Early Colorectal Responses to HIV-1 and Modulation by Antiretroviral Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plasmids and Virus Culture Conditions
2.3. Patients and Tissue Explants
2.4. Tissue Digestion and Flow Cytometry Analysis
2.5. Infectivity and Drug Exposure Assays
2.6. Immunohistochemistry
2.7. Imaging
2.8. Multiplex Cytokine Analysis
2.9. Microarray Analysis of Gene Expression
2.9.1. Total RNA Extraction and Quality Control
2.9.2. RNA Microarray Analysis
2.10. Statistical Analysis
3. Results
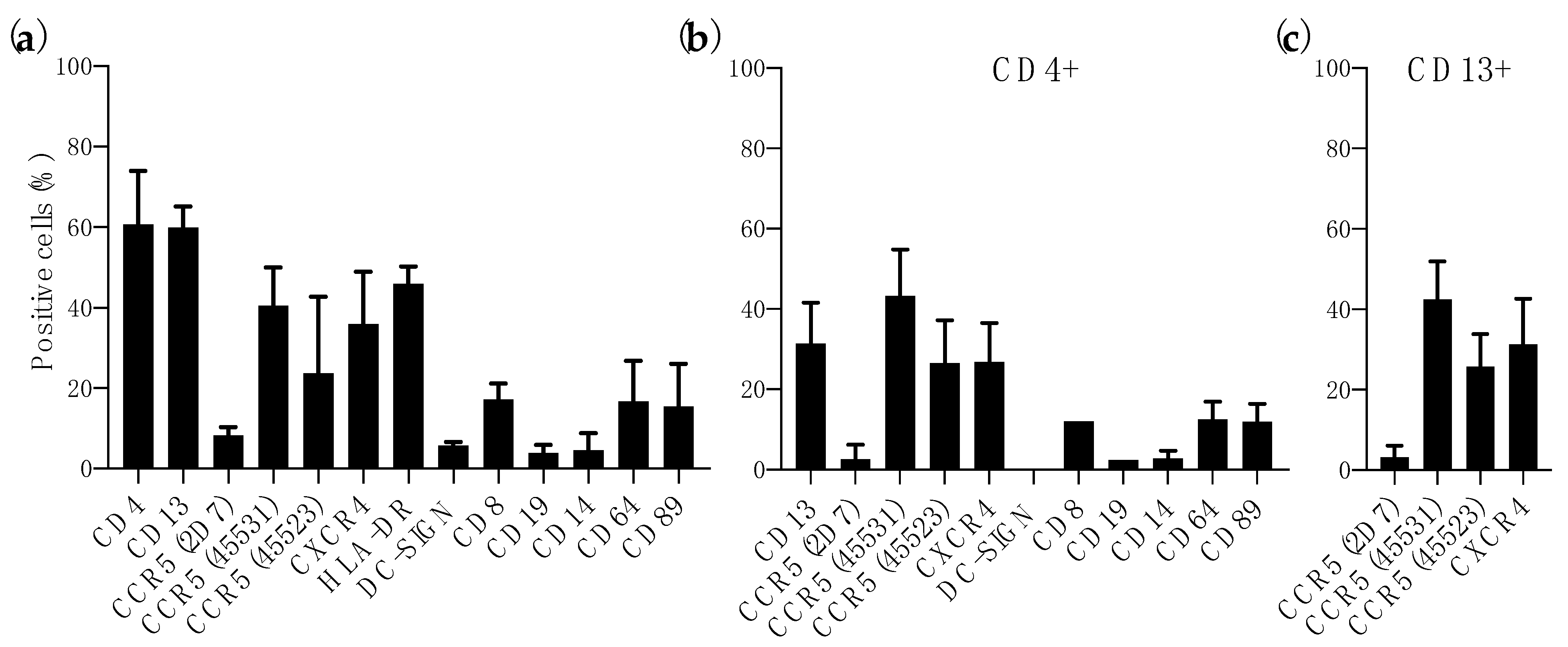
3.1. Expression of HIV-1 Receptor and Coreceptors in Colorectal Mucosa
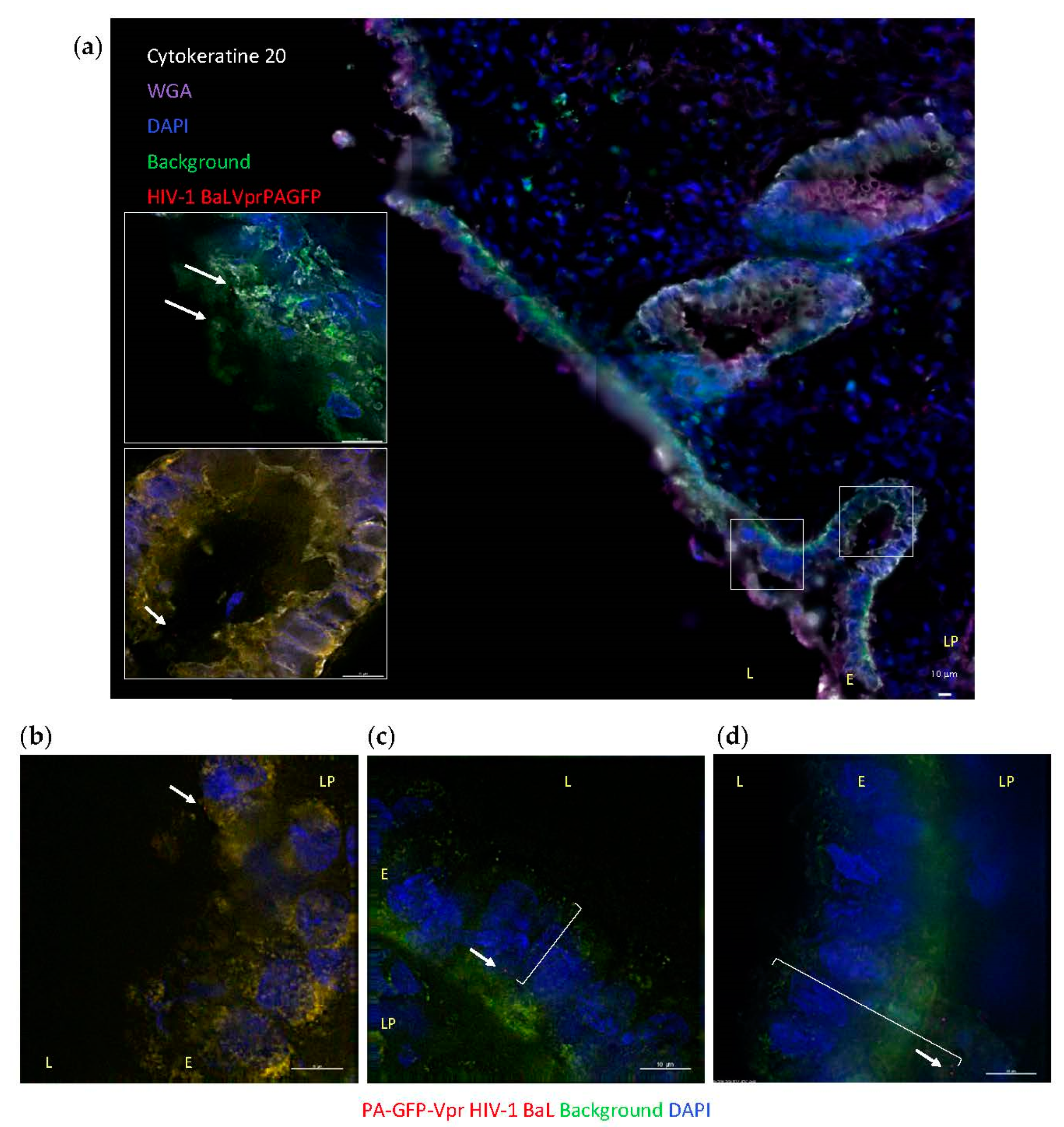
3.2. HIV-1 Penetration in Colorectal Explants
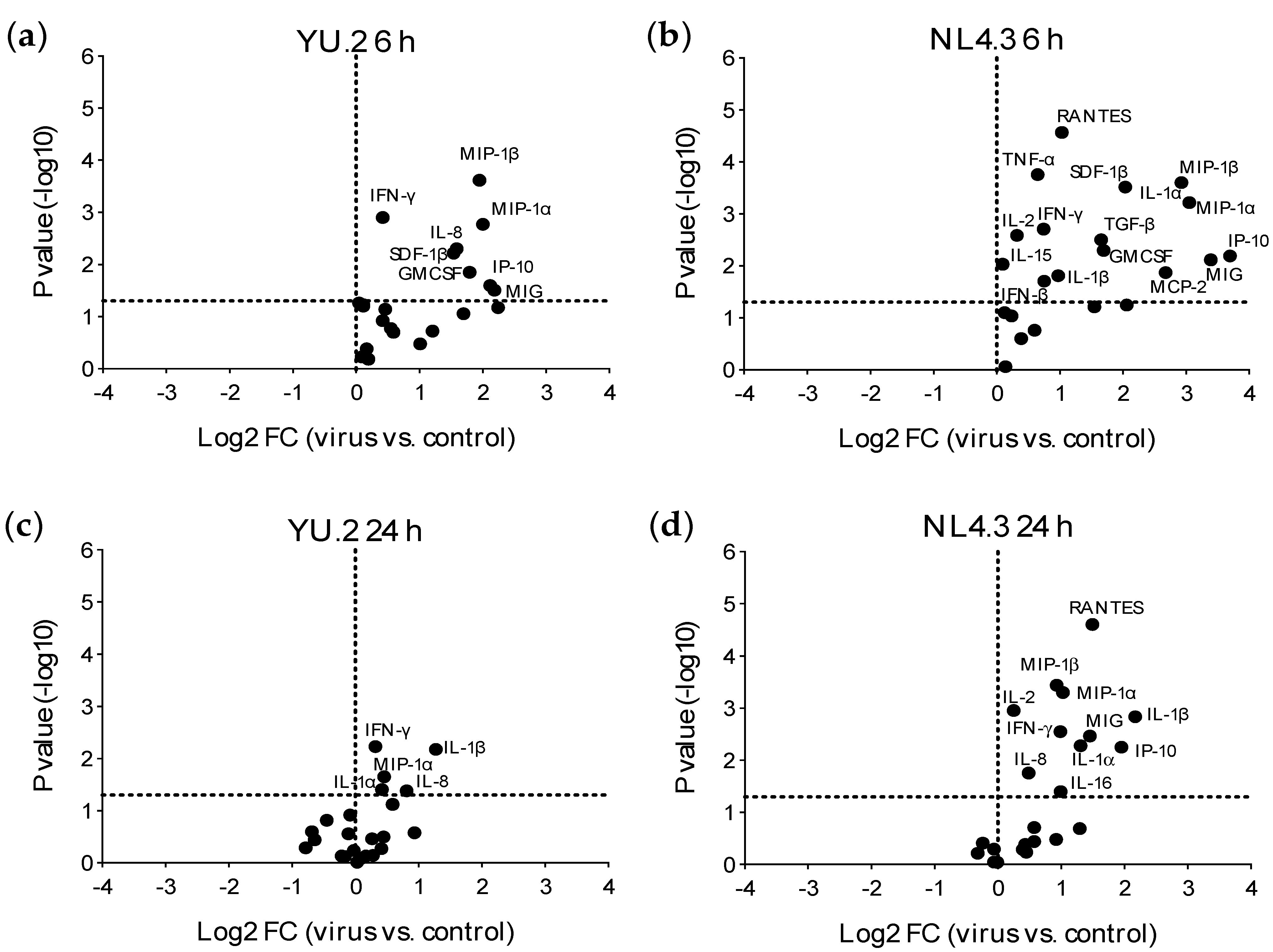
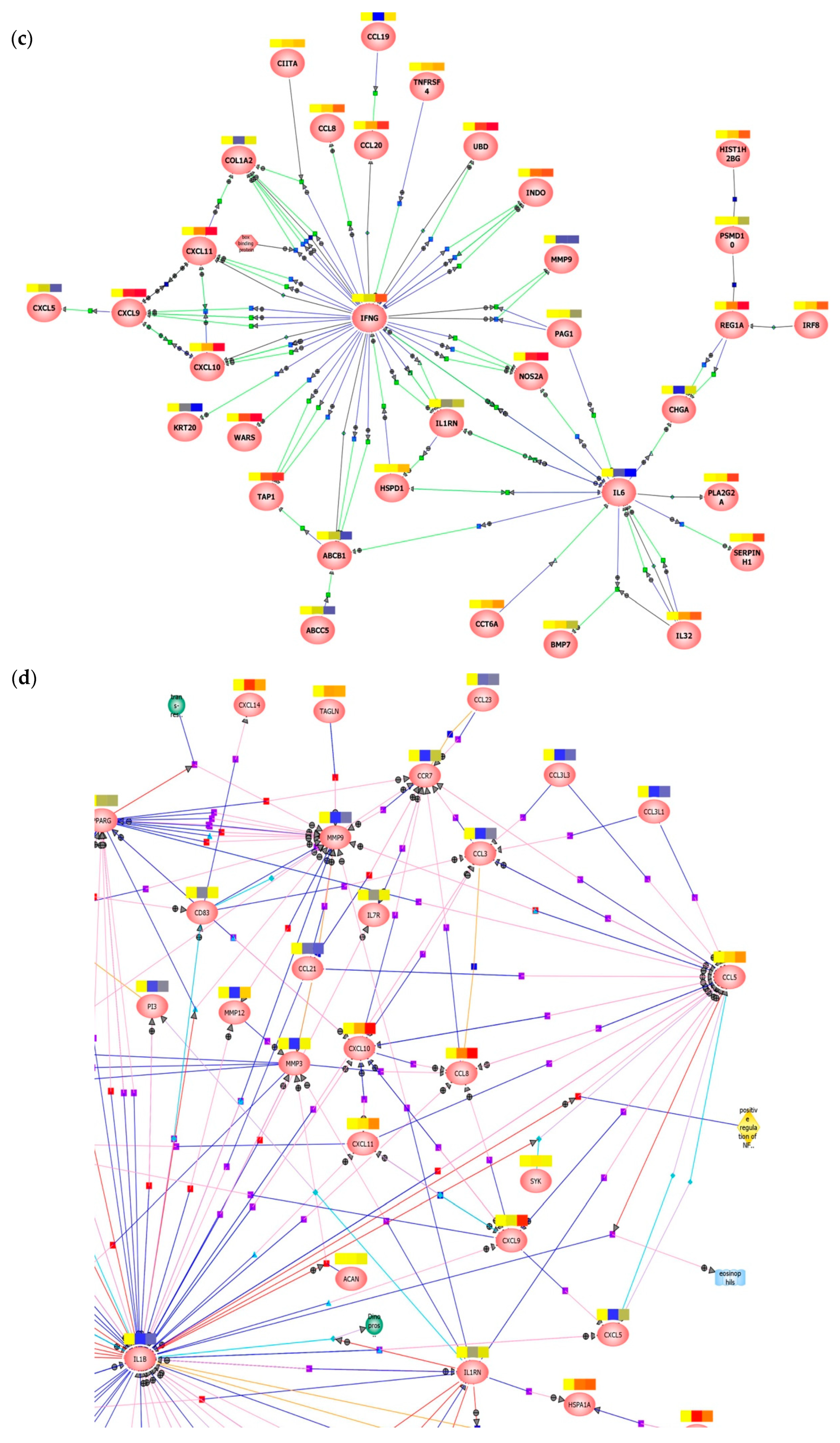
3.3. Early Responses Induced after Exposure of Colorectal Tissue to HIV-1
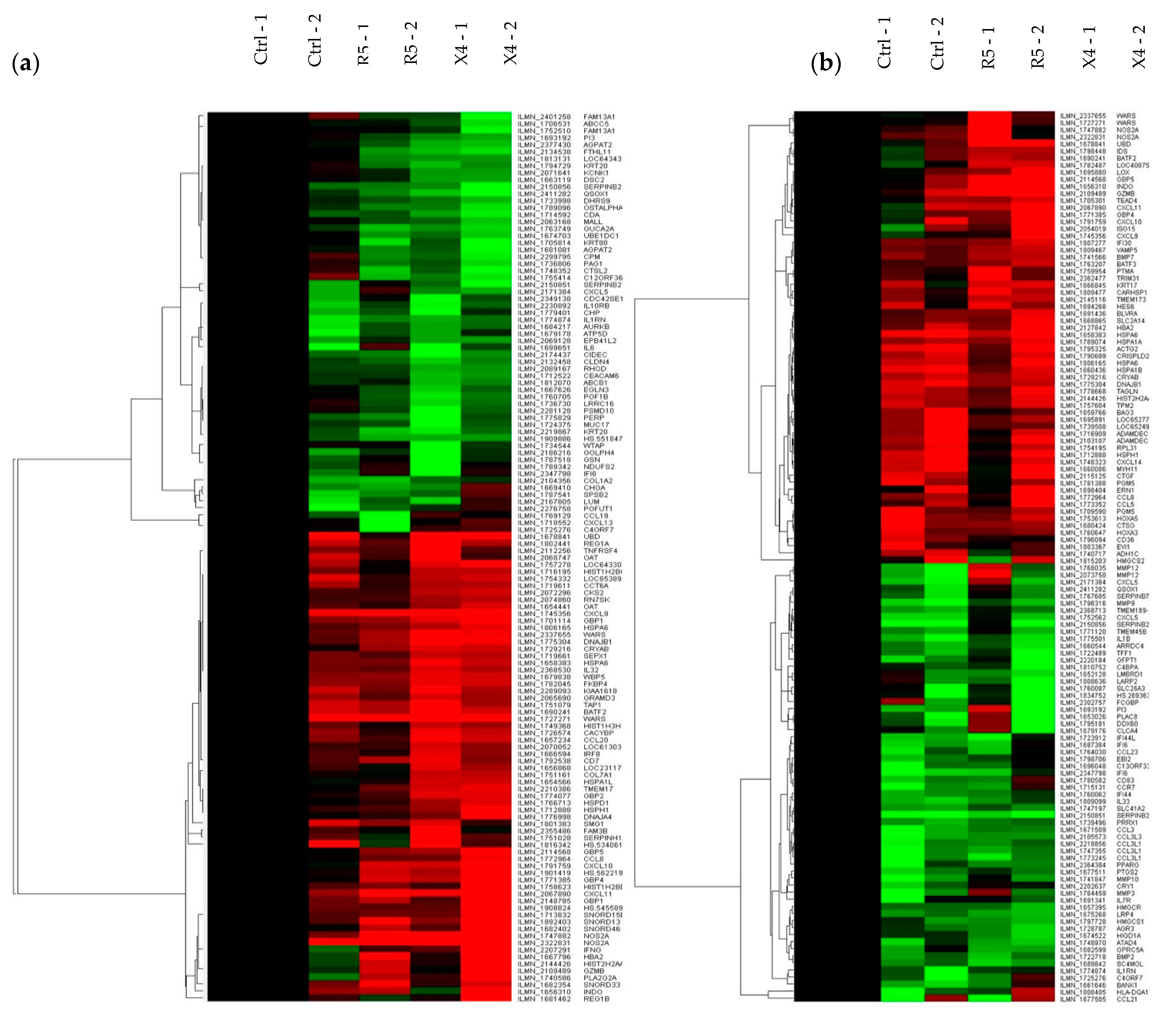
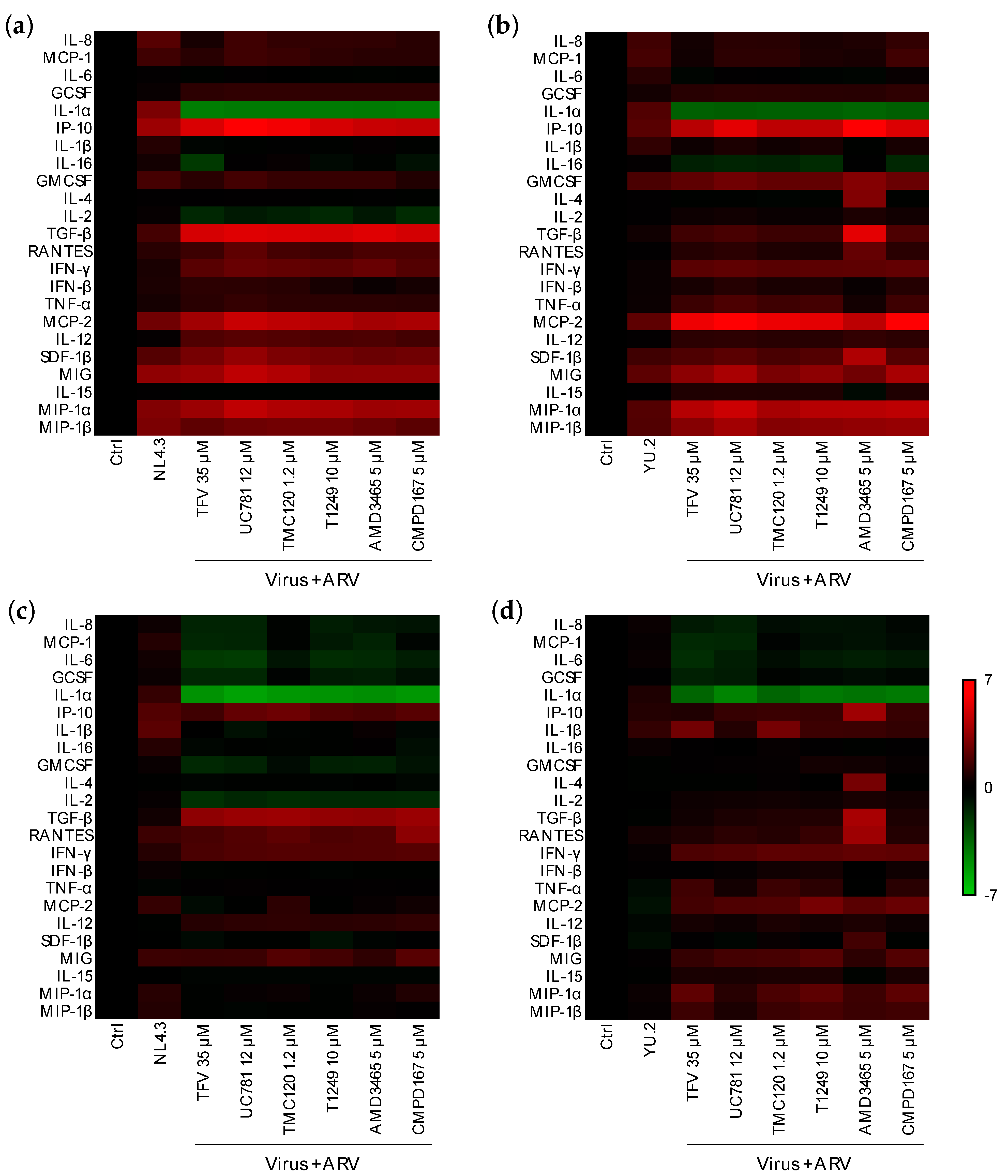
3.4. Modulation of Early Responses to HIV-1 Exposure by ARVs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baeten, J.M.; Donnell, D.; Ndase, P.; Mugo, N.R.; Campbell, J.D.; Wangisi, J.; Tappero, J.W.; Bukusi, E.A.; Cohen, C.R.; Katabira, E.; et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N. Engl. J. Med. 2012, 367, 399–410. [Google Scholar] [CrossRef] [Green Version]
- Grant, R.M.; Lama, J.R.; Anderson, P.L.; McMahan, V.; Liu, A.Y.; Vargas, L.; Goicochea, P.; Casapia, M.; Guanira-Carranza, J.V.; Ramirez-Cardich, M.E.; et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 2010, 363, 2587–2599. [Google Scholar] [CrossRef] [Green Version]
- Thigpen, M.C.; Kebaabetswe, P.M.; Paxton, L.A.; Smith, D.K.; Rose, C.E.; Segolodi, T.M.; Henderson, F.L.; Pathak, S.R.; Soud, F.A.; Chillag, K.L.; et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N. Engl. J. Med. 2012, 367, 423–434. [Google Scholar] [CrossRef]
- Nicol, M.R.; Corbino, J.A.; Cottrell, M.L. Pharmacology of Antiretrovirals in the Female Genital Tract for HIV Prevention. J. Clin. Pharmacol. 2018, 58, 1381–1395. [Google Scholar] [CrossRef]
- Deeks, S.G.; Tracy, R.; Douek, D.C. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013, 39, 633–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stacey, A.R.; Norris, P.J.; Qin, L.; Haygreen, E.A.; Taylor, E.; Heitman, J.; Lebedeva, M.; DeCamp, A.; Li, D.; Grove, D.; et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J. Virol. 2009, 83, 3719–3733. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Estes, J.D.; Schlievert, P.M.; Duan, L.; Brosnahan, A.J.; Southern, P.J.; Reilly, C.S.; Peterson, M.L.; Schultz-Darken, N.; Brunner, K.G.; et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature 2009, 458, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Abel, K.; Rocke, D.M.; Chohan, B.; Fritts, L.; Miller, C.J. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J. Virol. 2005, 79, 12164–12172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalichman, S.C.; Rompa, D.; Luke, W.; Austin, J. HIV transmission risk behaviours among HIV-positive persons in serodiscordant relationships. Int. J. STD AIDS 2002, 13, 677–682. [Google Scholar] [CrossRef]
- Leynaert, B.; Downs, A.M.; de Vincenzi, I. Heterosexual transmission of human immunodeficiency virus: Variability of infectivity throughout the course of infection. European Study Group on Heterosexual Transmission of HIV. Am. J. Epidemiol. 1998, 148, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Padian, N.S.; Shiboski, S.C.; Glass, S.O.; Vittinghoff, E. Heterosexual transmission of human immunodeficiency virus (HIV) in northern California: Results from a ten-year study. Am. J. Epidemiol. 1997, 146, 350–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vittinghoff, E.; Douglas, J.; Judson, F.; McKirnan, D.; MacQueen, K.; Buchbinder, S.P. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am. J. Epidemiol. 1999, 150, 306–311. [Google Scholar] [CrossRef] [Green Version]
- Brenchley, J.M.; Price, D.A.; Douek, D.C. HIV disease: Fallout from a mucosal catastrophe? Nat. Immunol. 2006, 7, 235–239. [Google Scholar] [CrossRef]
- Li, Y.; Hui, H.; Burgess, C.J.; Price, R.W.; Sharp, P.M.; Hahn, B.H.; Shaw, G.M. Complete nucleotide sequence, genome organization, and biological properties of human immunodeficiency virus type 1 in vivo: Evidence for limited defectiveness and complementation. J. Virol. 1992, 66, 6587–6600. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Kappes, J.C.; Conway, J.A.; Price, R.W.; Shaw, G.M.; Hahn, B.H. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: Identification of replication-competent and -defective viral genomes. J. Virol. 1991, 65, 3973–3985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adachi, A.; Gendelman, H.E.; Koenig, S.; Folks, T.; Willey, R.; Rabson, A.; Martin, M.A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 1986, 59, 284–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carias, A.M.; McCoombe, S.; McRaven, M.; Anderson, M.; Galloway, N.; Vandergrift, N.; Fought, A.J.; Lurain, J.; Duplantis, M.; Veazey, R.S.; et al. Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. J. Virol. 2013, 87, 11388–11400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, D.; Vodicka, M.A.; Lucero, G.; Svitkina, T.M.; Borisy, G.G.; Emerman, M.; Hope, T.J. Visualization of the intracellular behavior of HIV in living cells. J. Cell Biol. 2002, 159, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Patterson, G.H.; Lippincott-Schwartz, J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science 2002, 297, 1873–1877. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J.; Muesing, M.A.; Proudfoot, A.E.; Power, C.A.; Moore, J.P.; Trkola, A. Enhancement of human immunodeficiency virus type 1 infection by the CC-chemokine RANTES is independent of the mechanism of virus-cell fusion. J. Virol. 1999, 73, 684–694. [Google Scholar] [CrossRef] [Green Version]
- Rossio, J.L.; Esser, M.T.; Suryanarayana, K.; Schneider, D.K.; Bess, J.W., Jr.; Vasquez, G.M.; Wiltrout, T.A.; Chertova, E.; Grimes, M.K.; Sattentau, Q.; et al. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J. Virol. 1998, 72, 7992–8001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, P.S.; Elliott, J.; Grivel, J.C.; Margolis, L.; Anton, P.; McGowan, I.; Shattock, R.J. Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. Aids 2006, 20, 1237–1245. [Google Scholar] [CrossRef]
- Smythies, L.E.; Wahl, L.M.; Smith, P.D. Isolation and purification of human intestinal macrophages. Curr. Protoc. Immunol. 2006, 70, 7.6B.1–7.6B.9. [Google Scholar] [CrossRef] [PubMed]
- Marozsan, A.J.; Kuhmann, S.E.; Morgan, T.; Herrera, C.; Rivera-Troche, E.; Xu, S.; Baroudy, B.M.; Strizki, J.; Moore, J.P. Generation and properties of a human immunodeficiency virus type 1 isolate resistant to the small molecule CCR5 inhibitor, SCH-417690 (SCH-D). Virology 2005, 338, 182–199. [Google Scholar] [CrossRef] [Green Version]
- Herrera, C.; Cranage, M.; McGowan, I.; Anton, P.; Shattock, R.J. Reverse transcriptase inhibitors as potential colorectal microbicides. Antimicrob. Agents Chemother. 2009, 53, 1797–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, S.C.; Hou, Y.; Baisley, K.; van de Wijgert, J.; Watson-Jones, D.; Ao, T.T.; Herrera, C.; Maganja, K.; Andreasen, A.; Kapiga, S.; et al. Immune Activation in the Female Genital Tract: Expression Profiles of Soluble Proteins in Women at High Risk for HIV Infection. PLoS ONE 2016, 11, e0143109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, R.; Kappes, J.C.; Smythies, L.E.; Richter, H.E.; Novak, L.; Smith, P.D. Vaginal myeloid dendritic cells transmit founder HIV-1. J. Virol. 2014, 88, 7683–7688. [Google Scholar] [CrossRef] [Green Version]
- Shen, R.; Meng, G.; Ochsenbauer, C.; Clapham, P.R.; Grams, J.; Novak, L.; Kappes, J.C.; Smythies, L.E.; Smith, P.D. Stromal down-regulation of macrophage CD4/CCR5 expression and NF-kappaB activation mediates HIV-1 non-permissiveness in intestinal macrophages. PLoS Pathog. 2011, 7, e1002060. [Google Scholar] [CrossRef]
- Shen, R.; Richter, H.E.; Clements, R.H.; Novak, L.; Huff, K.; Bimczok, D.; Sankaran-Walters, S.; Dandekar, S.; Clapham, P.R.; Smythies, L.E.; et al. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J. Virol. 2009, 83, 3258–3267. [Google Scholar] [CrossRef] [Green Version]
- Shen, R.; Smythies, L.E.; Clements, R.H.; Novak, L.; Smith, P.D. Dendritic cells transmit HIV-1 through human small intestinal mucosa. J. Leukoc. Biol. 2010, 87, 663–670. [Google Scholar] [CrossRef]
- Shanmugasundaram, U.; Critchfield, J.W.; Pannell, J.; Perry, J.; Giudice, L.C.; Smith-McCune, K.; Greenblatt, R.M.; Shacklett, B.L. Phenotype and functionality of CD4+ and CD8+ T cells in the upper reproductive tract of healthy premenopausal women. Am. J. Reprod. Immunol. 2014, 71, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Hladik, F.; McElrath, M.J. Setting the stage: Host invasion by HIV. Nat. Rev. Immunol. 2008, 8, 447–457. [Google Scholar] [CrossRef] [Green Version]
- Anton, P.A.; Elliott, J.; Poles, M.A.; McGowan, I.M.; Matud, J.; Hultin, L.E.; Grovit-Ferbas, K.; Mackay, C.R.; Chen, I.S.Y.; Giorgi, J.V. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. Aids 2000, 14, 1761–1765. [Google Scholar] [CrossRef] [PubMed]
- Lapenta, C.; Boirivant, M.; Marini, M.; Santini, S.M.; Logozzi, M.; Viora, M.; Belardelli, F.; Fais, S. Human intestinal lamina propria lymphocytes are naturally permissive to HIV-1 infection. Eur. J. Immunol. 1999, 29, 1202–1208. [Google Scholar] [CrossRef]
- Poles, M.A.; Elliott, J.; Taing, P.; Anton, P.A.; Chen, I.S. A preponderance of CCR5(+) CXCR4(+) mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J. Virol. 2001, 75, 8390–8399. [Google Scholar] [CrossRef] [Green Version]
- Colin, P.; Benureau, Y.; Staropoli, I.; Wang, Y.; Gonzalez, N.; Alcami, J.; Hartley, O.; Brelot, A.; Arenzana-Seisdedos, F.; Lagane, B. HIV-1 exploits CCR5 conformational heterogeneity to escape inhibition by chemokines. Proc. Natl. Acad. Sci. USA 2013, 110, 9475–9480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.; Sharron, M.; Blanpain, C.; Doranz, B.J.; Vakili, J.; Setoh, P.; Berg, E.; Liu, G.; Guy, H.R.; Durell, S.R.; et al. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J. Biol. Chem. 1999, 274, 9617–9626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grivel, J.C.; Shattock, R.J.; Margolis, L.B. Selective transmission of R5 HIV-1 variants: Where is the gatekeeper? J. Transl. Med. 2011, 9, S6. [Google Scholar] [CrossRef] [Green Version]
- Salazar-Gonzalez, J.F.; Salazar, M.G.; Keele, B.F.; Learn, G.H.; Giorgi, E.E.; Li, H.; Decker, J.M.; Wang, S.; Baalwa, J.; Kraus, M.H.; et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 2009, 206, 1273–1289. [Google Scholar] [CrossRef]
- Harman, S.; Herrera, C.; Armanasco, N.; Nuttall, J.; Shattock, R.J. Preclinical evaluation of the HIV-1 fusion inhibitor L’644 as a potential candidate microbicide. Antimicrob. Agents Chemother. 2012, 56, 2347–2356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, N.; Rodriguez-Garcia, M.; Shen, Z.; Crist, S.G.; Bodwell, J.E.; Fahey, J.V.; Wira, C.R. Effects of tenofovir on cytokines and nucleotidases in HIV-1 target cells and the mucosal tissue environment in the female reproductive tract. Antimicrob. Agents Chemother. 2014, 58, 6444–6453. [Google Scholar] [CrossRef] [Green Version]
- Kelley, C.F.; Kraft, C.S.; de Man, T.J.; Duphare, C.; Lee, H.W.; Yang, J.; Easley, K.A.; Tharp, G.K.; Mulligan, M.J.; Sullivan, P.S.; et al. The rectal mucosa and condomless receptive anal intercourse in HIV-negative MSM: Implications for HIV transmission and prevention. Mucosal. Immunol. 2017, 10, 996–1007. [Google Scholar] [CrossRef] [Green Version]
- Olsson, J.; Poles, M.; Spetz, A.L.; Elliott, J.; Hultin, L.; Giorgi, J.; Andersson, J.; Anton, P. Human immunodeficiency virus type 1 infection is associated with significant mucosal inflammation characterized by increased expression of CCR5, CXCR4, and beta-chemokines. J. Infect. Dis. 2000, 182, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.M.; Biswas, N.; Siegel, A.; Myerski, A.; Engstrom, J.; Jeffrey Metter, E.; Brand, R.E.; Cranston, R.D.; McGowan, I. Immunological responsiveness of intestinal tissue explants and mucosal mononuclear cells to ex vivo stimulation. J. Immunol. Methods 2018, 463, 39–46. [Google Scholar] [CrossRef]
- Hladik, F.; Hope, T.J. HIV infection of the genital mucosa in women. Curr. HIV/AIDS. Rep. 2009, 6, 20–28. [Google Scholar] [CrossRef]
- McClure, C.P.; Bowman, C.A.; Geary, I.; Ryan, C.; Ball, J.K.; Eley, A. HIV-1 co-receptor expression and epithelial immune cells of the cervix in asymptomatic women attending a genitourinary medicine clinic. HIV Med. 2013, 14, 108–114. [Google Scholar] [CrossRef]
- Ito, Y.; Grivel, J.C.; Chen, S.; Kiselyeva, Y.; Reichelderfer, P.; Margolis, L. CXCR4-tropic HIV-1 suppresses replication of CCR5-tropic HIV-1 in human lymphoid tissue by selective induction of CC-chemokines. J. Infect. Dis. 2004, 189, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Liu, Q.H.; Tomkowicz, B.; Yi, Y.; Freedman, B.D.; Collman, R.G. Macrophage activation through CCR5- and CXCR4-mediated gp120-elicited signaling pathways. J. Leukoc. Biol. 2003, 74, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaillon, A.; Gianella, S.; Little, S.J.; Caballero, G.; Barin, F.; Kosakovsky Pond, S.; Richman, D.D.; Smith, D.M.; Mehta, S.R. Characterizing the multiplicity of HIV founder variants during sexual transmission among MSM. Virus Evol. 2016, 2, vew012. [Google Scholar] [CrossRef] [Green Version]
- Keele, B.F.; Giorgi, E.E.; Salazar-Gonzalez, J.F.; Decker, J.M.; Pham, K.T.; Salazar, M.G.; Sun, C.; Grayson, T.; Wang, S.; Li, H.; et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. USA 2008, 105, 7552–7557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Bar, K.J.; Wang, S.; Decker, J.M.; Chen, Y.; Sun, C.; Salazar-Gonzalez, J.F.; Salazar, M.G.; Learn, G.H.; Morgan, C.J.; et al. High Multiplicity Infection by HIV-1 in Men Who Have Sex with Men. PLoS Pathog. 2010, 6, e1000890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrahams, M.R.; Anderson, J.A.; Giorgi, E.E.; Seoighe, C.; Mlisana, K.; Ping, L.H.; Athreya, G.S.; Treurnicht, F.K.; Keele, B.F.; Wood, N.; et al. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-poisson distribution of transmitted variants. J. Virol. 2009, 83, 3556–3567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bebell, L.M.; Passmore, J.A.; Williamson, C.; Mlisana, K.; Iriogbe, I.; van Loggerenberg, F.; Karim, Q.A.; Karim, S.A. Relationship between levels of inflammatory cytokines in the genital tract and CD4+ cell counts in women with acute HIV-1 infection. J. Infect. Dis. 2008, 198, 710–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, C.J.; Sattentau, Q.J. Viral determinants of HIV-1 macrophage tropism. Viruses 2011, 3, 2255–2279. [Google Scholar] [CrossRef]
- Hladik, F.; Burgener, A.; Ballweber, L.; Gottardo, R.; Vojtech, L.; Fourati, S.; Dai, J.Y.; Cameron, M.J.; Strobl, J.; Hughes, S.M.; et al. Correction: Musosal effects for tenofovir 1% gel. eLlife 2015, 4. [Google Scholar] [CrossRef]
- Hladik, F.; Burgener, A.; Ballweber, L.; Gottardo, R.; Vojtech, L.; Fourati, S.; Dai, J.Y.; Cameron, M.J.; Strobl, J.; Hughes, S.M.; et al. Mucosal effects of tenofovir 1% gel. eLlife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, C.; McRaven, M.D.; Laing, K.G.; Dennis, J.; Hope, T.J.; Shattock, R.J. Early Colorectal Responses to HIV-1 and Modulation by Antiretroviral Drugs. Vaccines 2021, 9, 231. https://doi.org/10.3390/vaccines9030231
Herrera C, McRaven MD, Laing KG, Dennis J, Hope TJ, Shattock RJ. Early Colorectal Responses to HIV-1 and Modulation by Antiretroviral Drugs. Vaccines. 2021; 9(3):231. https://doi.org/10.3390/vaccines9030231
Chicago/Turabian StyleHerrera, Carolina, Mike D. McRaven, Ken G. Laing, Jayne Dennis, Thomas J. Hope, and Robin J. Shattock. 2021. "Early Colorectal Responses to HIV-1 and Modulation by Antiretroviral Drugs" Vaccines 9, no. 3: 231. https://doi.org/10.3390/vaccines9030231
APA StyleHerrera, C., McRaven, M. D., Laing, K. G., Dennis, J., Hope, T. J., & Shattock, R. J. (2021). Early Colorectal Responses to HIV-1 and Modulation by Antiretroviral Drugs. Vaccines, 9(3), 231. https://doi.org/10.3390/vaccines9030231





