Clinical and Economic Outcomes Associated with Cell-Based Quadrivalent Influenza Vaccine vs. Standard-Dose Egg-Based Quadrivalent Influenza Vaccines during the 2018–19 Influenza Season in the United States
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Overview
2.2. Data Sources
2.3. Study Periods
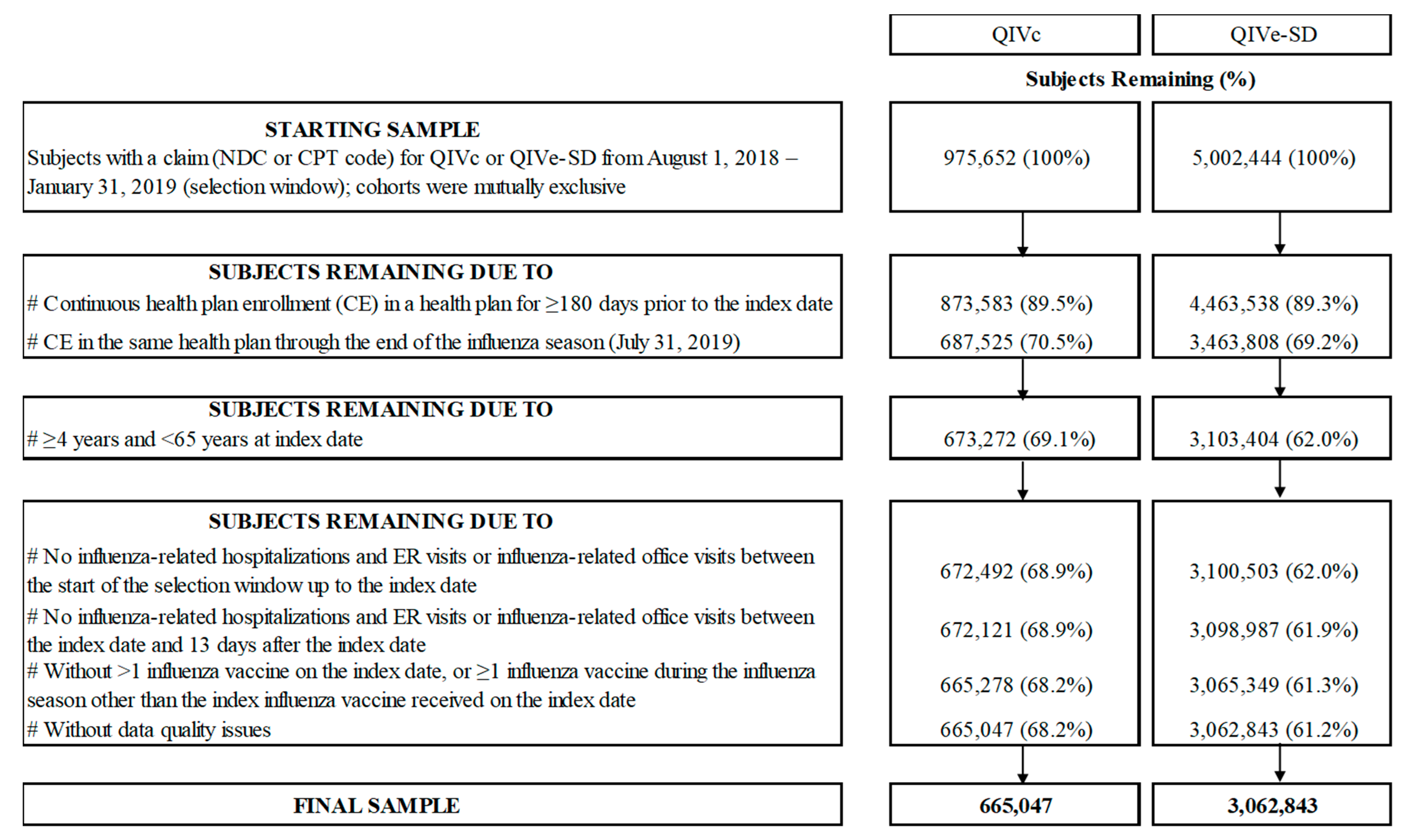
2.4. Study Population
2.5. Study Measures
Study Outcomes
2.6. Statistical Analysis
2.6.1. Clinical Outcomes Evaluation
2.6.2. Sensitivity Analysis
2.6.3. Economics Outcomes Evaluation
3. Results
3.1. Study Sample
3.2. Baseline Characteristics
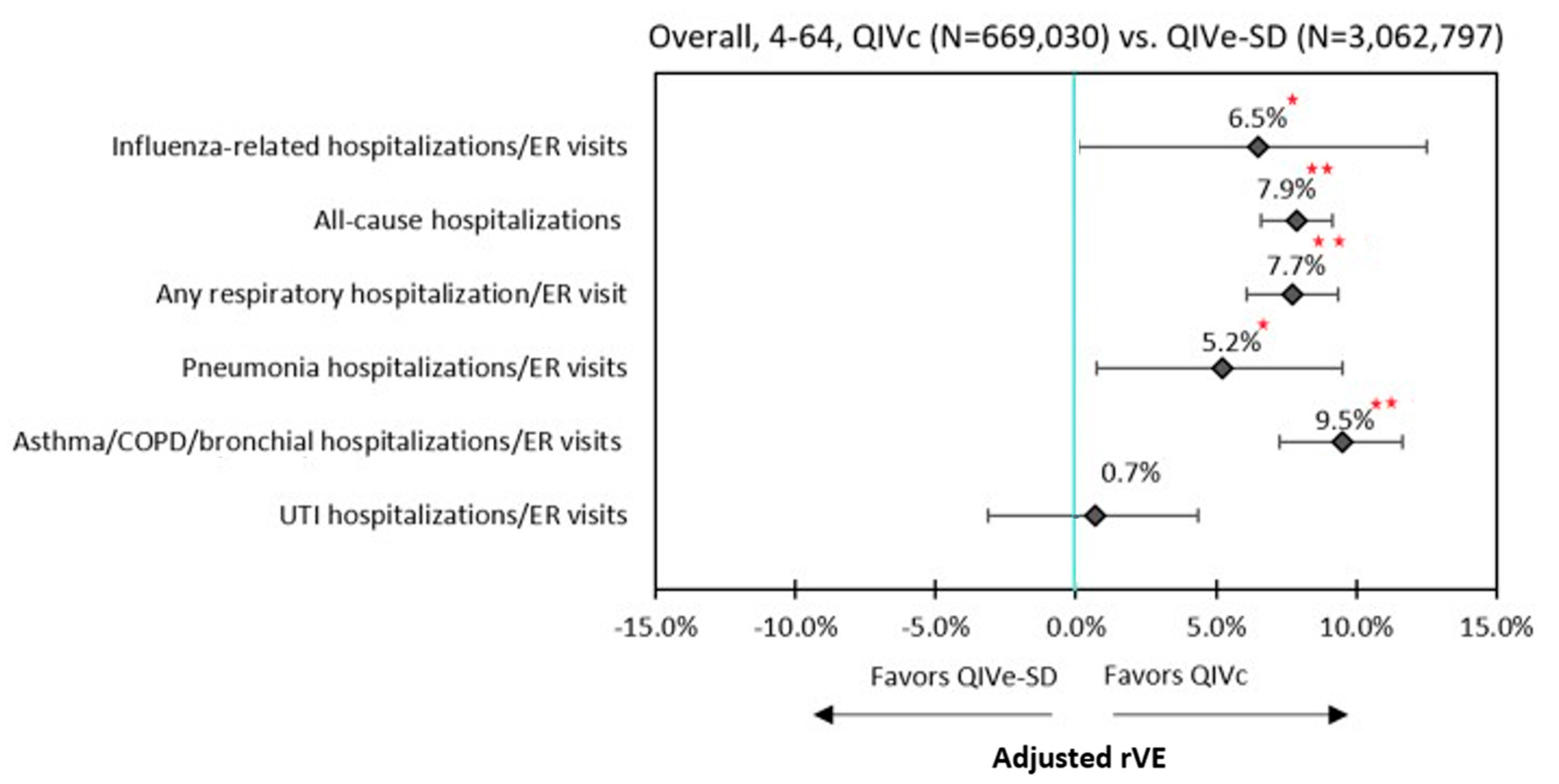
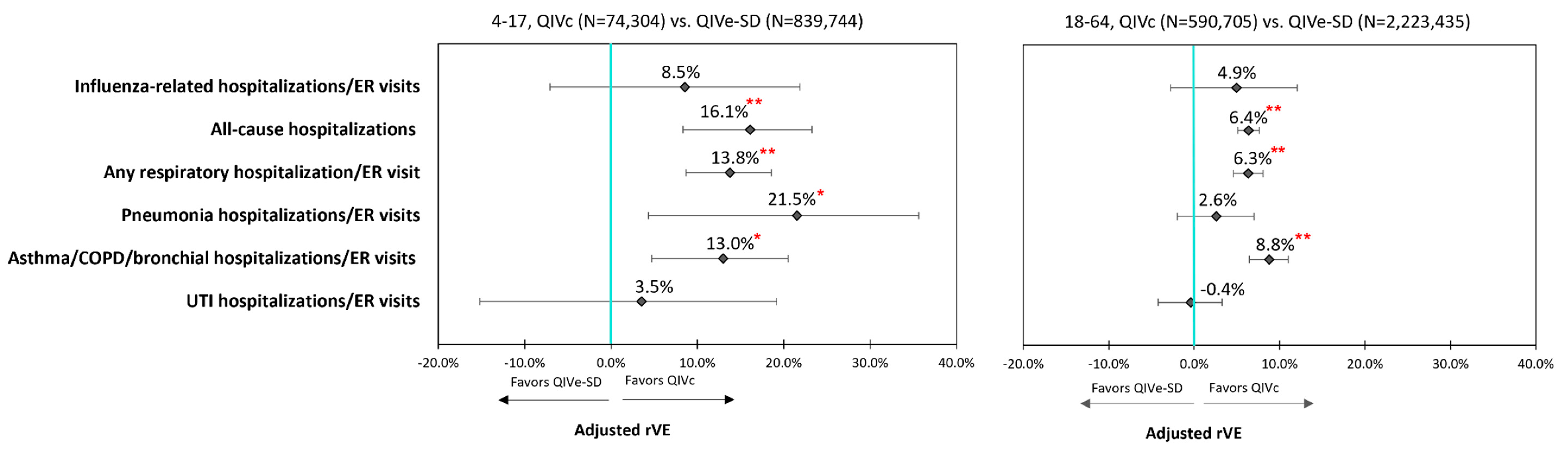
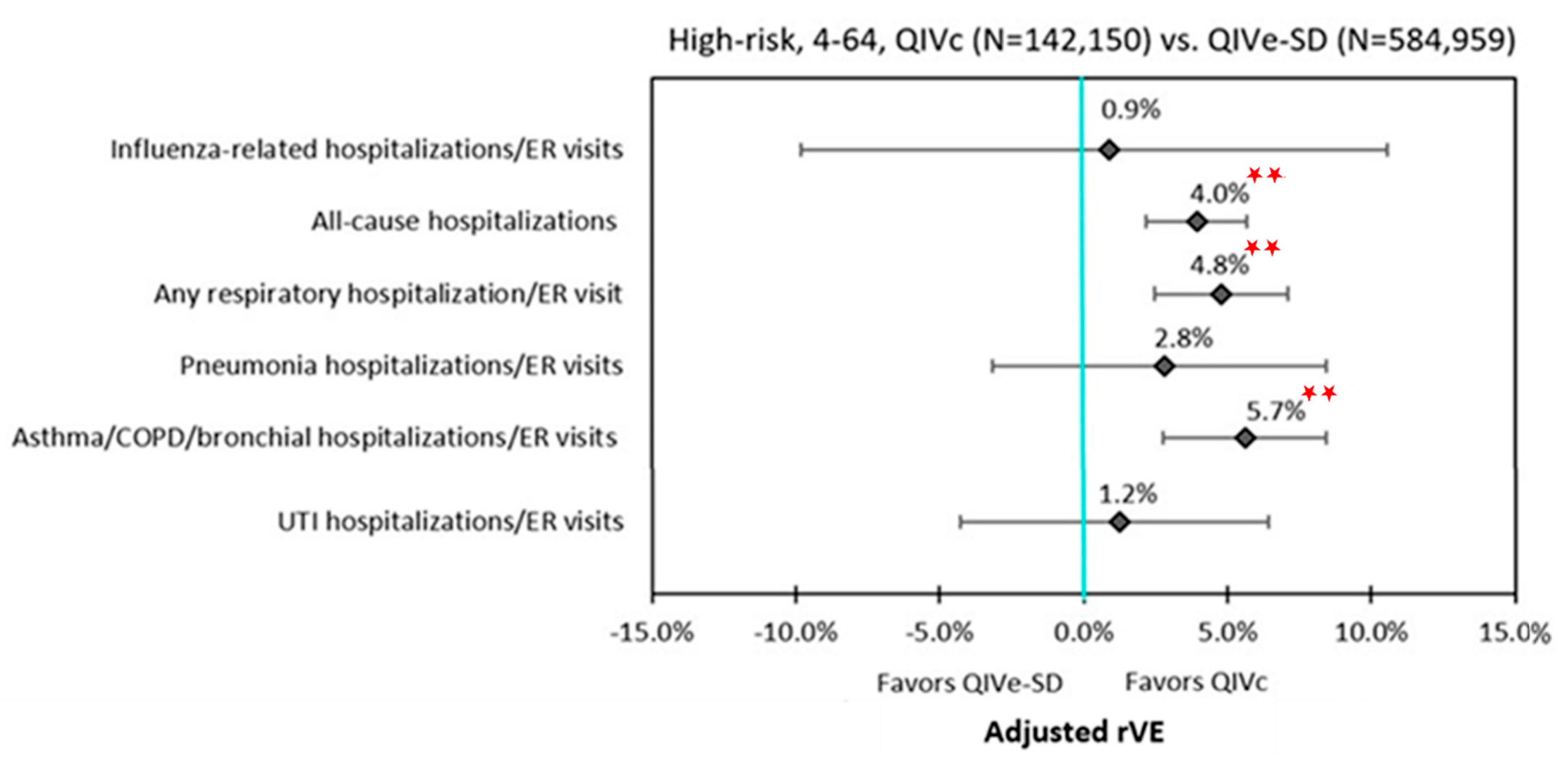
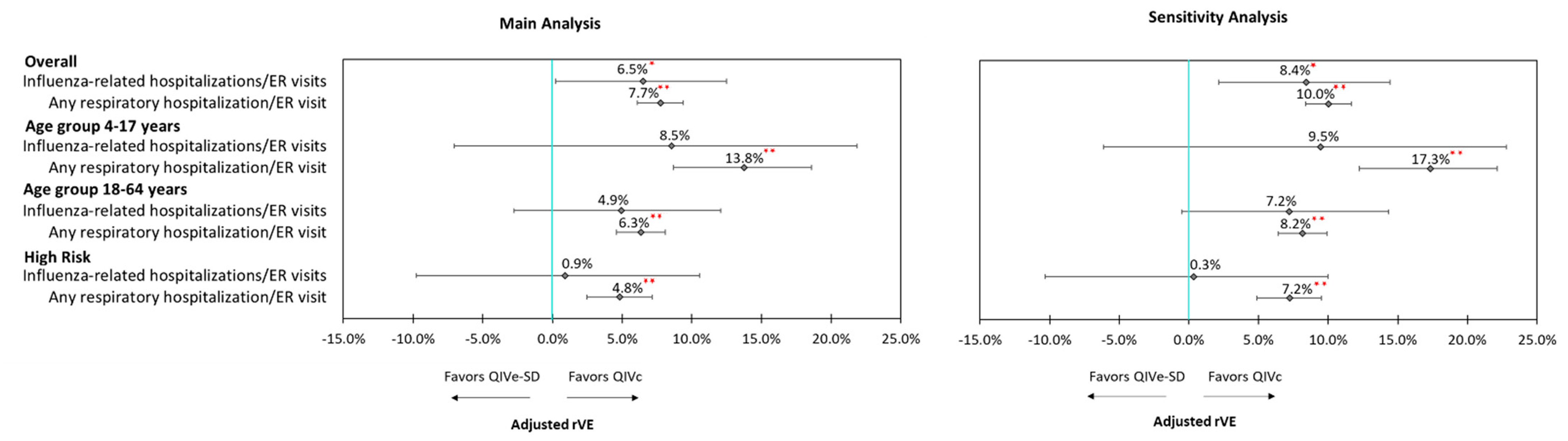
3.3. Clinical Outcomes
3.4. Sensitivity Analyses
3.5. Economic Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Disease Burden of Influenza. Available online: https://www.cdc.gov/flu/about/burden/index.html#:~:text=While%20the%20impact%20of%20flu,61%2C000%20deaths%20annually%20since%202010 (accessed on 5 October 2020).
- Estimated Influenza Illnesses, Medical visits, Hospitalizations, and Deaths in the United States—2019–2020 Influenza Season. Available online: https://www.cdc.gov/flu/about/burden/2019-2020.html (accessed on 5 October 2020).
- Rolfes, M.A.; Flannery, B.; Chung, J.R.; O’Halloran, A.; Garg, S.; Belongia, E.A.; Gaglani, M.; Zimmerman, R.K.; Jackson, M.L.; Monto, A.S.; et al. US Influenza Vaccine Effectiveness (Flu VE) Network, the Influenza Hospitalization Surveillance Network, and the Assessment Branch, Immunization Services Division, Centers for Disease Control and Prevention. Effects of Influenza Vaccination in the United States During the 2017–2018 Influenza Season. Clin. Infect. Dis. 2019, 69, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.R.; Rolfes, M.A.; Flannery, B.; Prasad, P.; O’Halloran, A.; Garg, S.; Fry, A.M.; Singleton, J.A.; Patel, M.; Reed, C. US Influenza Vaccine Effectiveness Network, the Influenza Hospitalization Surveillance Network, and the Assessment Branch, Immunization Services Division, Centers for Disease Control and Prevention. Effects of Influenza Vaccination in the United States During the 2018-2019 Influenza Season. Clin. Infect. Dis. 2020, 71, e368–e376. [Google Scholar] [CrossRef] [PubMed]
- Barr, I.G.; Donis, R.O.; Katz, J.M.; McCauley, J.W.; Odagiri, T.; Trusheim, H.; Tsai, T.F.; Wentworth, D.E. Cell culture-derived influenza vaccines in the severe 2017–2018 epidemic season: A step towards improved influenza vaccine effectiveness. NPJ Vaccines 2018, 3, 44. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.T.; Heaton, N.S. Efforts to Improve the Seasonal Influenza Vaccine. Vaccines 2018, 6, 19. [Google Scholar] [CrossRef]
- Frequently Asked Flu Questions 2018–2019 Influenza Season. Available online: https://www.cdc.gov/flu/about/season/flu-season-2018-2019.htm (accessed on 5 October 2020).
- Izurieta, H.S.; Chillarige, Y.; Kelman, J.; Wei, Y.; Lu, Y.; Xu, W.; Lu, M.; Pratt, D.; Chu, S.; Wernecke, M.; et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among the U.S. elderly, 2017–2018. J. Infect. Dis. 2019, 220, 1255–1264. [Google Scholar] [CrossRef]
- Divino, V.; Krishnarajah, G.; Pelton, S.I.; Mould-Quevedo, J.; Anupindi, V.R.; DeKoven, M.; Postma, M.J. A real-world study evaluating the relative vaccine effectiveness of a cell-based quadrivalent influenza vaccine compared to egg-based quadrivalent influenza vaccine in the US during the 2017–2018 influenza season. Vaccine 2020, 38, 6334–6343. [Google Scholar] [CrossRef]
- Bruxvoort, K.J.; Luo, Y.; Ackerson, B.; Tanenbaum, H.C.; Sy, L.S.; Gandhi, A.; Tseng, H.F. Comparison of vaccine effectiveness against influenza hospitalization of cell-based and egg-based influenza vaccines, 2017–2018. Vaccine 2019, 37, 5807–5811. [Google Scholar] [CrossRef]
- DeMarcus, L.; Shoubaki, L.; Federinko, S. Comparing influenza vaccine effectiveness between cell-derived and egg-derived vaccines, 2017–2018 influenza season. Vaccine 2019, 37, 4015–4021. [Google Scholar] [CrossRef]
- Boikos, C.; Sylvester, G.C.; Sampalis, J.S.; Mansi, J.A. Effectiveness of the Cell Culture- and Egg-Derived, Seasonal Influenza Vaccine During the 2017–2018 Northern Hemisphere Influenza Season. US National Foundation for Infectious Disease 2018 Clinical Vaccine. In Proceedings of the Canadian Immunization Conference, Ottawa, ON, Canada, 4–6 December 2018. [Google Scholar]
- Epperson, S.; Davis, C.T.; Brammer, L.; Elal, A.I.A.; Ajayi, N.; Barnes, J.; Budd, A.P.; Burns, E.; Daly, P.; Dugan, V.G.; et al. Update: Influenza Activity—United States and Worldwide, 19 May–28 September 2019, and Composition of the 2020 Southern Hemisphere Influenza Vaccine. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 880–884. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Walter, E.B.; Fry, A.M.; Jernigan, D.B. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2019–2020 Influenza Season. MMWR Recomm. Rep. 2019, 68, 1–21. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Blanton, L.H.; Fry, A.M.; Jernigan, D.B.; Atmar, R.L. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020–2021 Influenza Season. MMWR Recomm. Rep. 2020, 69, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Izurieta, H.S.; Chillarige, Y.; Kelman, J.; Wei, Y.; Lu, Y.; Xu, W.; Lu, M.; Pratt, D.; Wernecke, M.; MaCurdy, T.; et al. Relative effectiveness of influenza vaccines among the U.S. elderly, 2018–2019. J. Infect. Dis. 2020, 222, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Office for Human Research Protections. Updated FAQs on Informed Consent for Use of Biospecimens and Data. Available online: https://www.hhs.gov/ohrp/sachrp-committee/recommendations/attachment-c-faqs-recommendations-and-glossary-informed-consent-and-research-use-of-biospecimens-and-associated-data/index.html (accessed on 19 January 2021).
- Electronic Code of Federal Regulations. Protection of Human Subjects. Available online: https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=&SID=83cd09e1c0f5c6937cd9d7513160fc3f&pitd=20180719&n=pt45.1.46&r=PART&ty=HTML#se45.1.46_1102 (accessed on 19 January 2021).
- Public Health England. The Green Book. Chapter 19: Influenza. Published April 2019. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/796886/GreenBook_Chapter_19_Influenza_April_2019.pdf (accessed on 19 January 2021).
- Grohskopf, L.A.; Sokolow, L.Z.; Broder, K.R.; Walter, E.B.; Bresee, J.S.; Fry, A.M.; Jernigan, D.B. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2017–2018 Influenza Season. MMWR Recomm. Rep. 2017, 66, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, W.; Stirling, R.; on behalf of the National Advisory Committee on Immunization (NACI). Summary of the NACI Statement on Seasonal Influenza Vaccine for 2017–2018. Can. Commun. Dis. Rep. 2017, 43, 96–103. [Google Scholar] [CrossRef]
- van Aalst, R.; Gravenstein, S.; Mor, V.; Mahmud, S.M.; Wilschut, J.; Postma, M.; Chit, A. Comparative effectiveness of high dose versus adjuvanted influenza vaccine: A retrospective cohort study. Vaccine 2020, 38, 372–379. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Thoemmes, F.; Ong, A.D. A Primer on Inverse Probability of Treatment Weighting and Marginal Structural Models. Emerg. Adulthood 2016, 4, 40–59. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Lozano, J.E. Lozalojo/Mem: Second Release of the MEM R Library. Zenodo [Internet]. [cited 2017 February 1]. Available online: https://zenodo.org/record/165983 (accessed on 3 September 2020). [CrossRef]
- Biggerstaff, M.; Kniss, K.; Jernigan, D.B.; Brammer, L.; Bresee, J.; Garg, S.; Burns, E.; Reed, C. Systematic Assessment of Multiple Routine and Near Real-Time Indicators to Classify the Severity of Influenza Seasons and Pandemics in the United States, 2003–2004 Through 2015–2016. Am. J. Epidemiol. 2018, 187, 1040–1050. [Google Scholar] [CrossRef]
- Crown, W.H. Propensity-score matching in economic analyses: Comparison with regression models, instrumental variables, residual inclusion, differences-in-differences, and decomposition methods. Appl. Health Econ. Health Policy 2014, 12, 7–18. [Google Scholar] [CrossRef]
- Basu, A.; Rathouz, P.J. Estimating marginal and incremental effects on health outcomes using flexible link and variance function models. Biostatistics 2005, 6, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Vogt, A. Outliers: An evaluation of methodologies. In Proceedings of the Survey Research Methods. Joint Statistical Meeting, San Diego, CA, USA., 28 July–2 August 2012. [Google Scholar]
- Rothman, K.J.; Greenland, S.; Lash, T.L. Modern Epidemiology, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Budd, A.P.; Beacham, L.; Smith, C.B.; Garten, R.J.; Reed, C.; Kniss, K.; Mustaquim, D.; Ahmad, F.B.; Cummings, C.N.; Garg, S.; et al. Birth Cohort Effects in Influenza Surveillance Data: Evidence That First Influenza Infection Affects Later Influenza-Associated Illness. J. Infect. Dis. 2019, 220, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Uyeki, T.M.; Bernstein, H.H.; Bradley, J.S.; Englund, J.A.; File, T.M.; Fry, A.M.; Gravenstein, S.; Hayden, F.G.; Harper, S.A.; Hirshon, J.M.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza. Clin. Infect. Dis. 2019, 68, e1–e47. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | QIVc (N = 669,030) | QIVe-SD (N = 3,062,797) | SMD 1 |
|---|---|---|---|
| Mean Age (years) | 36.8 | 36.9 | 0.01 |
| SD | 19.2 | 19.2 | |
| Median | 39 | 40 | |
| Age group (%) | |||
| 4–8 years | 10.0% | 9.6% | −0.01 |
| 9–17 years | 15.3% | 14.9% | −0.01 |
| 18–49 years | 40.1% | 40.5% | 0.01 |
| 50–64 years | 34.6% | 35.0% | 0.01 |
| Female (%) | 55.5% | 55.3% | 0.00 |
| Payer type (%) | |||
| Commercial | 60.8% | 60.4% | −0.01 |
| Medicaid | 0.7% | 0.8% | 0.00 |
| Medicare Advantage | 0.0% | 0.0% | 0.00 |
| Self-Insured | 38.4% | 38.8% | 0.01 |
| Unknown | 0.0% | 0.0% | 0.00 |
| Health plan type (n, %) | |||
| Consumer directed health care | 0.1% | 0.1% | 0.02 |
| HMO | 7.7% | 8.4% | 0.02 |
| POS | 5.4% | 6.2% | 0.03 |
| PPO | 85.9% | 84.3% | −0.05 |
| Other/Unknown | 0.9% | 1.0% | 0.02 |
| Geographic region (n, %) | |||
| Northeast | 15.1% | 16.6% | 0.04 |
| Midwest | 35.1% | 33.3% | −0.04 |
| South | 39.0% | 38.8% | 0.00 |
| West | 10.8% | 11.3% | 0.02 |
| DHHS (U.S. Dept. of Health and Human Services) region (%) | |||
| Region 1: CT, ME, MA, NH, RI, VT | 6.1% | 6.6% | 0.02 |
| Region 2: NJ, NY, PR, VI | 2.7% | 2.9% | 0.02 |
| Region 3: DE, DC, MD, PA, VA, WV | 8.7% | 9.0% | 0.01 |
| Region 4: AL, FL, GA, KY, MS, NC, SC, TN | 23.5% | 24.0% | 0.01 |
| Region 5: IL, IN, MI, MN, OH, WI | 26.1% | 24.5% | −0.04 |
| Region 6: AR, LA, NM, OK, TX | 13.4% | 13.2% | 0.00 |
| Region 7: IA, KS, MO, NE | 7.6% | 7.6% | 0.00 |
| Region 8: CO, MT, ND, SD, UT, WY | 3.0% | 2.8% | −0.01 |
| Region 9: AZ, CA, HI, NV, AS, FS, GU, PU | 3.0% | 3.2% | 0.01 |
| Region 10: AK, ID, OR, WA | 6.0% | 6.1% | 0.01 |
| Characteristic | QIVc (N = 669,030) | QIVe-SD (N = 3,062,797) | SMD 1 |
|---|---|---|---|
| Month of flu vaccine (%) | |||
| August | 1.9% | 1.7% | −0.02 |
| September | 17.1% | 18.1% | 0.03 |
| October | 47.4% | 47.2% | 0.00 |
| November | 21.3% | 20.8% | −0.01 |
| December | 7.6% | 7.5% | 0.00 |
| January | 4.7% | 4.7% | 0.00 |
| Charlson Comorbidity Index (CCI) Score: (%) | |||
| 0 | 83.5% | 83.3% | −0.01 |
| 1 | 9.7% | 9.8% | 0.00 |
| 2 | 4.4% | 4.4% | 0.00 |
| 3+ | 2.5% | 2.5% | 0.00 |
| Mean CCI Score | 0.3 | 0.3 | 0.01 |
| SD | 0.8 | 0.8 | |
| Median | 0 | 0 | |
| Pre-index comorbid conditions of interest (%) | |||
| Asthma | 4.1% | 4.1% | 0.00 |
| Blood disorders | 0.1% | 0.1% | 0.00 |
| Chronic lung disease | 1.6% | 1.6% | 0.00 |
| Diabetes | 6.5% | 6.7% | 0.01 |
| Heart disease | 1.5% | 1.5% | 0.00 |
| Kidney disorders | 1.1% | 1.0% | −0.01 |
| Liver disorders | 1.5% | 1.5% | 0.00 |
| Neurological or neurodevelopmental conditions | 1.5% | 1.5% | 0.01 |
| Weakened immune system 2 | 2.6% | 2.7% | 0.01 |
| IBD | 0.6% | 0.6% | 0.00 |
| Composite of the above | 16.8% | 17.2% | 0.01 |
| Patients with pre-index hospitalization (%) | 2.0% | 2.1% | 0.01 |
| Mean Number of pre-index hospitalizations | 0.0 | 0.0 | 0.01 |
| SD | 0.2 | 0.2 | |
| Median | 0 | 0 | |
| Patients with pre-index ER visit (%) | 7.1% | 7.7% | 0.02 |
| Mean Number of pre-index ER visits | 0.1 | 0.1 | 0.02 |
| SD | 0.4 | 0.5 | |
| Median | 0 | 0 | |
| Patients with pre-index outpatient physician office visit (%) | 77.4% | 76.4% | −0.02 |
| Mean Number of outpatient physician office visit | 3.8 | 3.7 | −0.01 |
| SD | 7.0 | 6.5 | |
| Median | 2 | 2 | |
| Mean pre-index outpatient pharmacy costs | $1259 | $1304 | 0.01 |
| SD | $7566 | $8320 | |
| Median | $59 | $61 | |
| Mean pre-index inpatient costs | $570 | $641 | 0.01 |
| SD | $7575 | $8427 | |
| Median | $0 | $0 | |
| Mean pre-index outpatient medical costs | $1826 | $1869 | 0.01 |
| SD | $7656 | $6769 | |
| Median | $442 | $450 | |
| Mean ER costs | $119 | $136 | 0.02 |
| SD | $851 | $972 | |
| Median | $0 | $0 | |
| Mean TOTAL pre-index all-cause costs 3 | $4014 | $3732 | −0.08 |
| SD | $15476 | $15391 | |
| Median | $788 | $674 |
| Subgroup | Overall (4–64 Years) | 4–17 Years | 18–64 Years | High-Risk (4–64 Years) | ||||
|---|---|---|---|---|---|---|---|---|
| rVE | p-Value | rVE | p-Value | rVE | p-Value | rVE | p-Value | |
| Influenza-related hospitalizations and ER visits | 6.5% | 0.0475 | 8.5% | 0.2664 | 4.9% | 0.2024 | 0.9% | 0.8611 |
| All-cause hospitalizations | 7.9% | <0.0001 | 16.1% | 0.0001 | 6.4% | <0.0001 | 4.0% | <0.0001 |
| Respiratory hospitalizations/ER visits | ||||||||
| Any respiratory hospitalization/ER visit | 7.7% | <0.0001 | 13.8% | <0.0001 | 6.3% | <0.0001 | 4.8% | <0.0001 |
| Pneumonia hospitalizations/ER visits | 5.2% | 0.0234 | 21.5% | 0.0165 | 2.6% | 0.2617 | 2.8% | 0.3435 |
| Asthma/COPD/bronchial hospitalizations/ER visits | 9.5% | <0.0001 | 13.0% | 0.0026 | 8.8% | <0.0001 | 5.7% | 0.0001 |
| UTI hospitalizations/ER visits | 0.7% | 0.7211 | 3.5% | 0.6921 | −0.4% | 0.8329 | 1.2% | 0.6522 |
| Predicted Mean Annualized All-Cause Cost | QIVc N = 665,042 | QIVe-SD N = 665,042 | p-Value | Incremental Mean | ||
|---|---|---|---|---|---|---|
| Mean | 95% Cis * | Mean | 95% Cis * | |||
| TOTAL | $9277 | $9168–$9391 | $9738 | $9620–$9854 | <0.0001 | $461 |
| Inpatient | $1559 | $1532–$1589 | $1695 | $1667–$1725 | <0.0001 | $136 |
| Outpatient medical | $4375 | $4348–$4404 | $4612 | $4583–$4644 | <0.0001 | $238 |
| ER | $261 | $258–$265 | $291 | $288–$295 | <0.0001 | $30 |
| Outpatient pharmacy | $3182 | $3068–$3305 | $3193 | $3079–$3317 | 0.260 | $11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnarajah, G.; Divino, V.; Postma, M.J.; Pelton, S.I.; Anupindi, V.R.; DeKoven, M.; Mould-Quevedo, J. Clinical and Economic Outcomes Associated with Cell-Based Quadrivalent Influenza Vaccine vs. Standard-Dose Egg-Based Quadrivalent Influenza Vaccines during the 2018–19 Influenza Season in the United States. Vaccines 2021, 9, 80. https://doi.org/10.3390/vaccines9020080
Krishnarajah G, Divino V, Postma MJ, Pelton SI, Anupindi VR, DeKoven M, Mould-Quevedo J. Clinical and Economic Outcomes Associated with Cell-Based Quadrivalent Influenza Vaccine vs. Standard-Dose Egg-Based Quadrivalent Influenza Vaccines during the 2018–19 Influenza Season in the United States. Vaccines. 2021; 9(2):80. https://doi.org/10.3390/vaccines9020080
Chicago/Turabian StyleKrishnarajah, Girishanthy, Victoria Divino, Maarten J. Postma, Stephen I. Pelton, Vamshi Ruthwik Anupindi, Mitch DeKoven, and Joaquin Mould-Quevedo. 2021. "Clinical and Economic Outcomes Associated with Cell-Based Quadrivalent Influenza Vaccine vs. Standard-Dose Egg-Based Quadrivalent Influenza Vaccines during the 2018–19 Influenza Season in the United States" Vaccines 9, no. 2: 80. https://doi.org/10.3390/vaccines9020080
APA StyleKrishnarajah, G., Divino, V., Postma, M. J., Pelton, S. I., Anupindi, V. R., DeKoven, M., & Mould-Quevedo, J. (2021). Clinical and Economic Outcomes Associated with Cell-Based Quadrivalent Influenza Vaccine vs. Standard-Dose Egg-Based Quadrivalent Influenza Vaccines during the 2018–19 Influenza Season in the United States. Vaccines, 9(2), 80. https://doi.org/10.3390/vaccines9020080





