The Safe Baculovirus-Based PrM/E DNA Vaccine Protected Fetuses against Zika Virus in A129 Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Plasmid Construction and Gene Expression
2.3. Mice
2.4. Enzyme Linked Immunosorbent Assay (ELISA)
2.5. Enzyme-Linked ImmunoSpot (ELISPOT) Assay
2.6. Neutralizing Antibody Assay
2.7. ZIKV Challenge in A129 Mice
2.8. Virus Infectivity Test in Pregnant Mice
2.9. Data Analysis
3. Results
3.1. Construction and Assessment of ZIKV PrM/E Protein-Expressing Recombinant Baculoviruses Regulated by Different Signal Peptides
3.2. Recombinant ZIKV PrM/E-Expressing Baculoviruses Successfully Induce ZIKV-Specific Antibodies and T-Cell Responses
3.3. Mice Vaccinated with AcHERV-ZIKV Are Fully Protected against ZIKV Infection
3.4. AcHERV-ZIKV Protects Pregnant A129 Mice and Their Fetuses against ZIKV Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diamond, M.S.; Ledgerwood, J.E.; Pierson, T.C. Zika Virus Vaccine Development: Progress in the Face of New Challenges. Annu. Rev. Med. 2019, 70, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Dowd, K.A.; DeMaso, C.R.; Pelc, R.S.; Speer, S.D.; Smith, A.R.Y.; Goo, L.; Platt, D.J.; Mascola, J.R.; Graham, B.S.; Mulligan, M.J.; et al. Broadly Neutralizing Activity of Zika Virus-Immune Sera Identifies a Single Viral Serotype. Cell Rep. 2016, 16, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Haddow, A.D.; Schuh, A.J.; Yasuda, C.Y.; Kasper, M.R.; Heang, V.; Huy, R.; Guzman, H.; Tesh, R.B.; Weaver, S.C. Genetic characterization of Zika virus strains: Geographic expansion of the Asian lineage. PLoS Negl. Trop. Dis. 2012, 6, e1477. [Google Scholar] [CrossRef]
- Faria, N.R.; Azevedo, R.; Kraemer, M.U.G.; Souza, R.; Cunha, M.S.; Hill, S.C.; Theze, J.; Bonsall, M.B.; Bowden, T.A.; Rissanen, I.; et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science 2016, 352, 345–349. [Google Scholar] [CrossRef]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef]
- Cragan, J.D.; Mai, C.T.; Petersen, E.E.; Liberman, R.F.; Forestieri, N.E.; Stevens, A.C.; Delaney, A.; Dawson, A.L.; Ellington, S.R.; Shapiro-Mendoza, C.K.; et al. Baseline Prevalence of Birth Defects Associated with Congenital Zika Virus Infection—Massachusetts, North Carolina, and Atlanta, Georgia, 2013–2014. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 219–222. [Google Scholar] [CrossRef]
- Imperato, P.J. The Convergence of a Virus, Mosquitoes, and Human Travel in Globalizing the Zika Epidemic. J. Community Health 2016, 41, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Marston, H.D.; Lurie, N.; Borio, L.L.; Fauci, A.S. Considerations for Developing a Zika Virus Vaccine. N. Engl. J. Med. 2016, 375, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Dent, M.; Lai, H.; Sun, H.; Chen, Q. Immunization of Zika virus envelope protein domain III induces specific and neutralizing immune responses against Zika virus. Vaccine 2017, 35, 4287–4294. [Google Scholar] [CrossRef]
- Lazear, H.M.; Diamond, M.S. Zika Virus: New Clinical Syndromes and Its Emergence in the Western Hemisphere. J. Virol. 2016, 90, 4864–4875. [Google Scholar] [CrossRef]
- Yang, M.; Lai, H.F.; Sun, H.Y.; Chen, Q. Virus- like particles that display Zika virus envelope protein domain III induce potent neutralizing immune responses in mice. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 168, 1114–1125.e10. [Google Scholar] [CrossRef]
- Ura, T.; Okuda, K.; Shimada, M. Developments in Viral Vector-Based Vaccines. Vaccines 2014, 2, 624–641. [Google Scholar] [CrossRef]
- Robert-Guroff, M. Replicating and non-replicating viral vectors for vaccine development. Curr. Opin. Biotechnol. 2007, 18, 546–556. [Google Scholar] [CrossRef]
- Steffen, T.; Hassert, M.; Hoft, S.G.; Stone, E.T.; Zhang, J.; Geerling, E.; Grimberg, B.T.; Roberts, M.S.; Pinto, A.K.; Brien, J.D. Immunogenicity and Efficacy of a Recombinant Human Adenovirus Type 5 Vaccine against Zika Virus. Vaccines 2020, 8, 170. [Google Scholar] [CrossRef]
- Bullard, B.L.; Corder, B.N.; Gordon, D.N.; Pierson, T.C.; Weaver, E.A. Characterization of a Species E Adenovirus Vector as a Zika virus vaccine. Sci. Rep. 2020, 10, 3613. [Google Scholar] [CrossRef]
- Perez, P.; Marín, M.Q.; Lazaro-Frias, A.; de Oya, N.J.; Blazquez, A.B.; Escribano-Romero, E.; Sorzano, C.Ó.S.; Ortego, J.; Saiz, J.C.; Esteban, M.; et al. A Vaccine Based on a Modified Vaccinia Virus Ankara Vector Expressing Zika Virus Structural Proteins Controls Zika Virus Replication in Mice. Sci. Rep. 2018, 8, 17385. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Camacho, C.; Abbink, P.; Larocca, R.A.; Dejnirattisai, W.; Boyd, M.; Badamchi-Zadeh, A.; Wallace, Z.R.; Doig, J.; Velazquez, R.S.; Neto, R.D.L.; et al. Rational Zika vaccine design via the modulation of antigen membrane anchors in chimpanzee adenoviral vectors. Nat. Commun. 2018, 9, 2441. [Google Scholar] [CrossRef] [PubMed]
- Prow, N.A.; Liu, L.; Nakayama, E.; Cooper, T.H.; Yan, K.; Eldi, P.; Hazlewood, J.E.; Tang, B.; Le, T.T.; Setoh, Y.X.; et al. A vaccinia-based single vector construct multi-pathogen vaccine protects against both Zika and chikungunya viruses. Nat. Commun. 2018, 9, 1230. [Google Scholar] [CrossRef]
- Hofmann, C.; Sandig, V.; Jennings, G.; Rudolph, M.; Schlag, P.; Strauss, M. Efficient gene transfer into human hepatocytes by baculovirus vectors. Proc. Natl. Acad. Sci. USA 1995, 92, 10099–10103. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.Y.; Choi, H.; Kim, N.; Park, N.; Kim, H.; Kim, J.; Kim, Y.B. Unraveling the Genome-Wide Impact of Recombinant Baculovirus Infection in Mammalian Cells for Gene Delivery. Genes 2020, 11, 1306. [Google Scholar] [CrossRef]
- Cho, H.; Jang, Y.; Park, K.H.; Choi, H.; Nowakowska, A.; Lee, H.J.; Kim, M.; Kang, M.H.; Kim, J.H.; Shin, H.Y.; et al. Human endogenous retrovirus-enveloped baculoviral DNA vaccines against MERS-CoV and SARS-CoV2. NPJ Vaccines 2021, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Yockey, L.J.; Varela, L.; Rakib, T.; Khoury-Hanold, W.; Fink, S.L.; Stutz, B.; Szigeti-Buck, K.; Van den Pol, A.; Lindenbach, B.D.; Horvath, T.L.; et al. Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell 2016, 166, 1247–1256.e4. [Google Scholar] [CrossRef]
- Richner, J.M.; Jagger, B.W.; Shan, C.; Fontes, C.R.; Dowd, K.A.; Cao, B.; Himansu, S.; Caine, E.A.; Nunes, B.T.D.; Medeiros, D.B.A.; et al. Vaccine Mediated Protection Against Zika Virus-Induced Congenital Disease. Cell 2017, 170, 273–283.e12. [Google Scholar] [CrossRef] [PubMed]
- Ostrand-Rosenberg, S.; Sinha, P.; Figley, C.; Long, R.; Park, D.; Carter, D.; Clements, V.K. Frontline Science: Myeloid-derived suppressor cells (MDSCs) facilitate maternal-fetal tolerance in mice. J. Leukoc. Biol. 2017, 101, 1091–1101. [Google Scholar] [CrossRef]
- Trus, I.; Udenze, D.; Berube, N.; Wheler, C.; Martel, M.J.; Gerdts, V.; Karniychuk, U. CpG-Recoding in Zika Virus Genome Causes Host-Age-Dependent Attenuation of Infection With Protection Against Lethal Heterologous Challenge in Mice. Front. Immunol. 2019, 10, 3077. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, C.J.; Lawrence, S.; Smith, P.; Juengel, J.L.; McNatty, K.P. Active immunization against the proregions of GDF9 or BMP15 alters ovulation rate and litter size in mice. Reproduction 2012, 143, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika Virus and Birth Defects—Reviewing the Evidence for Causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef]
- Airenne, K.J.; Hu, Y.C.; Kost, T.A.; Smith, R.H.; Kotin, R.M.; Ono, C.; Matsuura, Y.; Wang, S.; Yla-Herttuala, S. Baculovirus: An insect-derived vector for diverse gene transfer applications. Mol. Ther. 2013, 21, 739–749. [Google Scholar] [CrossRef]
- Brave, A.; Ljungberg, K.; Wahren, B.; Liu, M.A. Vaccine delivery methods using viral vectors. Mol. Pharm. 2007, 4, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.F.; Ma, B.; Monie, A.; Tsen, S.W.; Wu, T.C. Therapeutic human papillomavirus vaccines: Current clinical trials and future directions. Expert Opin. Biol. Ther. 2008, 8, 421–439. [Google Scholar] [CrossRef]
- Lee, H.J.; Hur, Y.K.; Cho, Y.D.; Kim, M.G.; Lee, H.T.; Oh, Y.K.; Kim, Y.B. Immunogenicity of bivalent human papillomavirus DNA vaccine using human endogenous retrovirus envelope-coated baculoviral vectors in mice and pigs. PLoS ONE 2012, 7, e50296. [Google Scholar] [CrossRef]
- Grant, A.; Ponia, S.S.; Tripathi, S.; Balasubramaniam, V.; Miorin, L.; Sourisseau, M.; Schwarz, M.C.; Sanchez-Seco, M.P.; Evans, M.J.; Best, S.M.; et al. Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host Microbe 2016, 19, 882–890. [Google Scholar] [CrossRef]
- Elshahawi, H.; Syed Hassan, S.; Balasubramaniam, V. Importance of Zika Virus NS5 Protein for Viral Replication. Pathogens 2019, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yang, S.; He, J.; Guest, J.D.; Ma, Z.; Yang, L.; Pierce, B.G.; Tang, Q.; Zhang, Y.J. Zika virus NS5 protein antagonizes type I interferon production via blocking TBK1 activation. Virology 2019, 527, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.E.; Diamond, M.S. Animal Models of Zika Virus Infection, Pathogenesis, and Immunity. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Gorman, M.J.; Caine, E.A.; Zaitsev, K.; Begley, M.C.; Weger-Lucarelli, J.; Uccellini, M.B.; Tripathi, S.; Morrison, J.; Yount, B.L.; Dinnon, K.H., 3rd; et al. An Immunocompetent Mouse Model of Zika Virus Infection. Cell Host Microbe 2018, 23, 672–685.e6. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sastre, A. Ten Strategies of Interferon Evasion by Viruses. Cell Host Microbe 2017, 22, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Laurent-Rolle, M.; Morrison, J.; Rajsbaum, R.; Macleod, J.M.L.; Pisanelli, G.; Pham, A.; Ayllon, J.; Miorin, L.; Martinez-Romero, C.; tenOever, B.R.; et al. The Interferon Signaling Antagonist Function of Yellow Fever Virus NS5 Protein Is Activated by Type I Interferon. Cell Host Microbe 2014, 16, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Liu, X.; Zhang, P.; Wang, S.; Liu, Y.; Zhang, L.; Song, C. Porcine circovirus 3 Cap inhibits type I interferon signaling through interaction with STAT2. Virus Res. 2020, 275, 197804. [Google Scholar] [CrossRef]
- Lucas, C.G.O.; Kitoko, J.Z.; Ferreira, F.M.; Suzart, V.G.; Papa, M.P.; Coelho, S.V.A.; Cavazzoni, C.B.; Paula-Neto, H.A.; Olsen, P.C.; Iwasaki, A.; et al. Critical role of CD4(+) T cells and IFNgamma signaling in antibody-mediated resistance to Zika virus infection. Nat. Commun. 2018, 9, 3136. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A.; Kennedy, R.B.; Ovsyannikova, I.G.; Palacios, R.; Ho, P.L.; Kalil, J. Development of vaccines against Zika virus. Lancet Infect Dis. 2018, 18, e211–e219. [Google Scholar] [CrossRef]
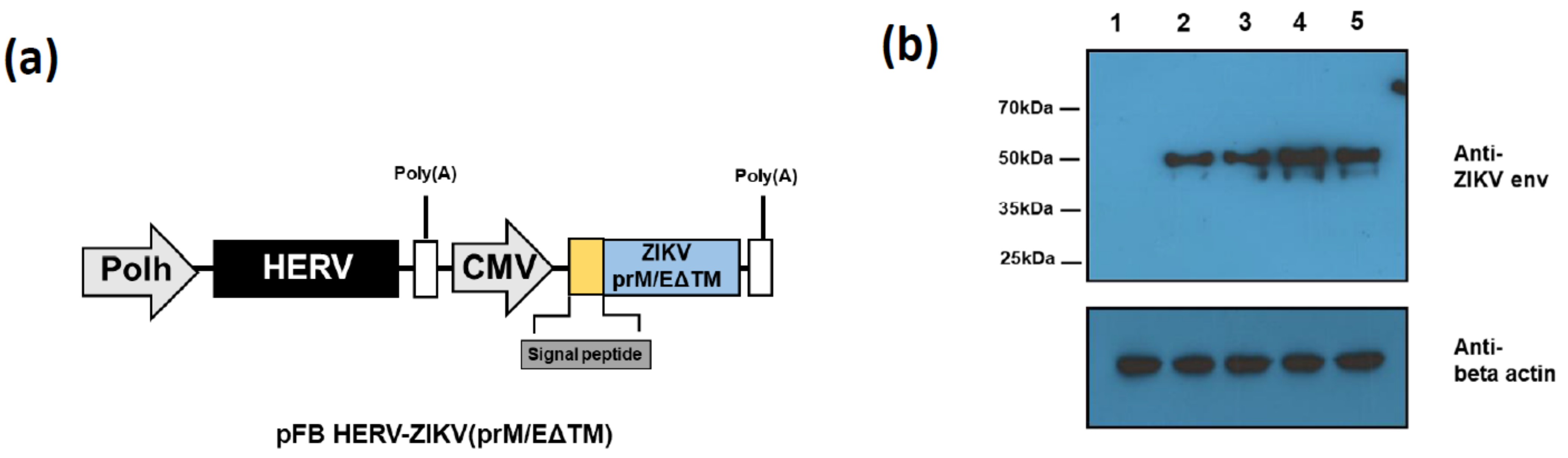
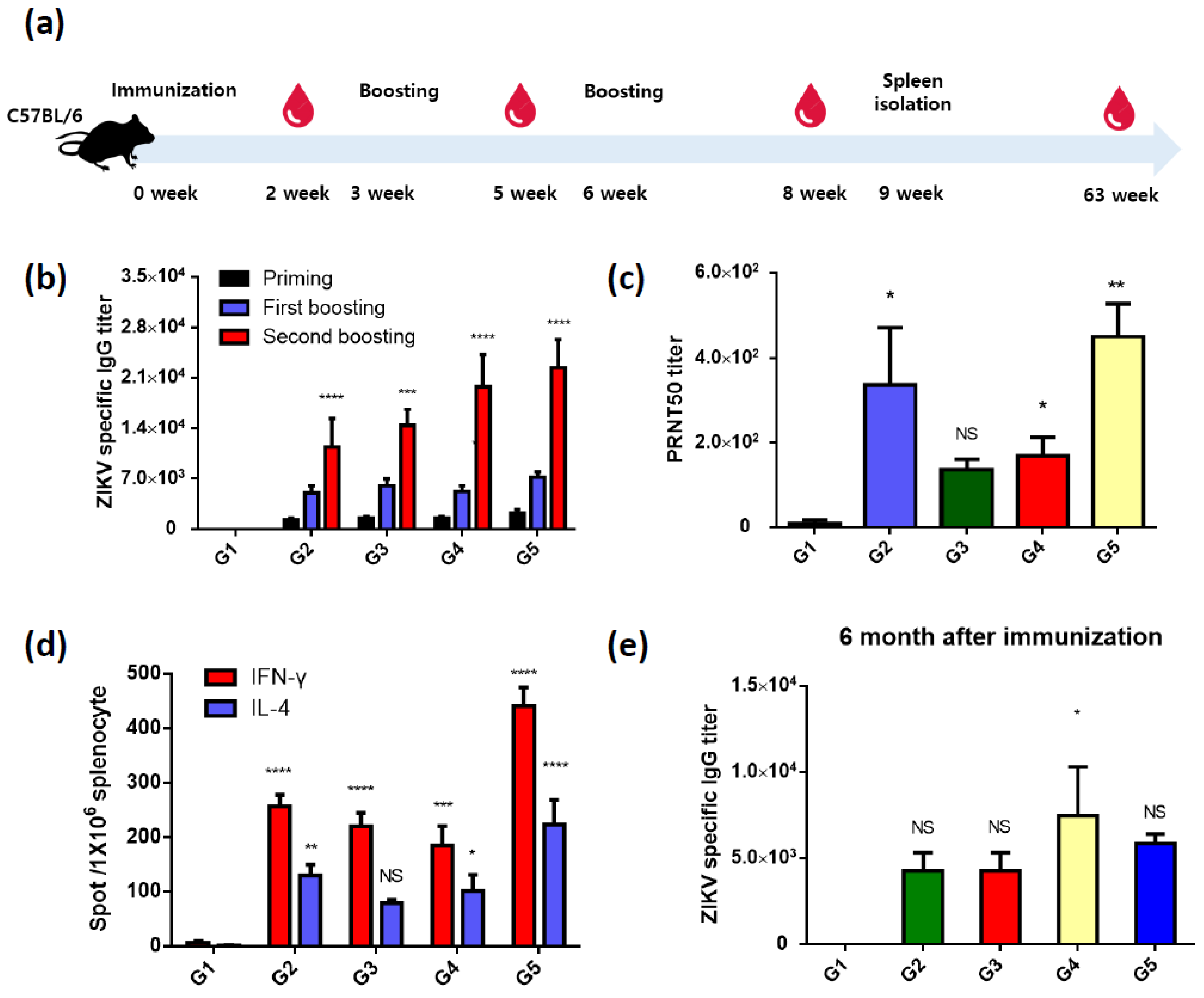
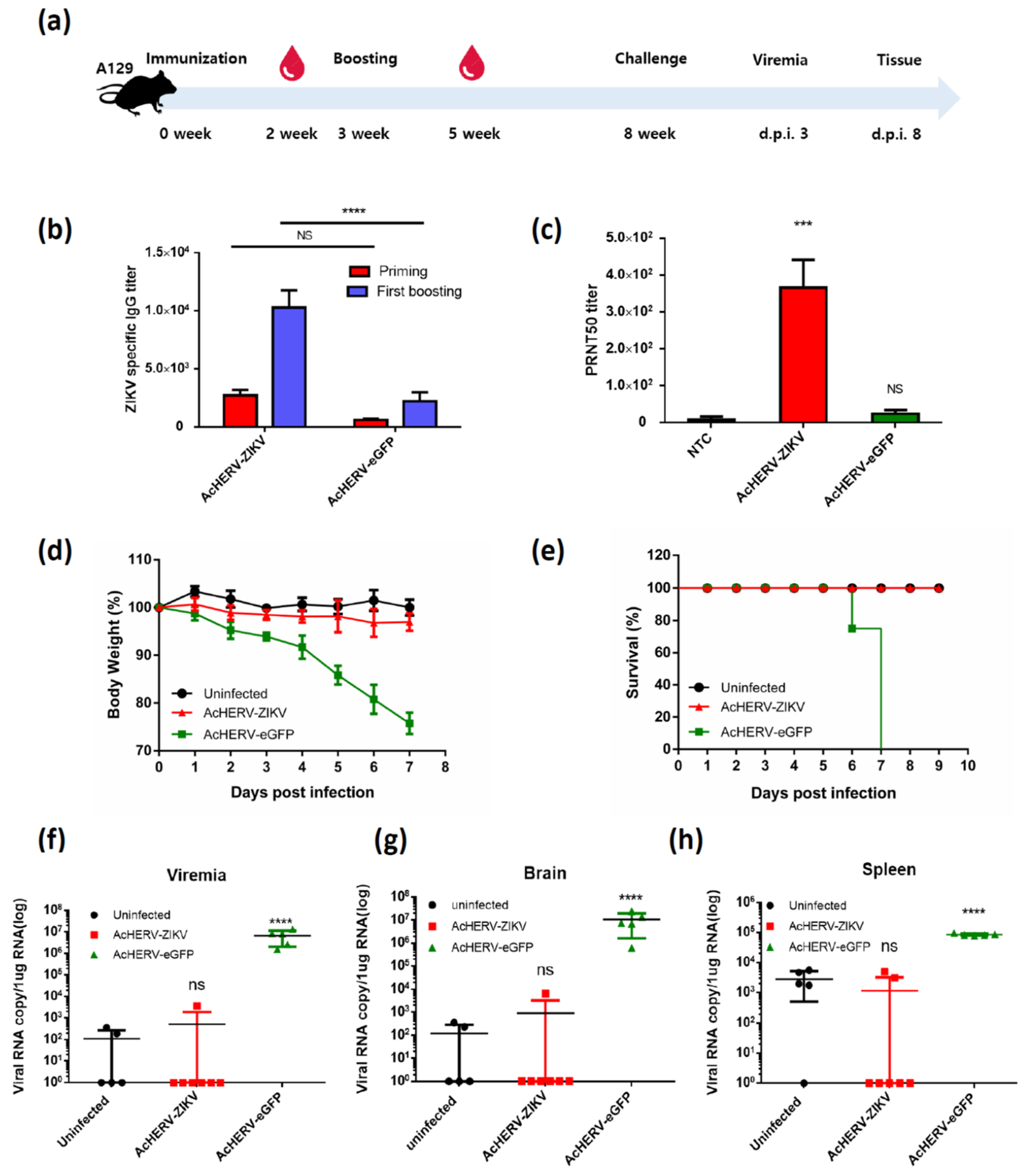
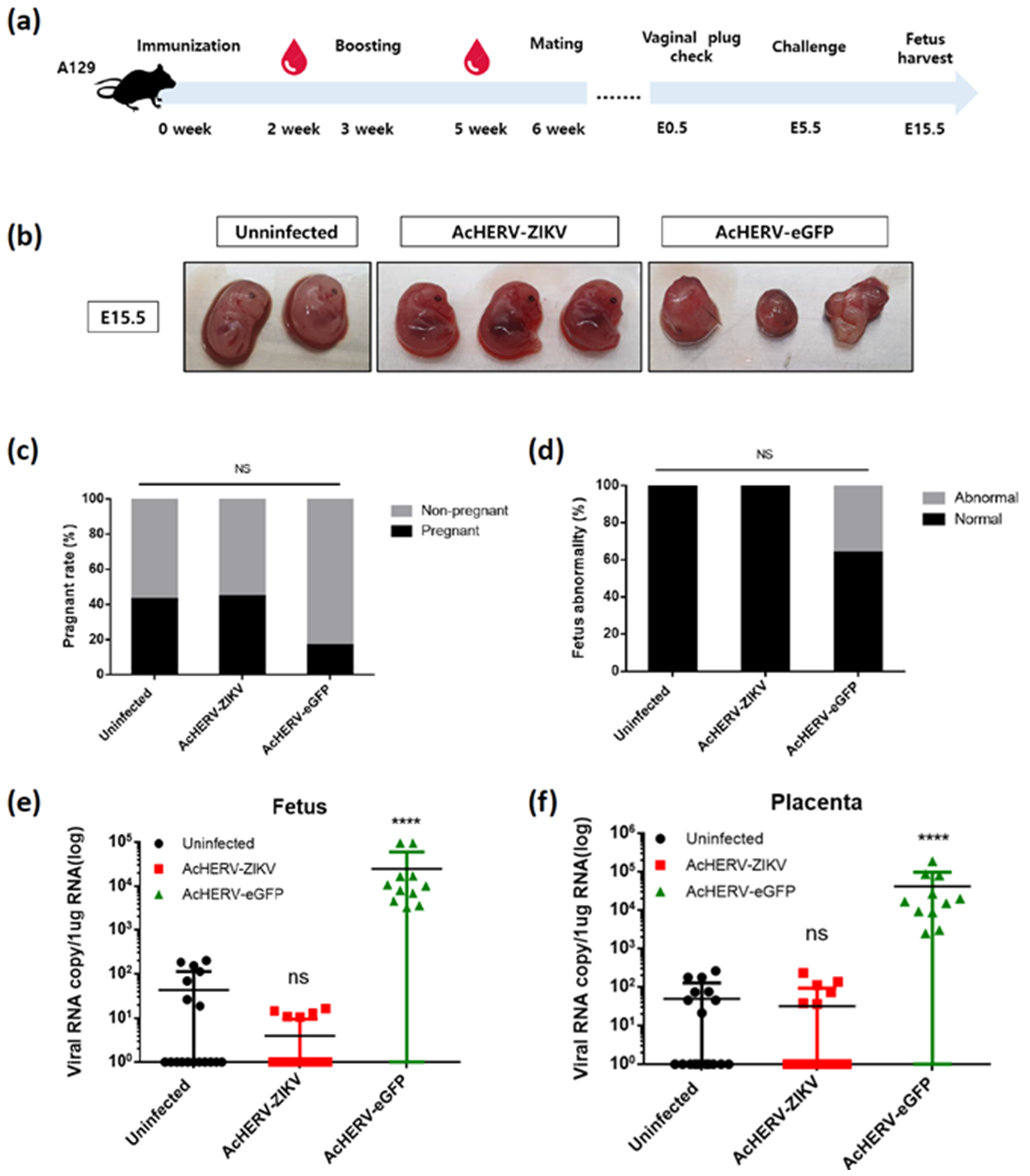
| Name | Amino Acid Sequence |
|---|---|
| ZIKV | MAAEVTRR |
| CD5 | MPMGSLQPLATLYLLGMLVAS |
| IgK | METDTLLLWVLLLWVPGSTG |
| IgM | MKFSWVMFFLMAVVTGVNSE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Chun, J.; Park, M.; Kim, S.; Kim, N.; Lee, H.-J.; Kim, M.; Shin, H.Y.; Oh, Y.-K.; Kim, Y.B. The Safe Baculovirus-Based PrM/E DNA Vaccine Protected Fetuses against Zika Virus in A129 Mice. Vaccines 2021, 9, 438. https://doi.org/10.3390/vaccines9050438
Choi H, Chun J, Park M, Kim S, Kim N, Lee H-J, Kim M, Shin HY, Oh Y-K, Kim YB. The Safe Baculovirus-Based PrM/E DNA Vaccine Protected Fetuses against Zika Virus in A129 Mice. Vaccines. 2021; 9(5):438. https://doi.org/10.3390/vaccines9050438
Chicago/Turabian StyleChoi, Hanul, Jungmin Chun, Mina Park, Suyeon Kim, Nahyun Kim, Hee-Jung Lee, Minjee Kim, Ha Youn Shin, Yu-Kyoung Oh, and Young Bong Kim. 2021. "The Safe Baculovirus-Based PrM/E DNA Vaccine Protected Fetuses against Zika Virus in A129 Mice" Vaccines 9, no. 5: 438. https://doi.org/10.3390/vaccines9050438
APA StyleChoi, H., Chun, J., Park, M., Kim, S., Kim, N., Lee, H.-J., Kim, M., Shin, H. Y., Oh, Y.-K., & Kim, Y. B. (2021). The Safe Baculovirus-Based PrM/E DNA Vaccine Protected Fetuses against Zika Virus in A129 Mice. Vaccines, 9(5), 438. https://doi.org/10.3390/vaccines9050438






