectious bronchitis in laying hens: Interference with response to emulsion vaccine by attenuated live vaccine. Avian Pathol. 1985, 14, 9–22. [Google Scholar] [CrossRef]
- Dos Santos, R.M.; Fernando, F.S.; Montassier, M.d.F.S.; Silva, K.R.; Lopes, P.D.; Pavani, C.; Borzi, M.M.; Okino, C.H.; Montassier, H.J. Memory immune responses and protection of chickens against a nephropathogenic infectious bronchitis virus strain by combining live heterologous and inactivated homologous vaccines. J. Vet. Med. Sci. 2019, 81, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Crinion, R.A.; Hofstad, M.S. Pathogenicity of Four Serotypes of Avian Infectious Bronchitis Virus for the Oviduct of Young Chickens of Various Ages. Avian Dis. 1972, 16, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Laconi, A.; Weerts, E.; Bloodgood, J.C.G.; Deniz Marrero, J.P.; Berends, A.J.; Cocciolo, G.; de Wit, J.J.; Verheije, M.H. Attenuated live infectious bronchitis virus QX vaccine disseminates slowly to target organs distant from the site of inoculation. Vaccine 2020, 38, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, A.; Senapathi, U.d.S.; Abdul-Cader, M.S.; Popowich, S.; Marshall, F.; Cork, S.C.; Van Der Meer, F.; Gomis, S.; Abdul-Careem, M.F. Comparative features of infections of two Massachusetts (Mass) infectious bronchitis virus (IBV) variants isolated from Western Canadian layer flocks. BMC Vet. Res. 2018, 14, 391. [Google Scholar] [CrossRef]
- Petrik, M. Clinical Presentation of False Layer Syndrome Caused by Infectious Bronchitis. In Proceedings of the AVMA/American Association of Avian Pathology Annual Meeting, Denver, CO, USA, 13–17 July 2018. [Google Scholar]
- Cottral, G.E. (Ed.) Serology. In Manual of Standardized Methods for Veterinary Microbiology; Cornell University Press: Ithaca, NY, USA, 1978; pp. 60–93. [Google Scholar]
- Senapathi, U.d.S.; Abdul-Cader, M.S.; Amarasinghe, A.; Van Marle, G.; Czub, M.; Gomis, S.; Abdul-Careem, M.F. The In Ovo Delivery of CpG Oligonucleotides Protects against Infectious Bronchitis with the Recruitment of Immune Cells into the Respiratory Tract of Chickens. Viruses 2018, 10, 635. [Google Scholar] [CrossRef]
- Forlenza, M.; Kaiser, T.; Savelkoul, H.F.; Wiegertjes, G.F. The use of real-time quantitative PCR for the analysis of cytokine mRNA levels. Methods Mol. Biol. 2012, 820, 7–23. [Google Scholar]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Seo, S.H.; Collisson, E.W. Specific cytotoxic T lymphocytes are involved in in vivo clearance of infectious bronchitis virus. J. Virol. 1997, 71, 5173–5177. [Google Scholar] [CrossRef]
- Collisson, E.W.; Pei, J.; Dzielawa, J.; Seo, S.H. Cytotoxic T lymphocytes are critical in the control of infectious bronchitis virus in poultry. Dev. Comp. Immunol. 2000, 24, 187–200. [Google Scholar] [CrossRef]
- Seo, S.H.; Pei, J.; Briles, W.E.; Dzielawa, J.; Collisson, E.W. Adoptive transfer of infectious bronchitis virus primed alphabeta T cells bearing CD8 antigen protects chicks from acute infection. Virology 2000, 269, 183–189. [Google Scholar] [CrossRef][Green Version]
- Ghani, S.; Feuerer, M.; Doebis, C.; Lauer, U.; Loddenkemper, C.; Huehn, J.; Hamann, A.; Syrbe, U. T cells as pioneers: Antigen-specific T cells condition inflamed sites for high-rate antigen-non-specific effector cell recruitment. Immunology 2009, 128, e870–e880. [Google Scholar] [CrossRef]
- Awad, F.; Hutton, S.; Forrester, A.; Baylis, M.; Ganapathy, K. Heterologous live infectious bronchitis virus vaccination in day-old commercial broiler chicks: Clinical signs, ciliary health, immune responses and protection against variant infectious bronchitis viruses. Avian Pathol. 2016, 45, 169–177. [Google Scholar] [CrossRef]
- Le Page, C.; Génin, P.; Baines, M.G.; Hiscott, J. Interferon activation and innate immunity. Rev. Immunogenet. 2000, 2, 374–386. [Google Scholar]
- Okino, C.H.; Alessi, A.C.; Montassier, M.d.F.; Rosa, A.J.; Wang, X.; Montassier, H.J. Humoral and Cell-Mediated Immune Responses to Different Doses of Attenuated Vaccine against Avian Infectious Bronchitis Virus. Viral Immunol. 2013, 26, 259–267. [Google Scholar] [CrossRef]
| Group | Live Attenuated Vaccine | Inactivated Vaccine | ||||
|---|---|---|---|---|---|---|
| Mass 3 Weeks | Mass + Conn 5 Weeks | Mass 8 Weeks | Mass 12 Weeks | Mass 16 Weeks | Mass 16 Weeks | |
| Group 1 (n = 5) | X | X | X | X | X | |
| Group 2 (n = 7) | X | X | X | X | X | |
| Group 3 (n = 5) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buharideen, S.M.; Hassan, M.S.H.; Najimudeen, S.M.; Niu, D.; Czub, M.; Gomis, S.; Abdul-Careem, M.F. Immune Responses in Laying Hens after an Infectious Bronchitis Vaccination of Pullets: A Comparison of Two Vaccination Strategies. Vaccines 2021, 9, 531. https://doi.org/10.3390/vaccines9050531
Buharideen SM, Hassan MSH, Najimudeen SM, Niu D, Czub M, Gomis S, Abdul-Careem MF. Immune Responses in Laying Hens after an Infectious Bronchitis Vaccination of Pullets: A Comparison of Two Vaccination Strategies. Vaccines. 2021; 9(5):531. https://doi.org/10.3390/vaccines9050531
Chicago/Turabian StyleBuharideen, Sabrina M., Mohamed S. H. Hassan, Shahnas M. Najimudeen, Dongyan Niu, Markus Czub, Susantha Gomis, and Mohamed Faizal Abdul-Careem. 2021. "Immune Responses in Laying Hens after an Infectious Bronchitis Vaccination of Pullets: A Comparison of Two Vaccination Strategies" Vaccines 9, no. 5: 531. https://doi.org/10.3390/vaccines9050531
APA StyleBuharideen, S. M., Hassan, M. S. H., Najimudeen, S. M., Niu, D., Czub, M., Gomis, S., & Abdul-Careem, M. F. (2021). Immune Responses in Laying Hens after an Infectious Bronchitis Vaccination of Pullets: A Comparison of Two Vaccination Strategies. Vaccines, 9(5), 531. https://doi.org/10.3390/vaccines9050531







