Decrease of IL-5 Production by Naive T Cells Cocultured with IL-18-Producing BCG-Pulsed Dendritic Cells from Patients Allergic to House Dust Mite
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Human Donors
2.3. Human Monocyte-Derived Dendritic Cell (MD-DC) Preparation
2.4. Preparation of Bacteria
2.5. Dendritic Cell Activation
2.6. Dendritic Cell Surface Marker Analysis
2.7. Naive and Memory CD4+ T Cell Isolation
2.8. DC-T Cell Cocultures
2.9. Statistical Analysis
3. Results
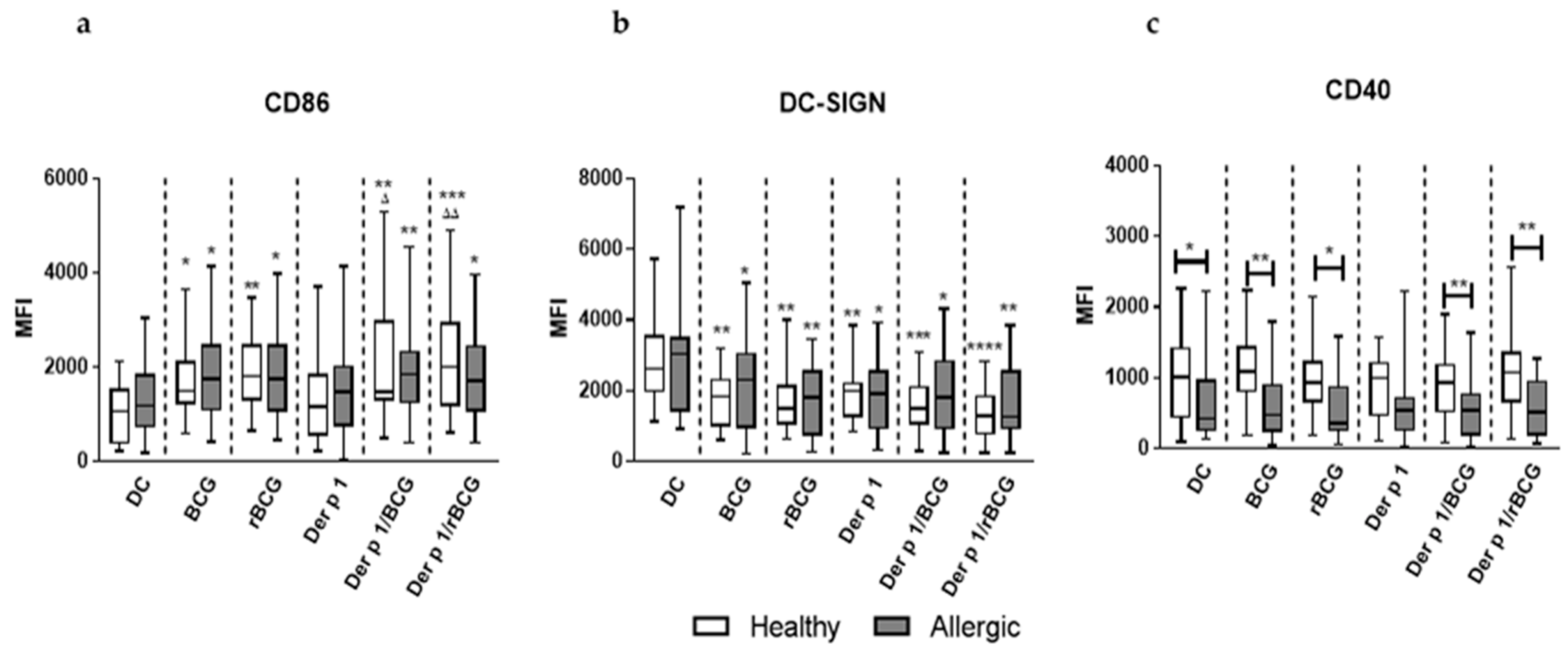
3.1. rBCG-hIL-18 and BCG Induce the Maturation of MD-DCs in the Presence of Der p 1
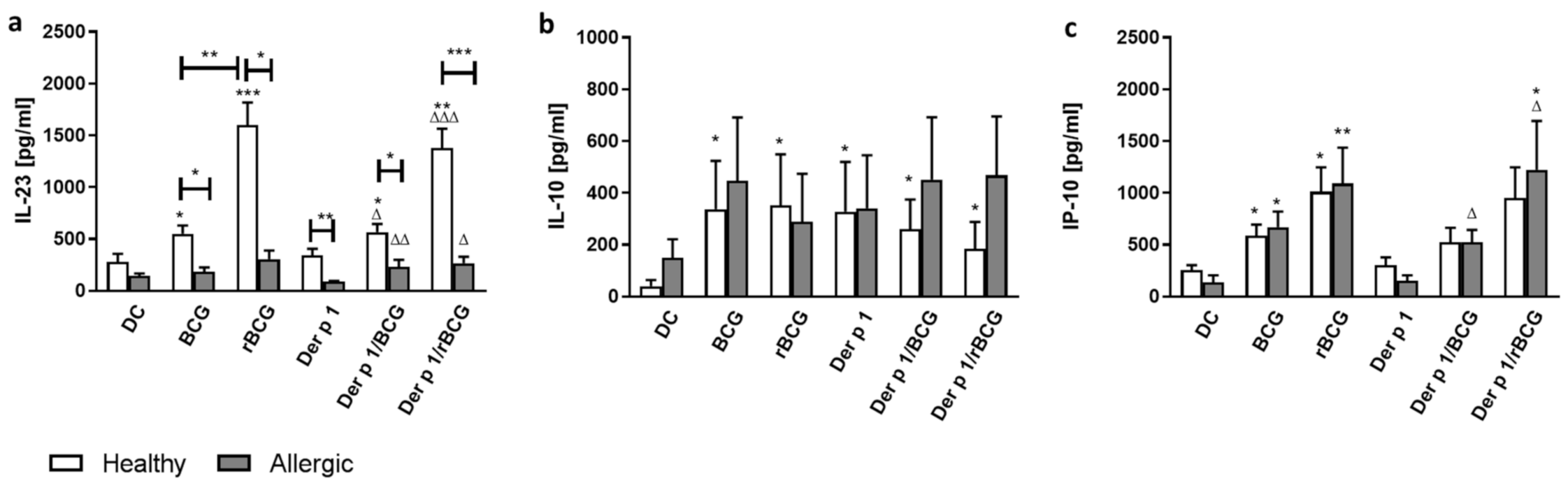
3.2. Effect of BCG and rBCG-hIL-18 on Cytokine and Chemokine Production by DCs in the Presence of Der p 1
3.3. IFN-γ Production by CD4+ Naive and Memory T Cells Cocultured with Der p 1-Treated DCs in the Presence of BCG or rBCG-hIL-18
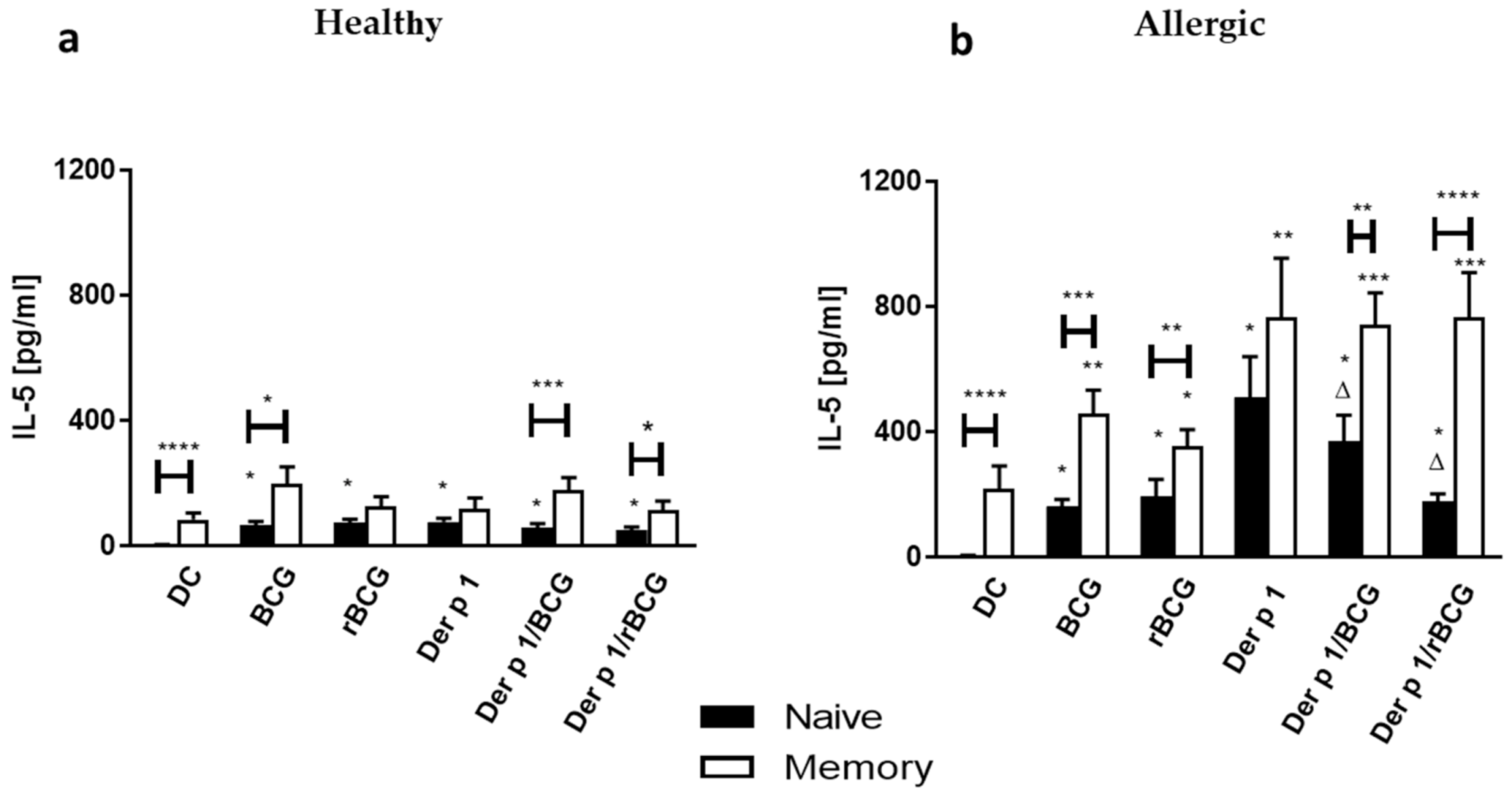
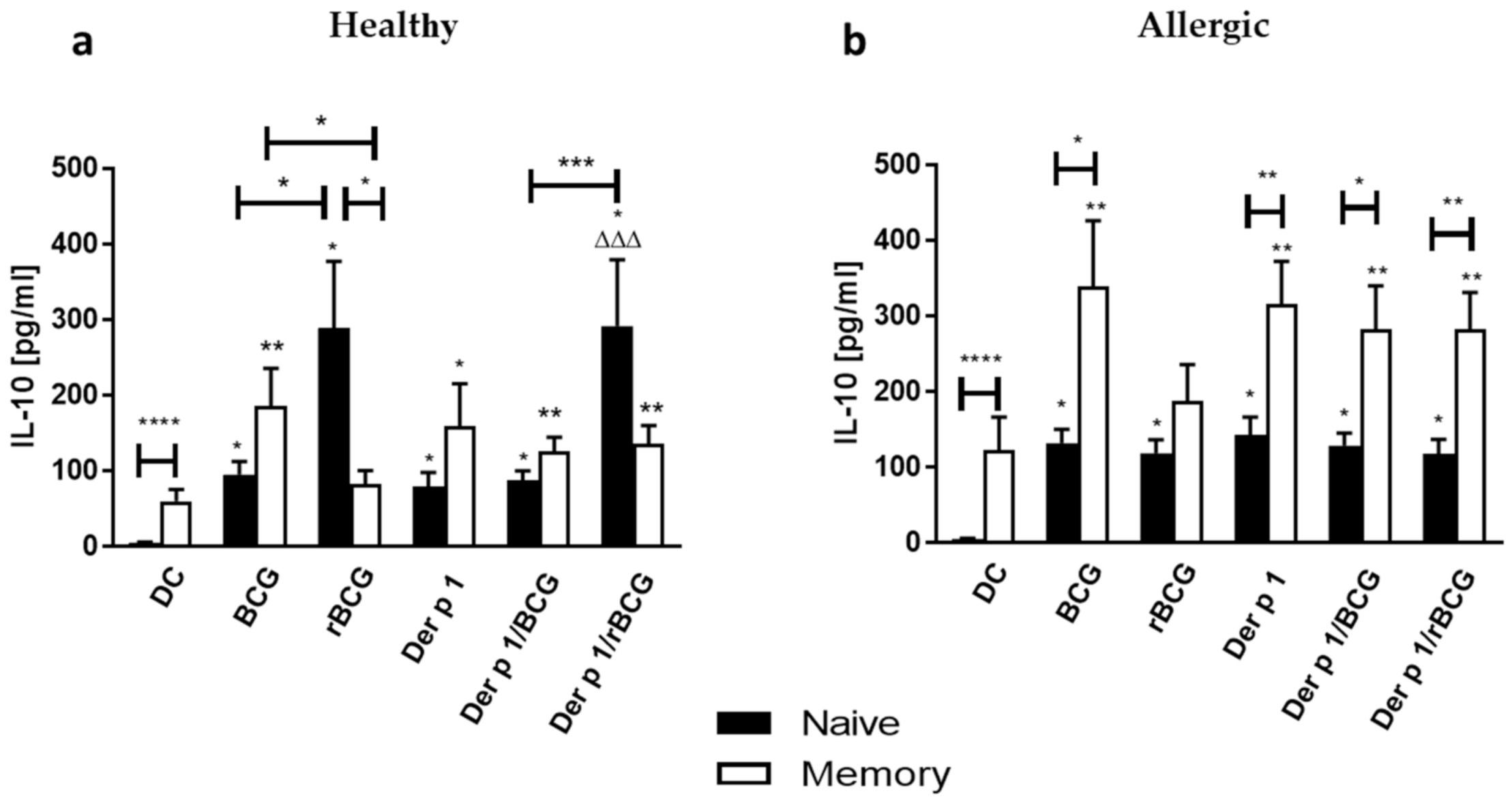
3.4. Effect of BCG and rBCG-hIL-18 on IL-5 and IL-10 Production by CD4+ Naive and Memory T Cells Cocultured with Der p 1-Treated DCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kowalewicz-Kulbat, M.; Locht, C. BCG and protection against inflammatory and auto-immune diseases. Expert Rev. Vaccines 2017, 16, 699–708. [Google Scholar] [CrossRef]
- Thomsen, S.F. Epidemiology and natural history of atopic diseases. Eur. Clin. Respir. J. 2015, 2, 24642. [Google Scholar] [CrossRef]
- El Biaze, M.; Boniface, S.; Koscher, V.; Mamessier, E.; Dupuy, P.; Milhe, F.; Ramadour, M.; Vervloet, D.; Magnan, A. T cell activation, from atopy to asthma: More a paradox than a paradigm. Allergy 2003, 58, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Till, S.; Dickason, R.; Huston, D.; Humbert, M.; Robinson, D.; Larché, M.; Durham, S.; Kay, A.; Corrigan, C. IL-5 secretion by allergen-stimulated CD4+ T cells in primary culture: Relationship to expression of allergic disease. J. Allergy Clin. Immunol. 1997, 99, 563–569. [Google Scholar] [CrossRef]
- Ebner, C.; Schenk, S.; Najafian, N.; Siemann, U.; Steiner, R.; Fischer, G.W.; Hoffmann, K.; Szépfalusi, Z.; Scheiner, O.; Kraft, D. Nonallergic individuals recognize the same T cell epitopes of Bet v 1, the major birch pollen allergen, as atopic patients. J. Immunol. 1995, 154, 1932–1940. [Google Scholar]
- Akdis, M.; Verhagen, J.; Taylor, A.; Karamloo, F.; Karagiannidis, C.; Crameri, R.; Thunberg, S.; Deniz, G.; Valenta, R.; Fiebig, H.; et al. Immune Responses in Healthy and Allergic Individuals Are Characterized by a Fine Balance between Allergen-specific T Regulatory 1 and T Helper 2 Cells. J. Exp. Med. 2004, 199, 1567–1575. [Google Scholar] [CrossRef]
- Van Overtvelt, L.; Wambre, E.; Maillère, B.; Von Hofe, E.; Louise, A.; Balazuc, A.M.; Bohle, B.; Ebo, D.; Leboulaire, C.; Garcia, G.; et al. Assessment of Bet v 1-specific CD4+ T cell responses in allergic and nonallergic individuals using MHC class II peptide tetramers. J. Immunol. 2008, 180, 4514–4522. [Google Scholar] [CrossRef]
- Stewart, G.A.; Richardson, J.P.; Zhang, J.; Robinson, C. The structure and function of allergens. In Middleton’s Allergy—Principles and Practice, 8th ed.; Adkinson, N.F., Bochner, B.S., Burks, A.W., Busse, W.W., Holgate, S.T., Lemanske, R.F., O’Hehir, R.E., Eds.; Elsevier: Philadelphia, PA, USA, 2014; pp. 398–429. [Google Scholar]
- Chapman, M.D.; Wünschmann, S.; Pomés, A. Proteases as Th2 adjuvants. Curr. Allergy Asthma Rep. 2007, 7, 363–367. [Google Scholar] [CrossRef]
- Erb, K.J.; Holloway, J.W.; Sobeck, A.; Moll, H.; Le Gros, G. Infection of Mice with Mycobacterium bovis–Bacillus Calmette-Guérin (BCG) Suppresses Allergen-induced Airway Eosinophilia. J. Exp. Med. 1998, 187, 561–569. [Google Scholar] [CrossRef]
- Biet, F.; Kremer, L.; Wolowczuk, I.; Delacre, M.; Locht, C. Mycobacterium bovis BCG Producing Interleukin-18 Increases Antigen-Specific Gamma Interferon Production in Mice. Infect. Immun. 2002, 70, 6549–6557. [Google Scholar] [CrossRef]
- Biet, F.; Duez, C.; Kremer, L.; Marquillies, P.; Amniai, L.; Tonnel, A.-B.; Locht, C.; Pestel, J. Recombinant Mycobacterium bovis BCG producing IL-18 reduces IL-5 production and bronchoalveolar eosinophilia induced by an allergic reaction. Allergy 2005, 60, 1065–1072. [Google Scholar] [CrossRef]
- Szpakowski, P.; Biet, F.; Locht, C.; Paszkiewicz, M.; Rudnicka, W.; Druszczyńska, M.; Allain, F.; Fol, M.; Pestel, J.; Kowalewicz-Kulbat, M. Dendritic Cell Activity Driven by Recombinant Mycobacterium bovisBCG Producing Human IL-18, in Healthy BCG Vaccinated Adults. J. Immunol. Res. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Lambrecht, B.N. The dendritic cell in allergic airway diseases: A new player to the game. Clin. Exp. Allergy 2001, 31, 206–218. [Google Scholar] [CrossRef]
- Dreborg, S. EAACI Subcommittee on Skin Tests. Skin tests used in type I allergy testing. Position Paper. Allergy 1989, 44, S22–S31. [Google Scholar]
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Caraballo, L.; Villa, E.; Ebisawa, M.; Passalacqua, G.; Savi, E.; Ebo, D.; Gómez, R.M.; et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ. J. 2020, 13, 100080. [Google Scholar] [CrossRef]
- Pochard, P.; Hammad, H.; Ratajczak, C.; Charbonnier-Hatzfeld, A.-S.; Just, N.; Tonnel, A.-B.; Pestel, J. Direct regulatory immune activity of lactic acid bacteria on Der p 1–pulsed dendritic cells from allergic patients. J. Allergy Clin. Immunol. 2005, 116, 198–204. [Google Scholar] [CrossRef]
- Kowalewicz-Kulbat, M.; Szpakowski, P.; Locht, C.; Biet, F.; Kaplonek, P.; Krawczyk, K.T.; Pestel, J.; Rudnicka, W. Tuberculin skin test reaction is related to memory, but not naive CD4+ T cell responses to mycobacterial stimuli in BCG-vaccinated young adults. Vaccine 2018, 36, 4566–4577. [Google Scholar] [CrossRef]
- Jan, R.-H.; Lin, T.-Y.; Hsu, Y.-C.; Lee, S.-S.; Lo, S.-Y.; Chang, M.; Chen, L.-K.; Lin, Y.-L. Immuno-modulatory activity of Ganoderma lucidum-derived polysacharide on human monocytoid dendritic cells pulsed with Der p 1 allergen. BMC Immunol. 2011, 12, 31. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Na, H.; Cho, M.; Chung, Y. Regulation of Th2 Cell Immunity by Dendritic Cells. Immune Netw. 2016, 16, 1–12. [Google Scholar] [CrossRef]
- Shen, H.; Huang, H.; Wang, J.; Ye, S.; Li, W.; Wang, K.; Zhang, G.; Wang, P. Neonatal vaccination with Bacillus Calmette-Guérin elicits long-term protection in mouse-allergic responses. Allergy 2008, 63, 555–563. [Google Scholar] [CrossRef]
- Marchant, A.; Goetghebuer, T.; O Ota, M.; Wolfe, I.; Ceesay, S.J.; De Groote, D.; Corrah, T.; Bennett, S.; Wheeler, J.; Huygen, K.; et al. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guérin vaccination. J. Immunol. 1999, 163, 2249–2255. [Google Scholar]
- Choi, I.S.; Lin, X.-H.; Koh, Y.-A.; Cui, Y. BCG-Induced Dendritic Cell Responses and Suppression of Interleukin-5 Production from T Cells in Atopic Asthmatics. J. Korean Med Sci. 2008, 23, 628–634. [Google Scholar] [CrossRef]
- Caccamo, N.; Joosten, S.A.; Ottenhoff, T.H.M.; Dieli, F. Atypical Human Effector/Memory CD4+ T Cells With a Naive-Like Phenotype. Front. Immunol. 2018, 9, 2832. [Google Scholar] [CrossRef]
- Becattini, S.; Latorre, D.; Mele, F.; Foglierini, M.; De Gregorio, C.; Cassotta, A.; Fernandez, B.; Kelderman, S.; Schumacher, T.N.; Corti, D.; et al. Functional heterogeneity of human memory CD4+ T cell clones primed by pathogens or vaccines. Science 2014, 347, 400–406. [Google Scholar] [CrossRef]
- Weinreich, M.A.; Hogquist, K.A. Thymic Emigration: When and How T Cells Leave Home. J. Immunol. 2008, 181, 2265–2270. [Google Scholar] [CrossRef]
- Wambre, E.; Bajzik, V.; Delong, J.H.; O’Brien, K.; Nguyen, Q.-A.; Speake, C.; Gersuk, V.H.; DeBerg, H.A.; Whalen, E.; Veronique, B.; et al. A phenotypically and functionally distinct human T H 2 cell subpopulation is associated with allergic disorders. Sci. Transl. Med. 2017, 9, eaam9171. [Google Scholar] [CrossRef]
- Walsh, M.C.; Pearce, E.L.; Cejas, P.J.; Lee, J.; Wang, L.-S.; Choi, Y. IL-18 Synergizes with IL-7 To Drive Slow Proliferation of Naive CD8 T Cells by Costimulating Self-Peptide–Mediated TCR Signals. J. Immunol. 2014, 193, 3992–4001. [Google Scholar] [CrossRef]
- Tan, J.T.; Dudl, E.; Leroy, E.; Murray, R.; Sprent, J.; Weinberg, K.I.; Surh, C.D. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl. Acad. Sci. USA 2001, 98, 8732–8737. [Google Scholar] [CrossRef]
- Lantz, O.; Grandjean, I.; Matzinger, P.; Di Santo, J.P. γ chain required for naïve CD4+ T cell survival but not for antigen proliferation. Nat. Immunol. 2000, 1, 54–58. [Google Scholar] [CrossRef]
- Sanders, N.L.; Mishra, A. Role of interleukin-18 in the pathophysiology of allergic diseases. Cytokine Growth Factor Rev. 2016, 32, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Tsutsui, H.; Komatsu, T.; Yutsudo, M.; Hakura, A.; Tanimoto, T.; Torigoe, K.; Okura, T.; Nukada, Y.; Hattori, K.; et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nat. Cell Biol. 1995, 378, 88–91. [Google Scholar] [CrossRef]
- Sims, J. IL-1 and IL-18 receptors, and their extended family. Curr. Opin. Immunol. 2002, 14, 117–122. [Google Scholar] [CrossRef]
- Hofstra, C.L.; Van Ark, I.; Hofman, G.; Kool, M.; Nijkamp, F.P.; Van Oosterhout, A.J. Prevention of Th2-like cell responses by coadministration of IL-12 and IL-18 is associated with inhibition of antigen-induced airway hyperresponsiveness, eosinophilia, and serum IgE levels. J. Immunol. 1998, 161, 5054–5060. [Google Scholar]
- Kohno, K.; Kataoka, J.; Ohtsuki, T.; Suemoto, Y.; Okamoto, I.; Usui, M.; Ikeda, M.; Kurimoto, M. IFN-gamma-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J. Immunol. 1997, 158, 1541–1550. [Google Scholar]
- Wild, J.S.; Sigounas, A.; Sur, N.; Siddiqui, M.S.; Alam, R.; Kurimoto, M.; Sur, S. IFN-γ-Inducing Factor (IL-18) Increases Allergic Sensitization, Serum IgE, Th2 Cytokines, and Airway Eosinophilia in a Mouse Model of Allergic Asthma. J. Immunol. 2000, 164, 2701–2710. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Samperio, P.; Trejo-Echeverria, A.; Ayala-Verdin, H. Regulation of Interleukin-12 Production in Human Cells Stimulated withMycobacterium bovisBCG. Cell. Immunol. 1998, 189, 25–30. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, W.; Shang, J.; Yang, J.; Zhang, L.; Bachert, C. Immunomodulatory effects of IL-23 and IL-17 in a mouse model of allergic rhinitis. Clin. Exp. Allergy 2013, 43, 956–966. [Google Scholar] [CrossRef]
- Wakashin, H.; Hirose, K.; Maezawa, Y.; Kagami, S.-I.; Suto, A.; Watanabe, N.; Saito, Y.; Hatano, M.; Tokuhisa, T.; Iwakura, Y.; et al. IL-23 and Th17 Cells Enhance Th2-Cell–mediated Eosinophilic Airway Inflammation in Mice. Am. J. Respir. Crit. Care Med. 2008, 178, 1023–1032. [Google Scholar] [CrossRef]
- Ogawa, R.; Suzuki, Y.; Kagawa, S.; Masaki, K.; Fukunaga, K.; Yoshimura, A.; Fujishima, S.; Terashima, T.; Betsuyaku, T.; Asano, K. Distinct effects of endogenous interleukin-23 on eosinophilic airway inflammation in response to different antigens. Allergol. Int. 2015, 64, S24–S29. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, D.-E.; Lee, J.-W.; Chang, Y.; Kim, H.Y.; Song, W.-J.; Kang, H.-R.; Park, H.-W.; Chang, Y.-S.; Cho, S.-H. IL-23 secreted by bronchial epithelial cells contributes to allergic sensitization in asthma model: Role of IL-23 secreted by bronchial epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2017, 312, L13–L21. [Google Scholar] [CrossRef] [PubMed]
- Alyasin, S.; Amin, R.; Fazel, A.; Karimi, M.H.; Nabavizadeh, S.H.; Esmaeilzadeh, H.; Babaei, M. IL-23 Gene and Protein Expression in Childhood Asthma. Iran. J. Immunol. 2017, 14, 73–80. [Google Scholar]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and Chemokine Receptors: Positioning Cells for Host Defense and Immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef] [PubMed]
- Borish, L.C.; Steinke, J.W. 2. Cytokines and chemokines. J. Allergy Clin. Immunol. 2003, 111, S460–S475. [Google Scholar] [CrossRef]
- Bisset, L.R.; Schmid-Grendelmeier, P. Chemokines and their receptors in the pathogenesis of allergic asthma: Progress and perspective. Curr. Opin. Pulm. Med. 2005, 11, 35–42. [Google Scholar] [CrossRef]
- Wark, P.A.; Bucchieri, F.; Johnston, S.L.; Gibson, P.G.; Hamilton, L.; Mimica, J.; Zummo, G.; Holgate, S.T.; Attia, J.; Thakkinstian, A.; et al. IFN-γ–induced protein 10 is a novel biomarker of rhinovirus-induced asthma exacerbations. J. Allergy Clin. Immunol. 2007, 120, 586–593. [Google Scholar] [CrossRef]
- Warwick, G.; Thomas, P.S.; Yates, D.H. Non-invasive biomarkers in exacerbations of obstructive lung disease. Respirology 2013, 18, 874–884. [Google Scholar] [CrossRef]
- Matsunaga, K.; Ichikawa, T.; Yanagisawa, S.; Akamatsu, K.; Koarai, A.; Hirano, T.; Sugiura, H.; Minakata, Y.; Ichinose, M. Clinical Application of Exhaled Breath Condensate Analysis in Asthma: Prediction of FEV1 Improvement by Steroid Therapy. Respirology 2009, 78, 393–398. [Google Scholar] [CrossRef]
- Ko, F.W.S.; Lun, S.W.M.; Wong, C.K.; Szeto, C.C.; Lam, C.W.K.; Leung, T.F.; Hui, D.S.C. Decreased T-bet expression and changes in chemokine levels in adults with asthma. Clin. Exp. Immunol. 2007, 147, 526–532. [Google Scholar] [CrossRef]
- Lun, S.W.M.; Wong, C.K.; Ko, F.W.S.; Ip, W.K.; Hui, D.S.C.; Lam, C.W.K. Aberrant Expression of CC and CXC Chemokines and Their Receptors in Patients with Asthma. J. Clin. Immunol. 2006, 26, 145–152. [Google Scholar] [CrossRef]
- Wada, K.; Kobayashi, T.; Matsuwaki, Y.; Moriyama, H.; Kita, H. AlternariaInhibits Double-Stranded RNA-Induced Cytokine Production through Toll-Like Receptor 3. Int. Arch. Allergy Immunol. 2013, 161, 75–83. [Google Scholar] [CrossRef]
- Henry, E.; Desmet, C.J.; Garzé, V.; Fiévez, L.; Bedoret, D.; Heirman, C.; Faisca, P.; Jaspar, F.J.; Gosset, P.; Jacquet, A.P.A.; et al. Dendritic Cells Genetically Engineered to Express IL-10 Induce Long-Lasting Antigen-Specific Tolerance in Experimental Asthma. J. Immunol. 2008, 181, 7230–7242. [Google Scholar] [CrossRef]
- Goel, C.; Gaur, S.; Bhati, G.; Arora, N. DC type 2 polarization depends on both the allergic status of the individual and protease activity of Per a 10. Immunobiology 2015, 220, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Sender, L.Y.; Gibbert, K.; Suezer, Y.; Radeke, H.H.; Kalinke, U.; Waibler, Z. CD40 ligand-triggered human dendritic cells mount interleukin-23 responses that are further enhanced by danger signals. Mol. Immunol. 2010, 47, 1255–1261. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalewicz-Kulbat, M.; Szpakowski, P.; Krawczyk, K.T.; Kowalski, M.L.; Kosinski, S.; Biet, F.; Rudnicka, W.; Locht, C. Decrease of IL-5 Production by Naive T Cells Cocultured with IL-18-Producing BCG-Pulsed Dendritic Cells from Patients Allergic to House Dust Mite. Vaccines 2021, 9, 277. https://doi.org/10.3390/vaccines9030277
Kowalewicz-Kulbat M, Szpakowski P, Krawczyk KT, Kowalski ML, Kosinski S, Biet F, Rudnicka W, Locht C. Decrease of IL-5 Production by Naive T Cells Cocultured with IL-18-Producing BCG-Pulsed Dendritic Cells from Patients Allergic to House Dust Mite. Vaccines. 2021; 9(3):277. https://doi.org/10.3390/vaccines9030277
Chicago/Turabian StyleKowalewicz-Kulbat, Magdalena, Piotr Szpakowski, Krzysztof T. Krawczyk, Marek L. Kowalski, Slawomir Kosinski, Franck Biet, Wieslawa Rudnicka, and Camille Locht. 2021. "Decrease of IL-5 Production by Naive T Cells Cocultured with IL-18-Producing BCG-Pulsed Dendritic Cells from Patients Allergic to House Dust Mite" Vaccines 9, no. 3: 277. https://doi.org/10.3390/vaccines9030277
APA StyleKowalewicz-Kulbat, M., Szpakowski, P., Krawczyk, K. T., Kowalski, M. L., Kosinski, S., Biet, F., Rudnicka, W., & Locht, C. (2021). Decrease of IL-5 Production by Naive T Cells Cocultured with IL-18-Producing BCG-Pulsed Dendritic Cells from Patients Allergic to House Dust Mite. Vaccines, 9(3), 277. https://doi.org/10.3390/vaccines9030277





