Influenza Vaccine Effectiveness in Mainland China: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
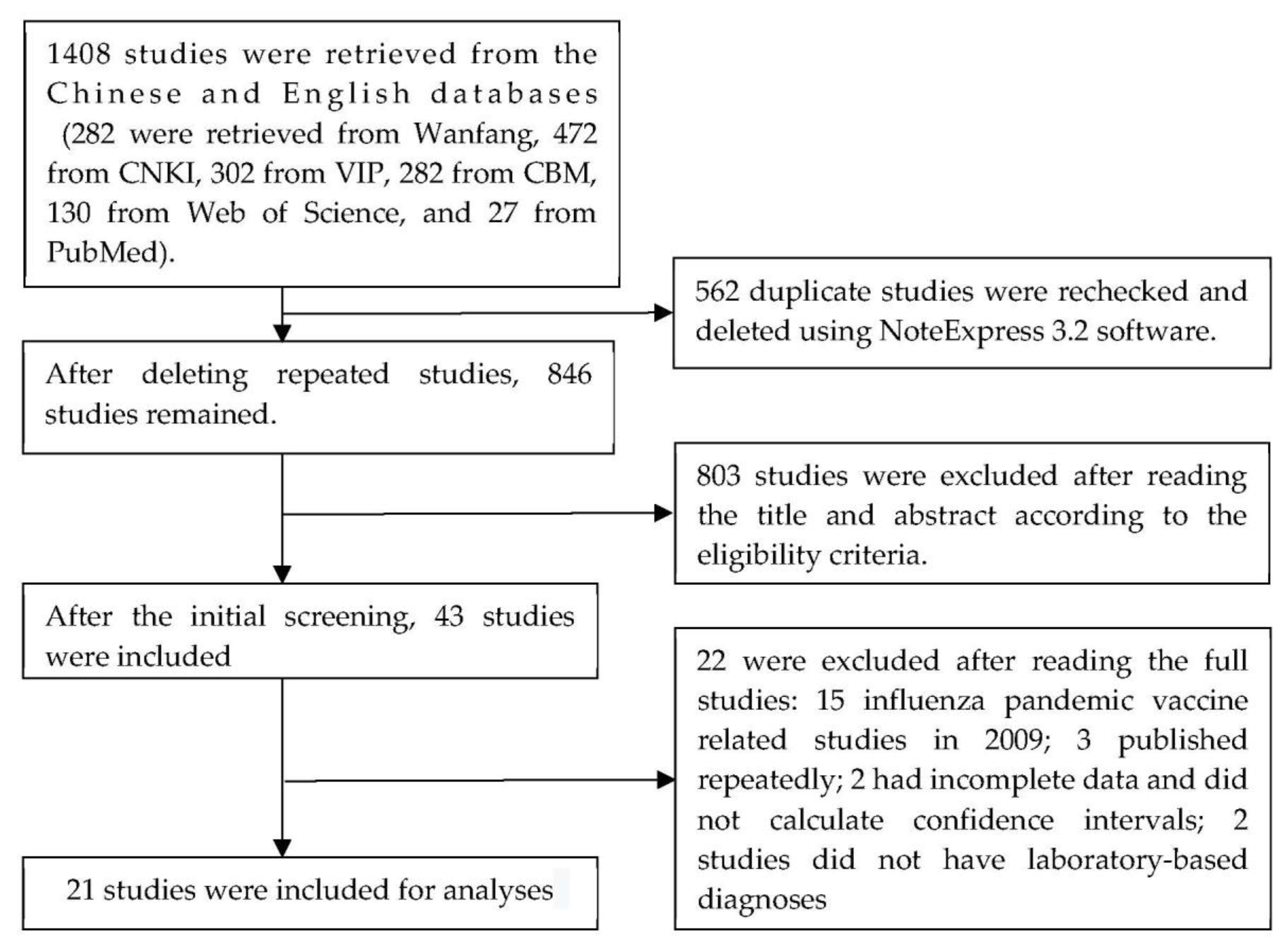
2.1. Literature Retrieval
2.2. Eligibility Criteria
2.3. Data Retrieval
2.4. Literature Quality Evaluation
2.5. Statistical Analyses
3. Results
3.1. Basic Information of Eligible Studies
3.2. Quality Evaluations
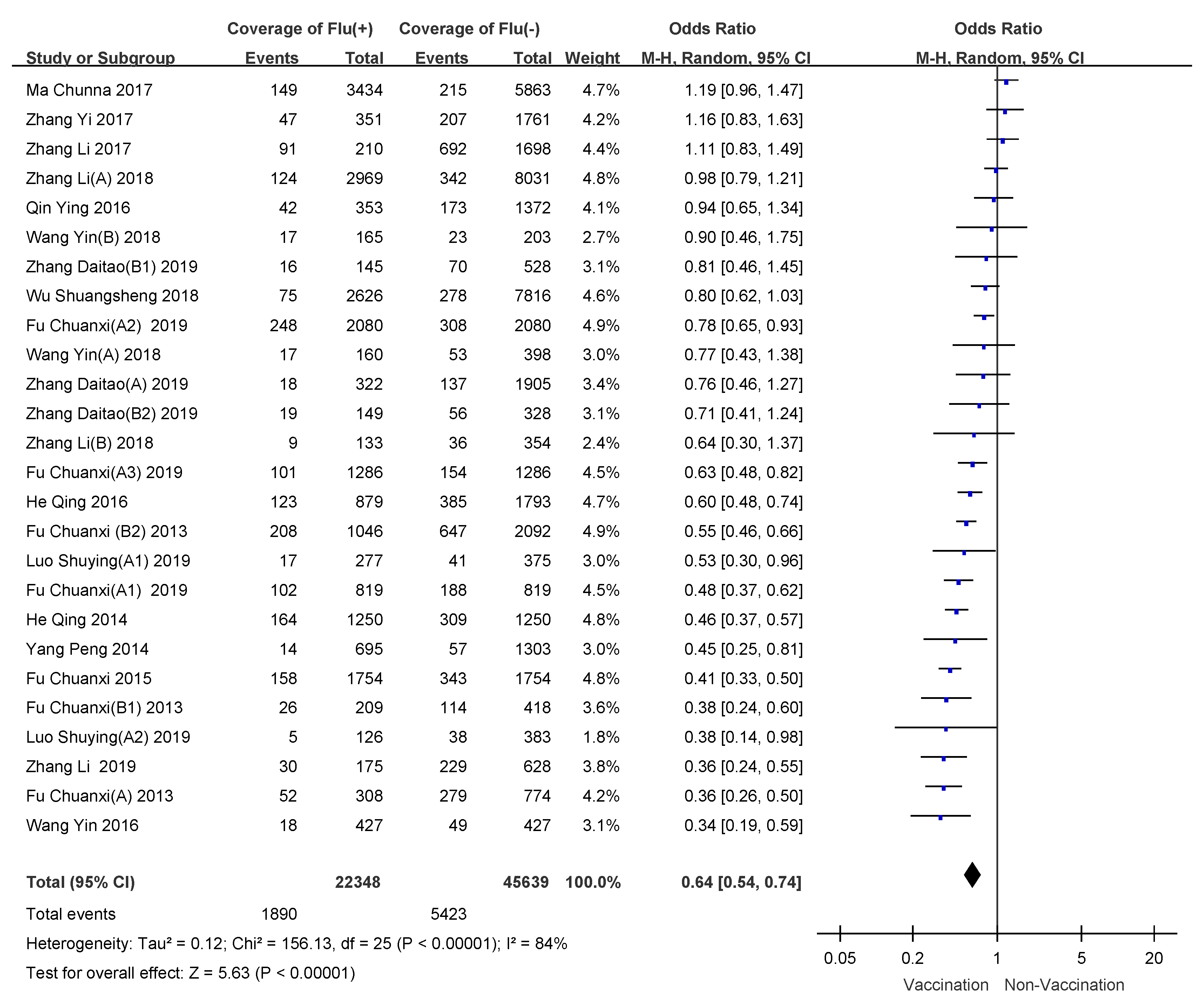
3.3. Meta Analysis of Influenza VE
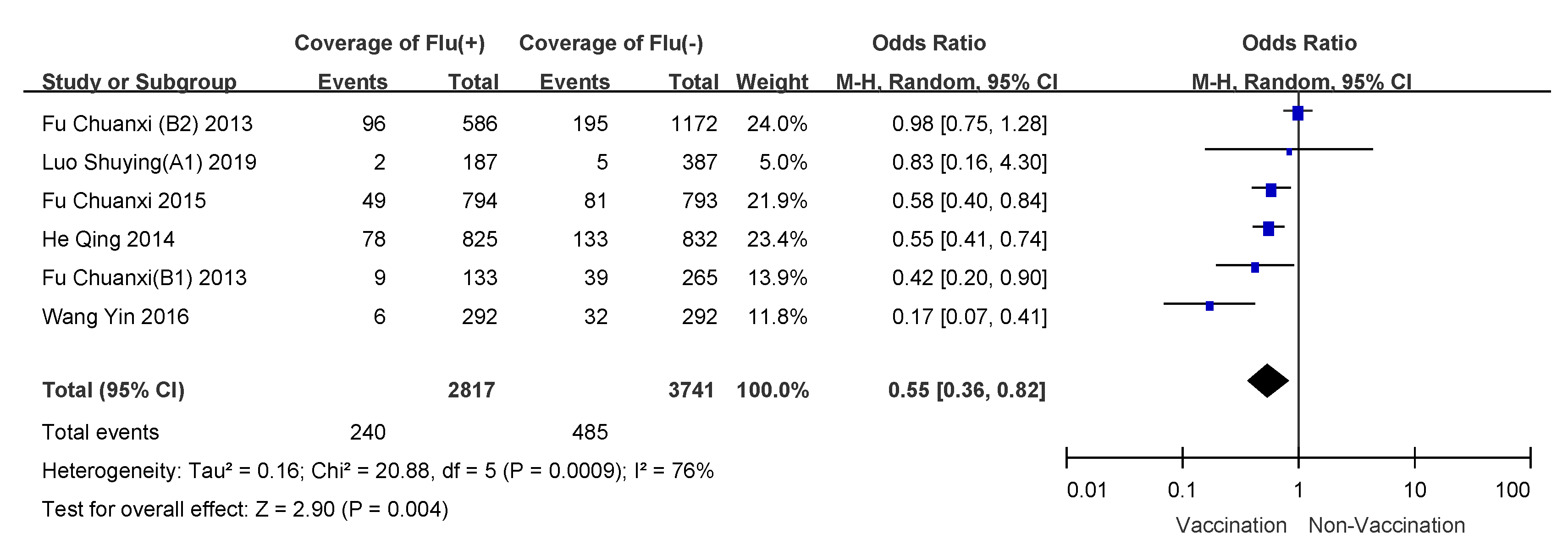
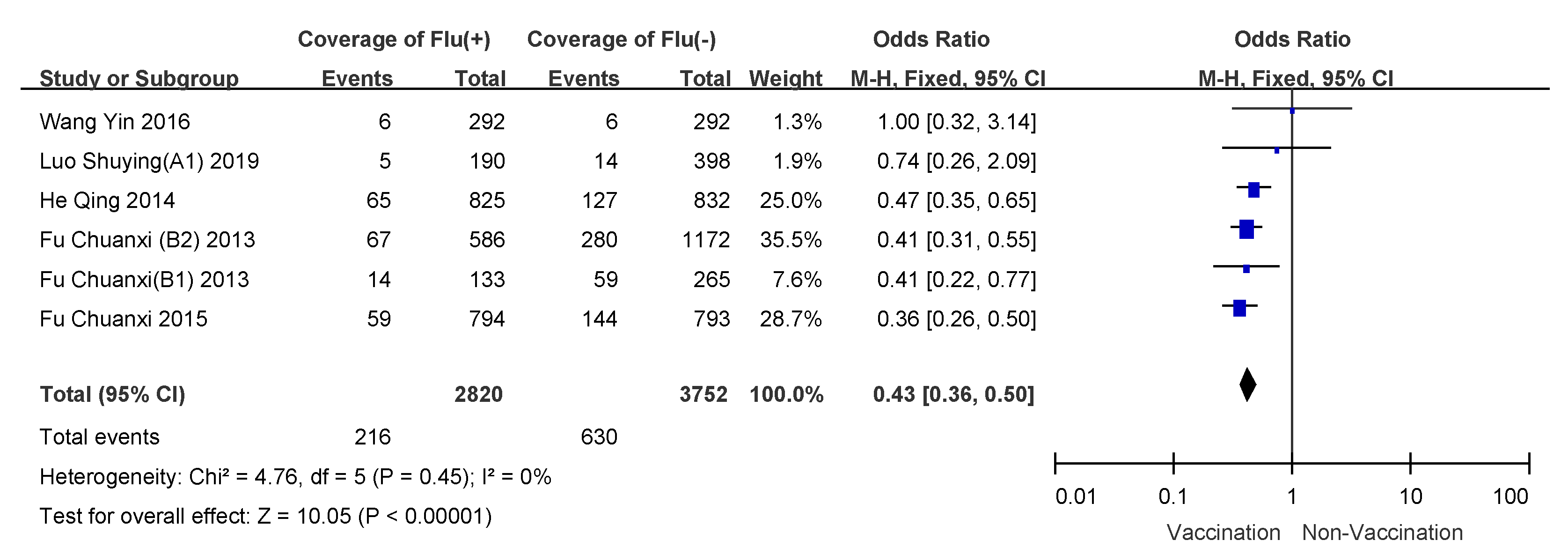
3.4. Subgroup Analyses
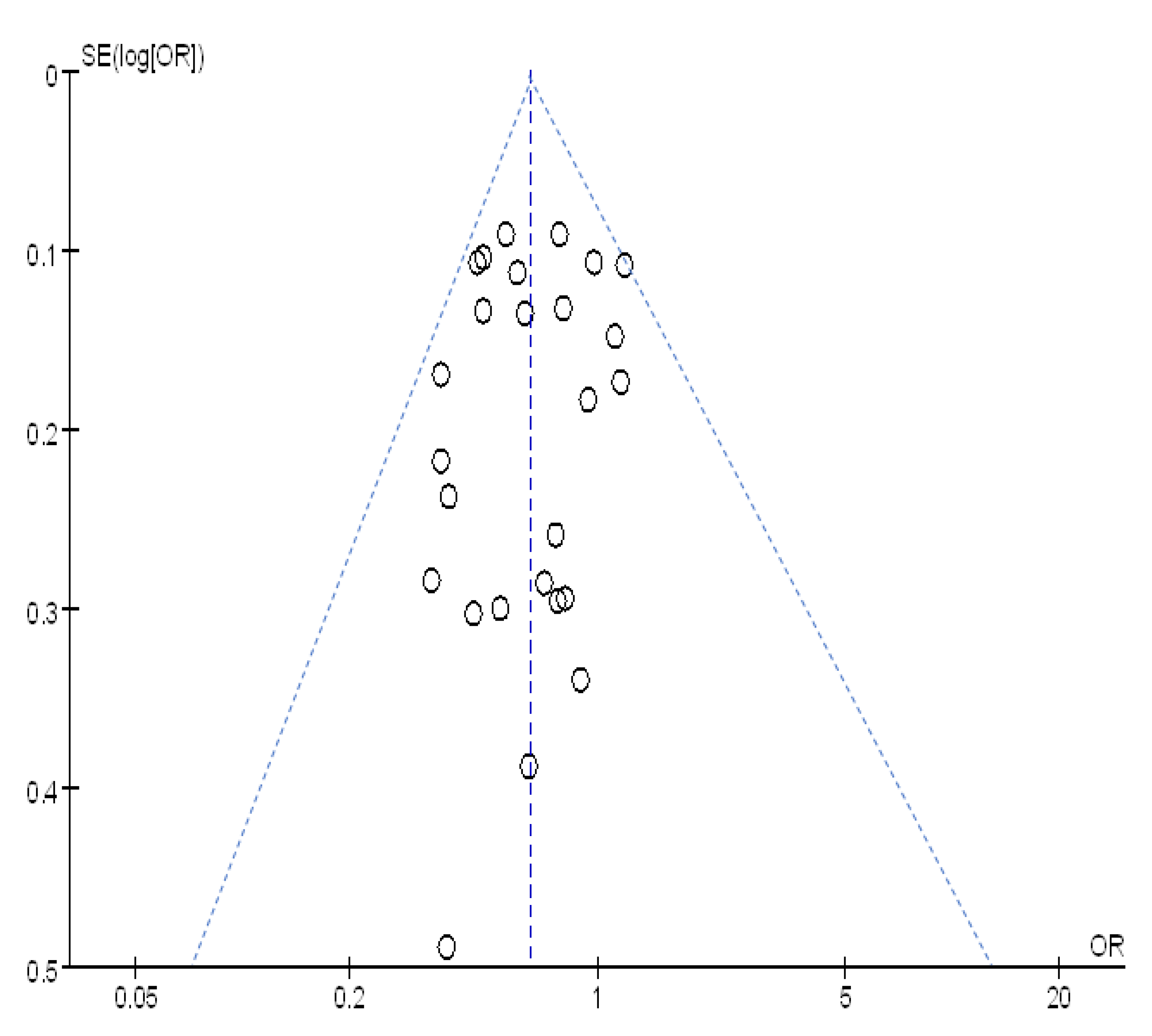
3.5. Sensitivity Analysis and Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 10 September 2019).
- Li, L.; Liu, Y.N.; Wu, P.; Peng, Z.B.; Wang, X.L.; Chen, T.; Wong, J.Y.T.; Yang, J.; Bond, H.S.; Wang, L.J.; et al. Influenza-associated excess respiratory mortality in China, 2010–15: A population-based study. Lancet Public Health 2019, 4, e473–e481. [Google Scholar] [CrossRef]
- Wang, Y.M.; Cao, B. Perspectives of antiviral drugs used on influenza. Zhonghua Liu Xing Bing Xue Za Zhi 2018, 39, 1051–1059. [Google Scholar] [PubMed]
- Paules, C.; Subbarao, K. Influenza. Lancet 2017, 390, 697–708. [Google Scholar] [CrossRef]
- Yang, J.; Atkins, K.E.; Feng, L.Z.; Pang, M.F.; Zheng, Y.M.; Liu, X.X.; Cowling, B.J.; Yu, H.J. Seasonal influenza vaccination in China: Landscape of diverse regional reimbursement policy, and budget impact analysis. Vaccine 2016, 34, 5724–5735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.R.; Zhu, X.P.; Zhou, L.J.; Liu, Y.Q.; Fan, Y.; Chen, G.; Chen, Z.; Liu, Y.; Sun, H.Y.; Wu, J.L. Safety and immunological effect of domestic split influenza virus vaccine. Zhonghua Yu Fang Yi Xue Za Zhi 2009, 43, 615–618. [Google Scholar] [PubMed]
- Duan, W.; Yang, P.; Shi, W.X.; Peng, X.M.; Liang, H.J.; Wang, Q.Y. Research on safety and cost-benefit of influenza vaccine. Guo Ji Bing Du Xue Za Zhi 2014, 21, 241–244. [Google Scholar]
- Wang, R.Q.; Tang, Y.Q.; Liu, Z.C. Safety and immunogenicity evaluation of domestic influenza split vaccine. Zhongguo Wei Sheng Jian Yan Za Zhi 2008, 18, 340–342. [Google Scholar]
- Wang, P.; Zhang, X.W.; Song, Y.F.; Yin, H.B.; Liu, L.J.; Che, L.; Li, H.; Liu, Y.; Chen, J.T. Safety and immunogenicity on the formulation of trivalent split influenza vaccine among healthy people aged over 18 years. Zhonghua Liu Xing Bing Xue Za Zhi 2011, 32, 120–124. [Google Scholar]
- Liu, M.; Liu, G.F.; Wang, Y.; Zhao, W.; Wang, L.; Shi, W.; Wen, S.Y. Study on the effectiveness and cost benefit of influenza vaccine on elderly population in Beijing city. Zhonghua Liu Xing Bing Xue Za Zhi 2005, 26, 412–416. [Google Scholar]
- Wang, J.Z. Observation on the preventive effect of influenza vaccine in children. Lin Chuang Yi Xue Yan Jiu Yu Shi Jian 2016, 1, 98. [Google Scholar]
- Bian, G.L.; Xu, G.Z.; Zhu, L.L. Study on the Efficacy of Infuenza Vaccine among Different populations. Zhejiang Yu Fang Yi Xue 2010, 22, 19. [Google Scholar]
- Merckx, J.; Wali, R.; Schiller, I.; Caya, C.; Gore, G.C.; Chartrand, C.; Dendukuri, N.; Papenburg, J. Diagnostic Accuracy of Novel and Traditional Rapid Tests for Influenza Infection Compared With Reverse Transcriptase Polymerase Chain Reaction: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2017, 167, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, Q.; Liang, H.M.; Zhou, T.X.; Zhang, X.B.; Peng, C.J. The clinical value of rapid diagnosis of influenza virus. Int. J. Lab. Med. 2012, 33, 1932–1933. [Google Scholar]
- Chen, C.; Fan, Q.Q.; Chen, G.; Zhu, M.Q.; Wu, J.; Zhang, W.H.; Jin, J.L. A new silver amplification immunochromatography system compared with conventional rapid antigen assay for the diagnosis of influenza A and B. Zhongguo Gan Ran Yu Hua Liao Za Zhi 2017, 17, 29–32. [Google Scholar]
- Zhang, X.; Yan, H.P.; Ma, Y.X.; Wang, Y.; Zhao, Y.; Zhang, H.P. Evaluation of nucleic acid amplification assay and rapid antigen assay of nasopharynx swabs and oropharynx swabs from fu-like patients in diagnosis of flu A. Zhonghua Chuan Ran Bing Za Zhi 2011, 29, 154–157. [Google Scholar]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 10 September 2019).
- McKenzie, J.E.; Beller, E.M.; Forbes, A.B. Introduction to systematic reviews and meta-analysis. Respirology 2016, 21, 626–637. [Google Scholar] [PubMed]
- Fu, C.X.; He, Q.; Li, Z.T.; Xu, J.X.; Hu, W.H.; Dong, Z.Q.; Liu, X.X.; Zhang, C.H.; Zhang, D.F.; Nie, J.; et al. A case-cohort study of the protection of the children against influenza after the licensure of influenza vaccine in 2010–2011. Re Dai Yi Xue Za Zhi 2013, 13, 301–303. [Google Scholar]
- Fu, C.X.; He, Q.; Li, Z.T.; Xu, J.X.; Li, Y.Q.; Lu, J.Y.; Li, K.B.; Yang, Q.Y.; Dong, Z.Q.; Liu, X.Y.; et al. Seasonal influenza vaccine effectiveness among children, 2010–2012. Influenza Other Respir. Viruses 2013, 7, 1168–1174. [Google Scholar] [CrossRef]
- He, Q.; Li, Z.T.; Lu, J.Y.; Li, K.B.; Xu, J.X.; Hu, W.H.; Dong, Z.Q.; Zhang, C.H.; Wang, M.; Zhang, D.F.; et al. A case-control study of seasonal influenza vaccine effectiveness for A(H1N1)pdm09 among children. Re Dai Yi Xue Za Zhi 2014, 14, 599–602. [Google Scholar]
- Fu, C.X.; Xu, J.X.; Lin, J.Y.; Wang, M.; Li, K.B.; Ge, J.; Thompson, M.G. Concurrent and cross-season protection of inactivated influenza vaccine against A(H1N1)pdm09 illness among young children: 2012–2013 case-control evaluation of influenza vaccine effectiveness. Vaccine 2015, 33, 2917–2921. [Google Scholar] [CrossRef]
- He, Q.; Xu, J.Q.; Wang, M.; Shen, J.C.; Zhang, C.H.; Fu, C.X. A case-control study of effectiveness of seasonal influenza vaccine in children in 2013–2014. Zhong Hua Ji Bing Kong Zhi Za Zhi 2016, 20, 1018–1021. [Google Scholar]
- Fu, C.X.; Greene, C.M.; He, Q.; Liao, Y.; Wan, Y.M.; Shen, J.C.; Rong, C.; Zhou, S.Z. Dose effect of influenza vaccine on protection against laboratory-confirmed influenza illness among children aged 6 months to 8 years of age in southern China, 2013/14–2015/16 seasons: A matched case-control study. Hum. Vaccin Immunother. 2020, 16, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Thompson, M.G.; Ma, C.N.; Shi, W.X.; Wu, S.S.; Zhang, D.T.; Wang, Q.Y. Influenza vaccine effectiveness against medically-attended influenza illness during the 2012–2013 season in Beijing, China. Vaccine 2014, 32, 5285–5289. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, Y.; Ma, C.N.; Duan, W.; Sun, Y.; Wu, S.S.; Zhang, M.; Tian, Y.; Zheng, Y.; Yang, P.; et al. Moderate influenza vaccine effectiveness against influenza A(H1N1)pdm09 virus and low effectiveness against A(H3N2) virus among older adults during 2013–2014 influenza season in Beijing, China. Hum. Vaccin Immunother. 2018, 14, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhang, Y.; Wu, P.; Feng, S.; Zheng, J.D.; Yang, P.; Pan, Y.; Wang, Q.Y.; Feng, L.Z.; Pang, X.H.; et al. Influenza vaccine effectiveness in preventing hospitalization among Beijing residents in China, 2013–2015. Vaccine 2016, 34, 2329–2333. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, P.; Thompson, M.G.; Pan, Y.; Ma, C.N.; Wu, S.S.; Sun, Y.; Zhang, M.; Duan, W.; Wang, Q.Y. Influenza Vaccine Effectiveness in Preventing Influenza Illness among Children during School-Based Outbreaks in the 2014–2015 Season in Beijing, China. Pediatr. Infect. Dis. J. 2017, 36, e69–e75. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.N.; Pan, Y.; Zhang, L.; Zhang, Y.; Wu, S.S.; Sun, Y.; Duan, W.; Zhang, M.; Wang, Q.Y.; Yang, P. Influenza vaccine effectiveness against medically attended influenza illness in Beijing, China, 2014/15 season. Hum. Vaccin Immunother. 2017, 13, 2379–2384. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, P.; Feng, L.Z.; Yang, P.; Pan, Y.; Feng, S.; Qin, Y.; Zheng, J.D.; Puig-Barberà, J.; Muscatello, D. Influenza vaccine effectiveness against influenza-associated hospitalization in 2015/16 season, Beijing, China. Vaccine 2017, 35, 3129–3134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pan, Y.; Hackert, V.; van der Hoek, W.; Meijer, A.; Krafft, T.; Yang, P.; Wang, Q.Y. The 2015–2016 influenza epidemic in Beijing, China: Unlike elsewhere, circulation of influenza A(H3N2) with moderate vaccine effectiveness. Vaccine 2018, 36, 4993–5001. [Google Scholar] [CrossRef]
- Wu, S.S.; Pan, Y.; Zhang, X.X.; Zhang, L.; Duan, W.; Ma, C.N.; Zhang, Y.; Zhang, M.; Sun, Y.; Yang, P.; et al. Influenza vaccine effectiveness in preventing laboratory-confirmed influenza in outpatient settings: A test-negative case-control study in Beijing, China, 2016/17 season. Vaccine 2018, 36, 5774–5780. [Google Scholar] [CrossRef]
- Zhang, D.T.; Chu, Y.H.; Li, H.J.; Zhao, X.J.; Liu, Z.C.; Zhou, L.; Song, X.X.; Chen, Y.L.; Han, J.T.; Liang, J.B.; et al. Effectiveness of seasonal infuenza vaccine against severe acute respiratory infections. Guo Ji Bing Du Xue Za Zhi 2019, 26, 77–81. [Google Scholar]
- Zhang, L.; van der Hoek, W.; Krafft, T.; Pilot, E.; Asten, L.V.; Lin, G.; Wu, S.; Duan, W.; Yang, P.; Wang, Q. Influenza vaccine effectiveness estimates against influenza A(H3N2) and A(H1N1)pdm09 among children during school-based outbreaks in the 2016–2017 season in Beijing, China. Hum. Vaccin Immunother. 2020, 16, 816–822. [Google Scholar] [CrossRef]
- Zhang, D.T.; Zhang, Y.; Wang, Q.Y.; Lock, J.; Pan, Y.; Cui, S.; Yang, P.; Hu, Y. The effectiveness of influenza vaccination in preventing hospitalizations in elderly in Beijing, 2016–18. Vaccine 2019, 37, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.Y.; Zhu, J.L.; Lv, M.Z.; Hu, Y.Q.; Cheng, H.; Zhang, G.M.; Chen, G.S.; Wu, X.H. Evaluation of the influenza vaccine effectiveness among children aged 6 to 72 months based on the test-negative case control study design. Zhong Hua Yu Fang Yi Xue Za Zhi 2019, 53, 576–580. [Google Scholar]
- Wang, Y.; Zhang, T.; Chen, L.L.; Greene, C.; Ding, Y.F.; Cheng, Y.; Yang, C.; Zeng, S.S.; Hua, J.; Zhou, S.Z. Seasonal influenza vaccine effectiveness against medically attended influenza illness among children aged 6–59 months, October 2011–September 2012: A matched test-negative case-control study in Suzhou, China. Vaccine 2016, 34, 2460–2465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.L.; Cheng, Y.J.; Zhou, S.Z.; Pang, Y.Y.; Zhang, J.; Greene, C.M.; Song, Y.; Zhang, T.; Zhao, G.M. Potential impact of B lineage mismatch on trivalent influenza vaccine effectiveness during the 2015–2016 influenza season among nursery school children in Suzhou, China. Hum. Vaccin Immunother. 2018, 14, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.L.; Yu, J.; Pang, Y.Y.; Zhang, J.; Zhang, T.; Zhao, G.M. The effectiveness of influenza vaccination among nursery school children in China during the 2016/17 influenza season. Vaccine 2018, 36, 2456–2461. [Google Scholar] [CrossRef]
- Eisenberg, K.W.; Szilagyi, P.G.; Fairbrother, G.; Griffin, M.R.; Staat, M.; Shone, L.P.; Weinberg, G.A.; Hall, C.B.; Poehling, K.A.; Edwards, K.M.; et al. Vaccine effectiveness against laboratory-confirmed influenza in children 6 to 59 months of age during the 2003–2004 and 2004–2005 influenza seasons. Pediatrics 2008, 122, 911–919. [Google Scholar] [CrossRef]
- Staat, M.A.; Griffin, M.R.; Donauer, S.; Edwards, K.M.; Szilagyi, P.G.; Weinberg, G.A.; Hall, C.B.; Prill, M.M.; Chaves, S.S.; Bridges, C.B.; et al. Vaccine effectiveness for laboratory-confirmed influenza in children 6–59 months of age, 2005–2007. Vaccine 2011, 29, 9005–9011. [Google Scholar] [CrossRef]
- Colucci, M.E.; Affanni, P.; Cantarelli, A.; Caruso, L.; Bracchi, M.T.; Capobianco, E.; Zoni, R.; Paini, G.; Odone, A.; Mohieldin, M.I.M.M.; et al. Influenza vaccine effectiveness in children: A retrospective study on eight post-pandemic seasons with trivalent inactivated vaccine. Acta Biomed. 2020, 91, 63–70. [Google Scholar]
- Derhovanessian, E.; Pawelec, G. Vaccination in the elderly. Microb. Biotechnol. 2012, 5, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Parodi, V.; de Florentiis, D.; Martini, M.; Filippo, A. Inactivated Influenza Vaccines. Drugs Aging 2011, 28, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Smetana, J.; Chlibek, R.; Shaw, J.; Splino, J.; Prymula, R. Influenza vaccination in the elderly. Hum. Vaccines Immunother. 2017, 14, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, K.; Viboud, C.; Simonsen, L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine 2006, 24, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Interim results: State-specific seasonal influenza vaccination coverage—United States, August 2009–January 2010. MMWR Morb. Mortal. Wkly. Rep. 2010, 59, 477–484. [Google Scholar]
- Wilkinson, K.; Wei, Y.C.; Szwajcer, A.; Rabbani, R.; Zarychanski, R.; Abou-Setta, A.M.; Mahmud, S.M. Efficacy and safety of high-dose influenza vaccine in elderly adults: A systematic review and meta-analysis. Vaccine 2017, 35, 2775–2780. [Google Scholar] [CrossRef]
- Falsey, A.R.; Treanor, J.J.; Tornieporth, N.; Capellan, J.; Gorse, G.J. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J. Infect. Dis. 2009, 200, 172–180. [Google Scholar] [CrossRef]
- Couch, R.B.; Winokur, P.; Brady, R.; Belshe, R.; Chen, W.H.; Cate, T.R.; Sigurdardottir, B.; Hoeper, A.; Graham, I.L.; Edelman, R.; et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 2007, 25, 7656–7663. [Google Scholar] [CrossRef]
- DiazGranados, C.A.; Dunning, A.J.; Kimmel, M.; Kirby, D.; Treanor, J.; Collins, A.; Pollak, R.; Christoff, J.; Earl, J.; Landolfi, V.; et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N. Engl. J. Med. 2014, 371, 635–645. [Google Scholar] [CrossRef]
- Shay, D.K.; Chillarige, Y.; Kelman, J.; Forshee, R.A.; Foppa, I.M.; Wernecke, M.; Lu, Y.; Ferdinands, J.M.; Iyengar, A.; Fry, A.M.; et al. Comparative Effectiveness of High-Dose Versus Standard-Dose Influenza Vaccines among US Medicare Beneficiaries in Preventing Post-Influenza Deaths During 2012–2013 and 2013–2014. J. Infect. Dis. 2017, 215, 510–517. [Google Scholar] [CrossRef]
- Chit, A.; Becker, D.L.; DiazGranados, C.A.; Maschio, M.; Yau, E.; Drummond, M. Cost-effectiveness of high-dose versus standard-dose inactivated influenza vaccine in adults aged 65 years and older: An economic evaluation of data from a randomised controlled trial. Lancet Infect. Dis. 2015, 15, 1459–1466. [Google Scholar] [CrossRef]
- Izurieta, H.S.; Thadani, N.; Shay, D.K.; Lu, Y.; Maurer, A.; Foppa, I.M.; Franks, R.; Pratt, D.; Forshee, R.A.; MaCurdy, T.; et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in US residents aged 65 years and older from 2012 to 2013 using Medicare data: A retrospective cohort analysis. Lancet Infect. Dis. 2015, 15, 293–300. [Google Scholar] [CrossRef]
- Feng, S.; Cowling, B.J.; Sullivan, S.G. Influenza vaccine effectiveness by test-negative design—Comparison of inpatient and outpatient settings. Vaccine 2016, 34, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Castilla, J.; Martínez-Baz, I.; Navascués, A.; Casado, I.; Aguinaga, A.; Díaz-González, J.; Delfrade, J.; Guevara, M.; Ezpeleta, C. Comparison of influenza vaccine effectiveness in preventing outpatient and inpatient influenza cases in older adults, northern Spain, 2010/11 to 2015/16. Euro Surveill. 2018, 23, 16-00780. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Chung, J.; Smith, E.R.; Talbot, H.K.; Trabue, C.H.; Zimmerman, R.K.; Silveira, F.P.; Gaglani, M.; Murthy, K.; Monto, A.S.; et al. Influenza vaccine effectiveness in inpatient and outpatient settings in the United States, 2015–2018. Clin. Infect. Dis. 2020, ciaa407. [Google Scholar] [CrossRef] [PubMed]
- Ainslie, K.E.C.; Haber, M.; Orenstein, W.A. Challenges in estimating influenza vaccine effectiveness. Expert Rev. Vaccines 2019, 18, 615–628. [Google Scholar] [CrossRef] [PubMed]

| (Ref.) | Published Year | Study Period | Study Place | Study Objects | Study Method | Vaccination Rate in Case Group | Vaccination Rate in Control Group |
|---|---|---|---|---|---|---|---|
| [19] | 2013 | 2010/09–2011/09 | Guang Zhou | 6 months~5 years community children | case–control study | 16.9% (52/308) | 36.0% (279/774) |
| [20] | 2013 | 2011/01–2011/06 | Guang Zhou | 6 months~5 years community children | 1:2 case–control study | 12.4% (26/209) | 27.3% (114/418) |
| [20] | 2013 | 2012/01–2012/06 | Guang Zhou | 6 months~5 years community children | 1:2 case–control study | 19.9% (208/1046) | 30.9% (647/2092) |
| [21] | 2014 | 2013/02–2013/06 | Guang Zhou | 6 months~5 years community children | 1:1 case–control study | 13.1% (164/1250) | 24.7% (309/1250) |
| [22] | 2015 | 2013/02–2013/06 | Guang Zhou | 8 months~6 years community children | 1:1 case–control study | 9.0% (158/1754) | 19.6% (343/1754) |
| [23] | 2016 | 2014/02–2014/07 | Guang Zhou | 6 months~5 years community children | 1:2 case–control study | 14.0% (123/879) | 21.5% (385/1793) |
| [24] | 2019 | 2014/02–2014/07 | Guang Zhou | 6 months~8 years community children | 1:1 case–control study | 12.5% (102/819) | 23.0% (188/819) |
| [24] | 2019 | 2015/03–2015/07 | Guang Zhou | 6 months~8 years community children | 1:1 case–control study | 12.0% (248/2080) | 14.8% (308/2080) |
| [24] | 2019 | 2016/03–2016/05 | Guang Zhou | 6 months~8 years community children | 1:1 case–control study | 7.9% (101/1286) | 12.0% (154/1286) |
| [25] * | 2014 | 2012/12–2013/01 | Bei Jing | Outpatients of all age groups | Test-negative design | 2.0% (14/695) | 4.4% (57/1303) |
| [26] | 2018 | 2013/11–2014/04 | Bei Jing | Outpatients of more than 60 years old | Test-negative design | 6.8% (9/133) | 10.2% (36/354) |
| [27] | 2016 | 2013/12–2015/05 | Bei Jing | Inpatients of all age groups | Test-negative design | 11.9% (42/353) | 12.6% (173/1372) |
| [28] | 2017 | 2014/11–2014/12 | Bei Jing | 6~18 years old students | case–control study | 43.3% (91/210) | 40.8% (692/1698) |
| [29] | 2017 | 2014/11–2015/04 | Bei Jing | Outpatients of all age groups | Test-negative design | 4.3% (149/3434) | 3.7% (215/5863) |
| [30] * | 2017 | 2015/10–2016/05 | Bei Jing | Outpatients of all age groups | Test-negative design | 13.4% (47/351) | 11.8% (207/1761) |
| [31] | 2018 | 2015/11–2016/03 | Bei Jing | Outpatients of all age groups | Test-negative design | 4.2% (124/2969) | 4.3% (342/8031) |
| [32] | 2018 | 2016/11–2017/04 | Bei Jing | Outpatients of all age groups | Test-negative design | 2.9% (75/2626) | 3.6% (278/7816) |
| [33] | 2019 | 2016/11–2017/04 | Bei Jing | Outpatients of all age groups | Test-negative design | 5.6% (18/322) | 7.2% (137/1905) |
| [34] | 2019 | 2016/11–2017/04 | Bei Jing | 6~18 years old students | case–control study | 17.0% (30/175) | 36.5% (229/628) |
| [35] | 2019 | 2016/11–2017/04 | Bei Jing | Inpatients of more than 60 years old | Test-negative design | 11.0% (16/145) | 13.3% (70/528) |
| [35] | 2019 | 2017/11–2018/04 | Bei Jing | Inpatients of more than 60 years old | Test-negative design | 12.8% (19/149) | 17.1% (56/328) |
| [36] | 2019 | 2016/10–2017/04 | Zhe Jiang | Outpatients of 6months~6 years | Test-negative design | 6.1% (17/277) | 10.9% (41/375) |
| [36] | 2019 | 2017/10–2018/04 | Zhe Jiang | Outpatients of 6months~6 years | Test-negative design | 4.0% (5/126) | 9.9% (38/383) |
| [37] | 2016 | 2011/10–2012/09 | Su Zhou | 6 months~5 years community children | 1:1 case–control study | 4.2% (18/427) | 11.5% (49/427) |
| [38] * | 2018 | 2015/10–2016/02 | Su Zhou | Kindergarten children aged 3–6 years | Test-negative design | 10.3%(17/165) * | 11.3% (23/203) * |
| [39] * | 2018 | 2016/10–2017/02 | Su Zhou | Kindergarten children aged 3–6 years | Test-negative design | 10.6%(17/160) * | 13.3% (53/398) * |
| [Ref.] | Published Year | Selection | Comparability | Exposure | Total Scores | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Is the Case Definition Adequate? | Representativeness of the Cases | Selection of Controls | Definition of Controls | Comparability of Cases and Controls on the Basis of the Design or Analysis | Ascertainment of Exposure | Same Method of Ascertainment for Cases and Controls | Non-Response Rate | |||
| [19] | 2013 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 7 |
| [20] | 2013 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 7 |
| [20] | 2013 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 7 |
| [21] | 2014 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 7 |
| [22] | 2015 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 7 |
| [23] | 2016 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 7 |
| [24] | 2019 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 7 |
| [24] | 2019 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 7 |
| [24] | 2019 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 7 |
| [25] | 2014 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 5 |
| [26] | 2018 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 5 |
| [27] | 2016 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 5 |
| [28] | 2017 | 1 | 0 | 1 | 0 | 2 | 1 | 1 | 0 | 6 |
| [29] | 2017 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 5 |
| [30] | 2017 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 5 |
| [31] | 2018 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 5 |
| [32] | 2018 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 5 |
| [33] | 2019 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 5 |
| [34] | 2019 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| [35] | 2019 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 5 |
| [35] | 2019 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 5 |
| [36] | 2019 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 5 |
| [36] | 2019 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 5 |
| [37] | 2016 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 7 |
| [38] | 2018 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 5 |
| [39] | 2018 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 5 |
| Study Subjects | Heterogeneity Test: χ2 (P) | I2 | Model Selection | OR (95%CI) | VE (95%CI) |
|---|---|---|---|---|---|
| Receipt of Two Doses of Vaccine * | |||||
| Children 6–35 months receiving one dose | 20.8 (0.0009) | 76% | random-effects model | 0.55 (0.36–0.82) | 45% (18–64%) |
| Children 6–35 months receiving two doses | 4.76 (0.45) | 0 | fixed-effects model | 0.43 (0.36–0.50) | 57% (50–64%) |
| Age Groups | |||||
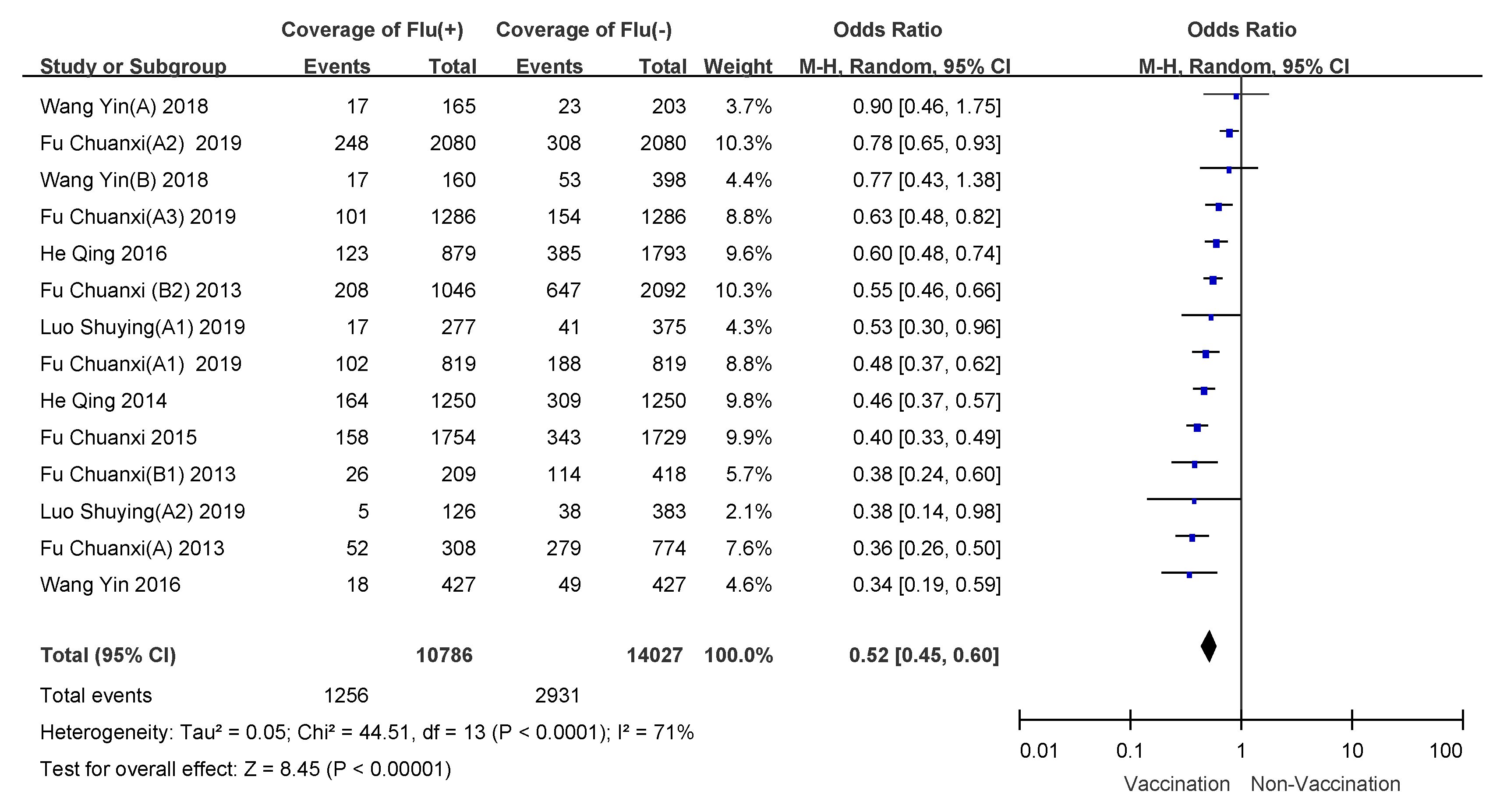
| 6 months to 8 years | 37.4 (0.0003) | 65% | random-effects model | 0.53 (0.46–0.61) | 47% (39–54%) |
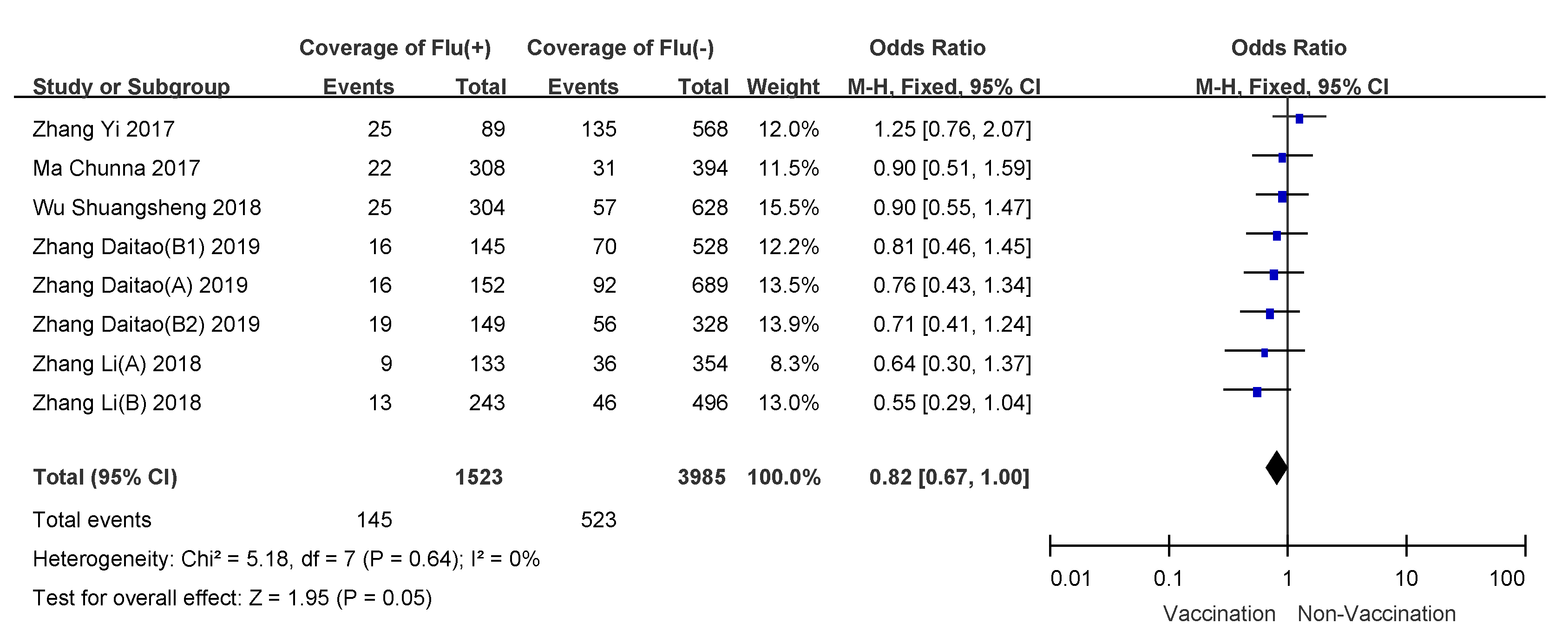
| More than 60 years | 5.18 (0.64) | 0 | fixed-effects model | 0.82 (0.67–1.00) | 18% (0–33%) |
| Disease Severity ** | |||||
| inpatients | 11.74 (0.02) | 66% | random-effects model | 0.79 (0.56–1.11) | 21% (−11–44%) |
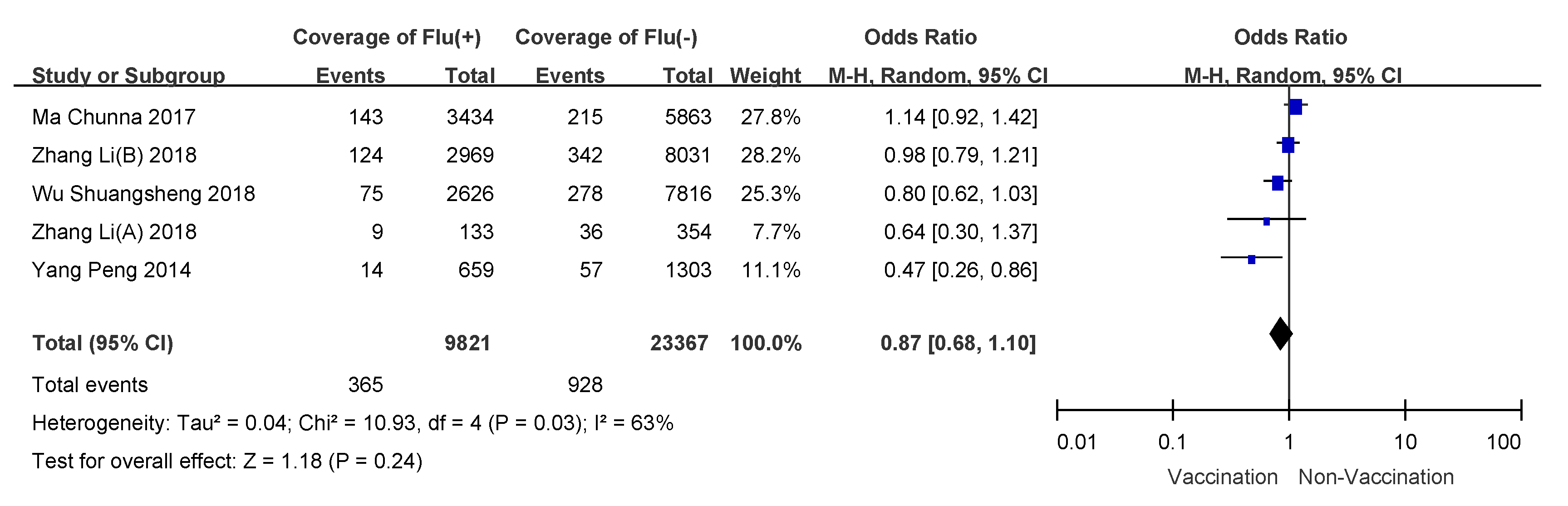
| outpatients | 10.93 (0.03) | 63% | random-effects model | 0.87(0.68–1.10) | 13% (−10–32%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhao, H.; Li, Z.; Zhu, A.; Ren, M.; Geng, M.; Li, Y.; Qin, Y.; Feng, L.; Peng, Z.; et al. Influenza Vaccine Effectiveness in Mainland China: A Systematic Review and Meta-Analysis. Vaccines 2021, 9, 79. https://doi.org/10.3390/vaccines9020079
Yang X, Zhao H, Li Z, Zhu A, Ren M, Geng M, Li Y, Qin Y, Feng L, Peng Z, et al. Influenza Vaccine Effectiveness in Mainland China: A Systematic Review and Meta-Analysis. Vaccines. 2021; 9(2):79. https://doi.org/10.3390/vaccines9020079
Chicago/Turabian StyleYang, Xiaokun, Hongting Zhao, Zhili Li, Aiqin Zhu, Minrui Ren, Mengjie Geng, Yu Li, Ying Qin, Luzhao Feng, Zhibin Peng, and et al. 2021. "Influenza Vaccine Effectiveness in Mainland China: A Systematic Review and Meta-Analysis" Vaccines 9, no. 2: 79. https://doi.org/10.3390/vaccines9020079
APA StyleYang, X., Zhao, H., Li, Z., Zhu, A., Ren, M., Geng, M., Li, Y., Qin, Y., Feng, L., Peng, Z., An, Z., Zheng, J., Li, Z., & Feng, Z. (2021). Influenza Vaccine Effectiveness in Mainland China: A Systematic Review and Meta-Analysis. Vaccines, 9(2), 79. https://doi.org/10.3390/vaccines9020079






