Stability of Outer Membrane Vesicles-Based Vaccines, Identifying the Most Appropriate Methods to Detect Changes in Vaccine Potency
Abstract
1. Introduction
2. Materials and Methods
2.1. GMMA Production
2.2. GMMA Formulation on Alhydrogel
2.3. Stability Studies
2.4. GMMA Characterization
2.4.1. GMMA Drug Substance Characterization
2.4.2. GMMA Drug Product Characterization
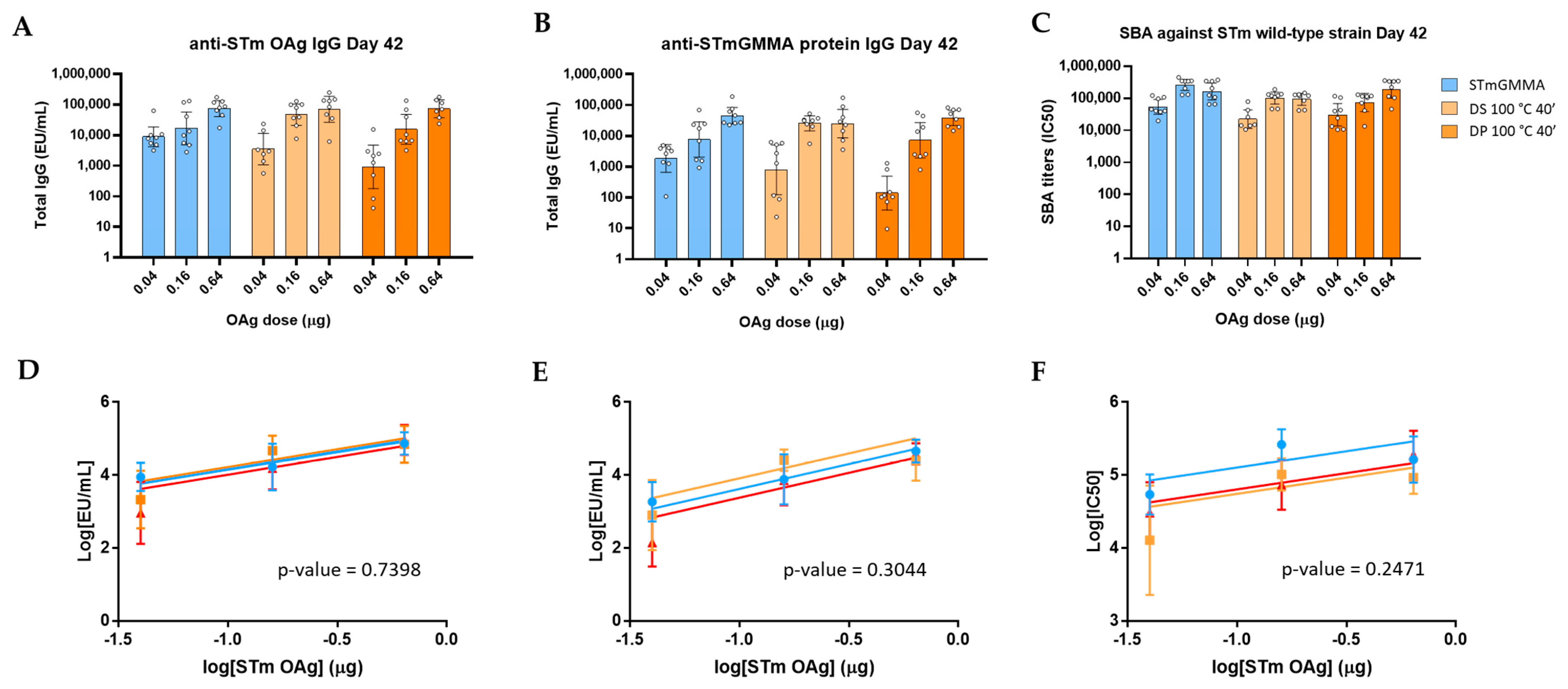
2.5. Immunogenicity Studies in Mice
2.6. Statistical Analysis
3. Results
3.1. GMMA Stressed at 100 °C
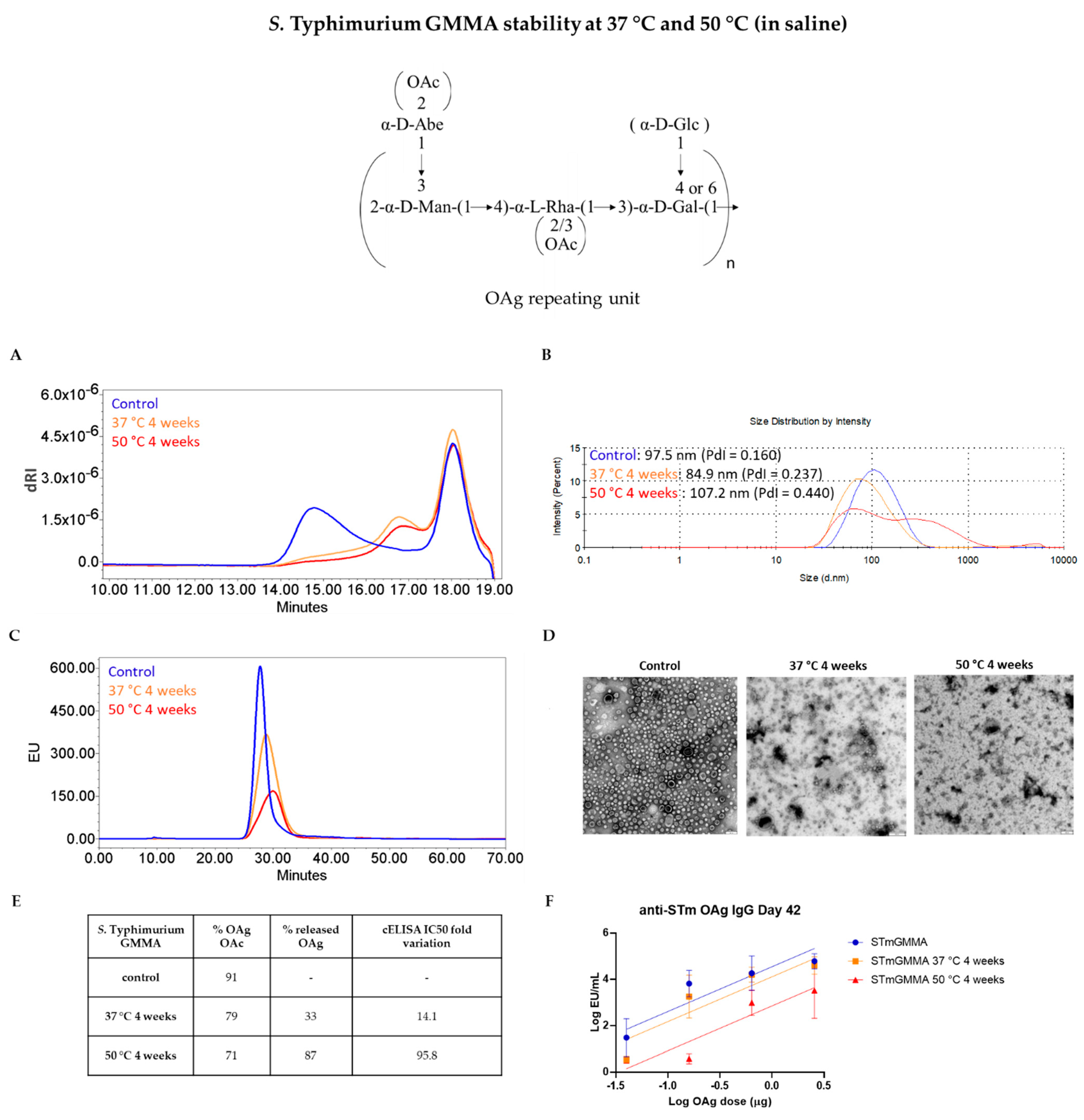
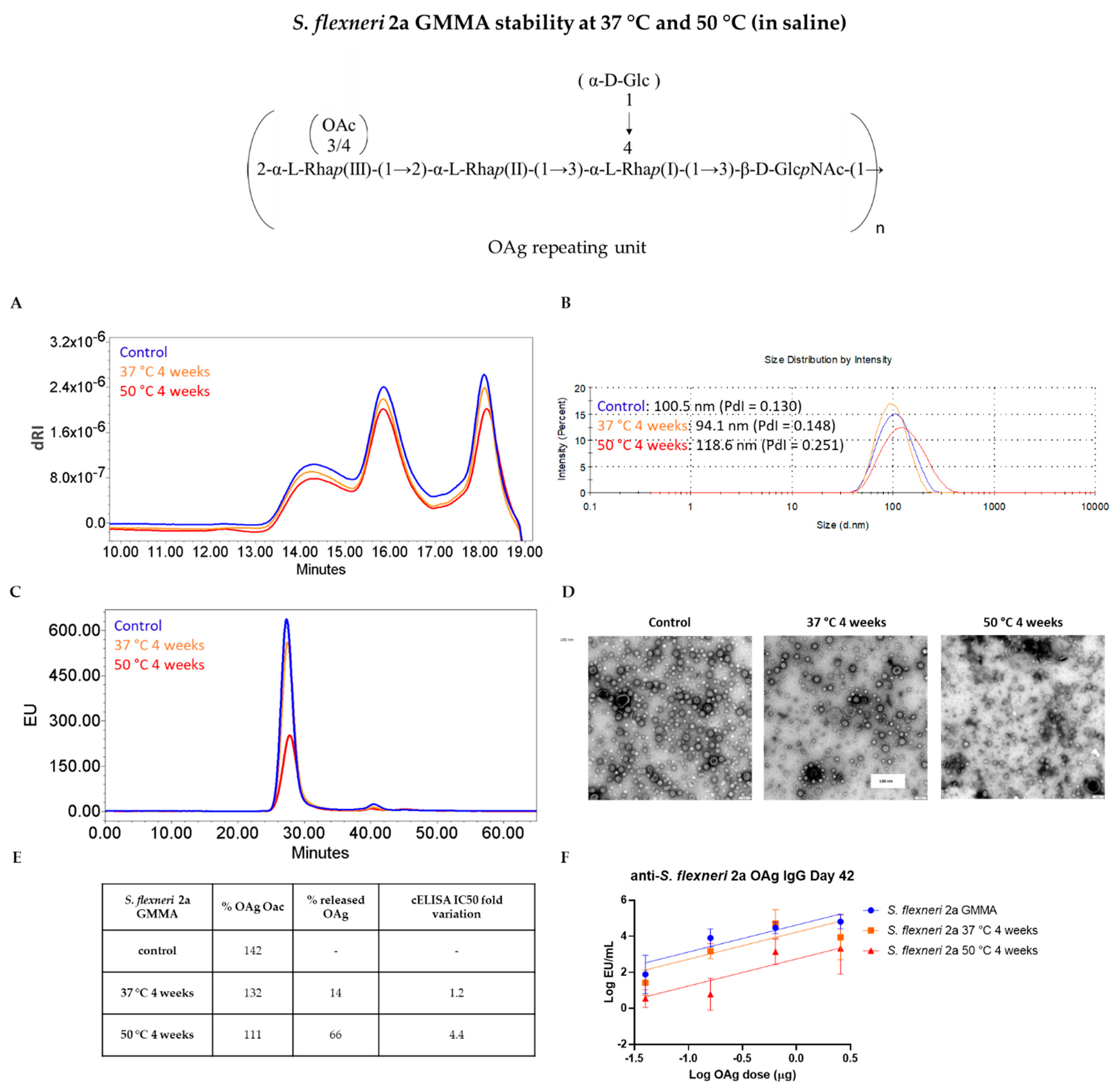
3.2. GMMA Stressed at 37 °C or 50 °C in Saline
3.3. GMMA Stressed at 37 °C or 50 °C in Buffer at pH 6.5
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.N.; Kuehn, M.J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. MMBR 2010, 74, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Rossi, O.; Necchi, F.; Micoli, F. OMV Vaccines and the Role of TLR Agonists in Immune Response. Int. J. Mol. Sci. 2020, 21, 4416. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, H.; Yang, C.; Wu, Y.; Zhou, X.; Liu, H.; Wang, Y. Bacterial outer membrane vesicles as a platform for biomedical applications: An update. J. Control. Release Off. J. Control. Release Soc. 2020, 323, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Van der Pol, L.; Stork, M.; van der Ley, P. Outer membrane vesicles as platform vaccine technology. Biotechnol. J. 2015, 10, 1689–1706. [Google Scholar] [CrossRef]
- O’Ryan, M.; Stoddard, J.; Toneatto, D.; Wassil, J.; Dull, P.M. A multi-component meningococcal serogroup B vaccine (4CMenB): The clinical development program. Drugs 2014, 74, 15–30. [Google Scholar] [CrossRef]
- Micoli, F.; MacLennan, C.A. Outer membrane vesicle vaccines. Semin. Immunol. 2020, 50, 101433. [Google Scholar] [CrossRef] [PubMed]
- Gerke, C.; Colucci, A.M.; Giannelli, C.; Sanzone, S.; Vitali, C.G.; Sollai, L.; Rossi, O.; Martin, L.B.; Auerbach, J.; Di Cioccio, V.; et al. Production of a Shigella sonnei Vaccine Based on Generalized Modules for Membrane Antigens (GMMA), 1790GAHB. PLoS ONE 2015, 10, e0134478. [Google Scholar] [CrossRef]
- Rossi, O.; Caboni, M.; Negrea, A.; Necchi, F.; Alfini, R.; Micoli, F.; Saul, A.; MacLennan, C.A.; Rondini, S.; Gerke, C. Toll-Like Receptor Activation by Generalized Modules for Membrane Antigens from Lipid A Mutants of Salmonella enterica Serovars Typhimurium and Enteritidis. Clin. Vaccine Immunol. CVI 2016, 23, 304–314. [Google Scholar] [CrossRef]
- Rossi, O.; Pesce, I.; Giannelli, C.; Aprea, S.; Caboni, M.; Citiulo, F.; Valentini, S.; Ferlenghi, I.; MacLennan, C.A.; D’Oro, U.; et al. Modulation of endotoxicity of Shigella generalized modules for membrane antigens (GMMA) by genetic lipid A modifications: Relative activation of TLR4 and TLR2 pathways in different mutants. J. Biol. Chem. 2014, 289, 24922–24935. [Google Scholar] [CrossRef] [PubMed]
- Kis, Z.; Shattock, R.; Shah, N.; Kontoravdi, C. Emerging Technologies for Low-Cost, Rapid Vaccine Manufacture. Biotechnol. J. 2019, 14, 1–2. [Google Scholar] [CrossRef]
- De Benedetto, G.; Alfini, R.; Cescutti, P.; Caboni, M.; Lanzilao, L.; Necchi, F.; Saul, A.; MacLennan, C.A.; Rondini, S.; Micoli, F. Characterization of O-Antigen delivered by Generalized Modules for Membrane Antigens (GMMA) vaccine candidates against nontyphoidal Salmonella. Vaccine 2017, 35, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Rondini, S.; Alfini, R.; Lanzilao, L.; Necchi, F.; Negrea, A.; Rossi, O.; Brandt, C.; Clare, S.; Mastroeni, P.; et al. Comparative immunogenicity and efficacy of equivalent outer membrane vesicle and glycoconjugate vaccines against nontyphoidal Salmonella. Proc. Natl. Acad. Sci. USA 2018, 115, 10428–10433. [Google Scholar] [CrossRef] [PubMed]
- Schager, A.E.; Dominguez-Medina, C.C.; Necchi, F.; Micoli, F.; Goh, Y.S.; Goodall, M.; Flores-Langarica, A.; Bobat, S.; Cook, C.N.L.; Arcuri, M.; et al. IgG Responses to Porins and Lipopolysaccharide within an Outer Membrane-Based Vaccine against Nontyphoidal Salmonella Develop at Discordant Rates. mBio 2018, 9, e02379-17. [Google Scholar] [CrossRef] [PubMed]
- Koeberling, O.; Ispasanie, E.; Hauser, J.; Rossi, O.; Pluschke, G.; Caugant, D.A.; Saul, A.; MacLennan, C.A. A broadly-protective vaccine against meningococcal disease in sub-Saharan Africa based on generalized modules for membrane antigens (GMMA). Vaccine 2014, 32, 2688–2695. [Google Scholar] [CrossRef] [PubMed]
- Raso, M.M.; Gasperini, G.; Alfini, R.; Schiavo, F.; Aruta, M.G.; Carducci, M.; Forgione, M.C.; Martini, S.; Cescutti, P.; Necchi, F.; et al. GMMA and Glycoconjugate Approaches Compared in Mice for the Development of a Vaccine against Shigella flexneri Serotype 6. Vaccines 2020, 8, 160. [Google Scholar] [CrossRef]
- Launay, O.; Lewis, D.J.M.; Anemona, A.; Loulergue, P.; Leahy, J.; Sciré, A.S.; Maugard, A.; Marchetti, E.; Zancan, S.; Huo, Z.; et al. Safety Profile and Immunologic Responses of a Novel Vaccine against Shigella sonnei Administered Intramuscularly, Intradermally and Intranasally: Results from Two Parallel Randomized Phase 1 Clinical Studies in Healthy Adult Volunteers in Europe. EBioMedicine 2017, 22, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Launay, O.; Ndiaye, A.G.W.; Conti, V.; Loulergue, P.; Sciré, A.S.; Landre, A.M.; Ferruzzi, P.; Nedjaai, N.; Schütte, L.D.; Auerbach, J.; et al. Booster Vaccination with GVGH Shigella sonnei 1790GAHB GMMA Vaccine Compared to Single Vaccination in Unvaccinated Healthy European Adults: Results from a Phase 1 Clinical Trial. Front. Immunol. 2019, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Obiero, C.W.; Ndiaye, A.G.W.; Sciré, A.S.; Kaunyangi, B.M.; Marchetti, E.; Gone, A.M.; Schütte, L.D.; Riccucci, D.; Auerbach, J.; Saul, A.; et al. A Phase 2a Randomized Study to Evaluate the Safety and Immunogenicity of the 1790GAHB Generalized Modules for Membrane Antigen Vaccine against Shigella sonnei Administered Intramuscularly to Adults from a Shigellosis-Endemic Country. Front. Immunol. 2017, 8, 1884. [Google Scholar] [CrossRef]
- Arigita, C.; Jiskoot, W.; Westdijk, J.; van Ingen, C.; Hennink, W.E.; Crommelin, D.J.; Kersten, G.F. Stability of mono- and trivalent meningococcal outer membrane vesicle vaccines. Vaccine 2004, 22, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Lanzilao, L.; Stefanetti, G.; Saul, A.; MacLennan, C.A.; Micoli, F.; Rondini, S. Strain Selection for Generation of O-Antigen-Based Glycoconjugate Vaccines against Invasive Nontyphoidal Salmonella Disease. PLoS ONE 2015, 10, e0139847. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Gasperini, G.; Rossi, O.; Aruta, M.G.; Raso, M.M.; Alfini, R.; Biagini, M.; Necchi, F.; Micoli, F. Dissecting the contribution of O-Antigen and proteins to the immunogenicity of Shigella sonnei generalized modules for membrane antigens (GMMA). Sci. Rep. 2021, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, C.; Raso, M.M.; Palmieri, E.; De Felice, A.; Pippi, F.; Micoli, F. Development of a Specific and Sensitive HPAEC-PAD Method for Quantification of Vi Polysaccharide Applicable to other Polysaccharides Containing Amino Uronic Acids. Anal. Chem. 2020, 92, 6304–6311. [Google Scholar] [CrossRef]
- De Benedetto, G.; Cescutti, P.; Giannelli, C.; Rizzo, R.; Micoli, F. Multiple Techniques for Size Determination of Generalized Modules for Membrane Antigens from Salmonella typhimurium and Salmonella enteritidis. ACS Omega 2017, 2, 8282–8289. [Google Scholar] [CrossRef]
- Rossi, O.; Aruta, M.G.; Acquaviva, A.; Mancini, F.; Micoli, F.; Necchi, F. Characterization of Competitive ELISA and Formulated Alhydrogel Competitive ELISA (FAcE) for Direct Quantification of Active Ingredients in GMMA-Based Vaccines. Methods Protoc. 2020, 3, 62. [Google Scholar] [CrossRef]
- Micoli, F.; Ravenscroft, N.; Cescutti, P.; Stefanetti, G.; Londero, S.; Rondini, S.; Maclennan, C.A. Structural analysis of O-polysaccharide chains extracted from different Salmonella Typhimurium strains. Carbohydr. Res. 2014, 385, 1–8. [Google Scholar] [CrossRef]
- Necchi, F.; Saul, A.; Rondini, S. Development of a high-throughput method to evaluate serum bactericidal activity using bacterial ATP measurement as survival readout. PLoS ONE 2017, 12, e0172163. [Google Scholar] [CrossRef] [PubMed]
- Rossi, O.; Molesti, E.; Saul, A.; Giannelli, C.; Micoli, F.; Necchi, F. Intra-Laboratory Evaluation of Luminescence Based High-Throughput Serum Bactericidal Assay (L-SBA) to Determine Bactericidal Activity of Human Sera against Shigella. High-Throughput 2020, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, C.A.; Martin, L.B.; Micoli, F. Vaccines against invasive Salmonella disease: Current status and future directions. Hum. Vaccines Immunother. 2014, 10, 1478–1493. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Wierzba, T.; Walker, R.I. Status of vaccine research and development for Shigella. Vaccine 2016, 34, 2887–2894. [Google Scholar] [CrossRef]
- Nakao, R.; Hasegawa, H.; Dongying, B.; Ohnishi, M.; Senpuku, H. Assessment of outer membrane vesicles of periodontopathic bacterium Porphyromonas gingivalis as possible mucosal immunogen. Vaccine 2016, 34, 4626–4634. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Rondini, S.; Gavini, M.; Pisoni, I.; Lanzilao, L.; Colucci, A.M.; Giannelli, C.; Pippi, F.; Sollai, L.; Pinto, V.; et al. A scalable method for O-antigen purification applied to various Salmonella serovars. Anal. Biochem. 2013, 434, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Gnopo, Y.M.D.; Misra, A.; Hsu, H.L.; DeLisa, M.P.; Daniel, S.; Putnam, D. Induced fusion and aggregation of bacterial outer membrane vesicles: Experimental and theoretical analysis. J. Colloid Interface Sci. 2020, 578, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, D.E.; Yethon, J.A.; Whitfield, C. Molecular basis for structural diversity in the core regions of the lipopolysaccharides of Escherichia coli and Salmonella enterica. Mol. Microbiol. 1998, 30, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. MMBR 2003, 67, 593–656. [Google Scholar] [CrossRef] [PubMed]
- Schindler, M.; Osborn, M.J. Interaction of divalent cations and polymyxin B with lipopolysaccharide. Biochemistry 1979, 18, 4425–4430. [Google Scholar] [CrossRef] [PubMed]
- Yethon, J.A.; Gunn, J.S.; Ernst, R.K.; Miller, S.I.; Laroche, L.; Malo, D.; Whitfield, C. Salmonella enterica serovar typhimurium waaP mutants show increased susceptibility to polymyxin and loss of virulence In vivo. Infect. Immun. 2000, 68, 4485–4491. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yethon, J.A.; Heinrichs, D.E.; Monteiro, M.A.; Perry, M.B.; Whitfield, C. Involvement of waaY, waaQ, and waaP in the modification of Escherichia coli lipopolysaccharide and their role in the formation of a stable outer membrane. J. Biol. Chem. 1998, 273, 26310–26316. [Google Scholar] [CrossRef] [PubMed]
- Yethon, J.A.; Whitfield, C. Purification and characterization of WaaP from Escherichia coli, a lipopolysaccharide kinase essential for outer membrane stability. J. Biol. Chem. 2001, 276, 5498–5504. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Chen, J.; Ernst, R.K.; Wang, X. Influence of lipid A acylation pattern on membrane permeability and innate immune stimulation. Mar. Drugs 2013, 11, 3197–3208. [Google Scholar] [CrossRef] [PubMed]
- Frasch, C.E.; Holst, J.v.A.L.; Poolman, J.; Rosenqvist, E. Preparation of outer membrane protein vaccines against meningococcal disease. In Methods in Molecular Medicine. Meningococcal Disease Protocols; Pollard, A.J., Maiden, M.C.J., Eds.; Humana Press: Totowa, NJ, USA, 2001; Volume 66, pp. 81–107. [Google Scholar]
- Fredriksen, J.H.; Rosenqvist, E.; Wedege, E.; Bryn, K.; Bjune, G.; Frøholm, L.O.; Lindbak, A.K.; Møgster, B.; Namork, E.; Rye, U.; et al. Production, characterization and control of MenB-vaccine “Folkehelsa”: An outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 1991, 14, 67–79. [Google Scholar] [PubMed]
- Van de Waterbeemd, B.; Zomer, G.; Kaaijk, P.; Ruiterkamp, N.; Wijffels, R.H.; van den Dobbelsteen, G.P.; van der Pol, L.A. Improved production process for native outer membrane vesicle vaccine against Neisseria meningitidis. PLoS ONE 2013, 8, e65157. [Google Scholar] [CrossRef] [PubMed]
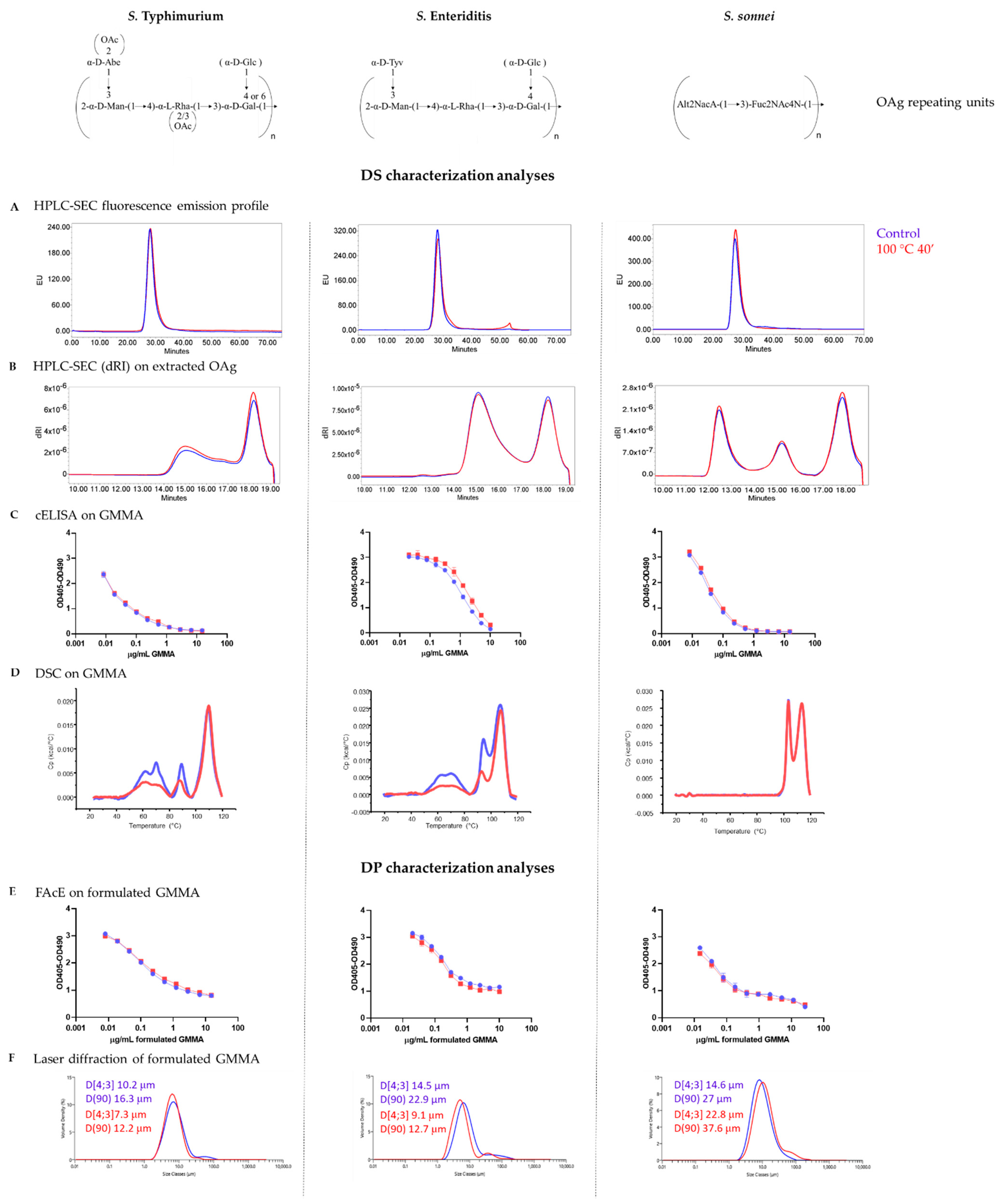
| Product Quality Attribute | Method | Reference | |
|---|---|---|---|
| Drug substance | Purity | HPLC-SEC (fluorescence emission profile, A260–A280 nm) | [12] |
| Size and aggregation status | SEC-MALS/DLS | [24] | |
| OAg identity and quantification | cELISA | [25] | |
| OAg quantification | HPAEC-PAD | [12,23,26] | |
| Total protein quantification | Micro BCA | - | |
| OAg length | HPLC-SEC on extracted OAg | [26] | |
| OAg O-acetylation content | 1H NMR on extracted OAg | [26] | |
| Drug product | OAg identity and quantification | FAcE | [25] |
| Size distribution | Laser diffraction | - | |
| OAg and protein not adsorbed to Alhydrogel | SDS-PAGE silver staining | - |
| Quality Attribute | Z-Average (d, nm) | OAg/Protein w/w Ratio | OAg O-Acetylation % | IC50 Fold Variation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stability Condition | Control | 37 °C4 w | 50 °C4 w | Control | 37 °C4 w | 50 °C4 w | Control | 37 °C4 w | 50 °C4 w | Control | 37 °C4 w | 50 °C4 w |
| STmGMMA | 93.84 (PdI = 0.188) | 80.22 (PdI = 0.223) | 82.31 (PdI = 0.262) | 0.70 | 0.71 | 0.74 | 87 | 35 | 15 | - | 4.9 | 164.6 |
| SEnGMMA | 88.36 (PdI = 0.152) | 83.79 (PdI = 0.161) | 83.65 (PdI = 0.191) | 1.76 | 1.84 | 2.01 | 2.48 | 1.77 | 1.23 | - | 1.8 | 1.9 |
| S. flexneri 2a GMMA | 92.12 (PdI = 0.100) | 87.76 (PdI = 0.129) | 95.71 (PdI = 0.177) | 0.96 | 0.98 | 1.06 | 183 | 131 | 34 | - | 9.2 | 20.2 |
| S. sonnei | 127.5 (PdI = 0.175) | 116.4 (PdI = 0.187) | 100.6 (PdI = 0.178) | 0.23 | 0.23 | 0.25 | - | - | - | - | 1.3 | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmieri, E.; Arato, V.; Oldrini, D.; Ricchetti, B.; Aruta, M.G.; Pansegrau, W.; Marchi, S.; Giusti, F.; Ferlenghi, I.; Rossi, O.; et al. Stability of Outer Membrane Vesicles-Based Vaccines, Identifying the Most Appropriate Methods to Detect Changes in Vaccine Potency. Vaccines 2021, 9, 229. https://doi.org/10.3390/vaccines9030229
Palmieri E, Arato V, Oldrini D, Ricchetti B, Aruta MG, Pansegrau W, Marchi S, Giusti F, Ferlenghi I, Rossi O, et al. Stability of Outer Membrane Vesicles-Based Vaccines, Identifying the Most Appropriate Methods to Detect Changes in Vaccine Potency. Vaccines. 2021; 9(3):229. https://doi.org/10.3390/vaccines9030229
Chicago/Turabian StylePalmieri, Elena, Vanessa Arato, Davide Oldrini, Beatrice Ricchetti, Maria Grazia Aruta, Werner Pansegrau, Sara Marchi, Fabiola Giusti, Ilaria Ferlenghi, Omar Rossi, and et al. 2021. "Stability of Outer Membrane Vesicles-Based Vaccines, Identifying the Most Appropriate Methods to Detect Changes in Vaccine Potency" Vaccines 9, no. 3: 229. https://doi.org/10.3390/vaccines9030229
APA StylePalmieri, E., Arato, V., Oldrini, D., Ricchetti, B., Aruta, M. G., Pansegrau, W., Marchi, S., Giusti, F., Ferlenghi, I., Rossi, O., Alfini, R., Giannelli, C., Gasperini, G., Necchi, F., & Micoli, F. (2021). Stability of Outer Membrane Vesicles-Based Vaccines, Identifying the Most Appropriate Methods to Detect Changes in Vaccine Potency. Vaccines, 9(3), 229. https://doi.org/10.3390/vaccines9030229










