COVID-19 Vaccination in Patients with Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension: Safety Profile and Reasons for Opting against Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. Study Group
3.2. Reasons and Factors Associated with Unwillingness to Vaccinate against COVID-19 in PAH/CTEPH Patients
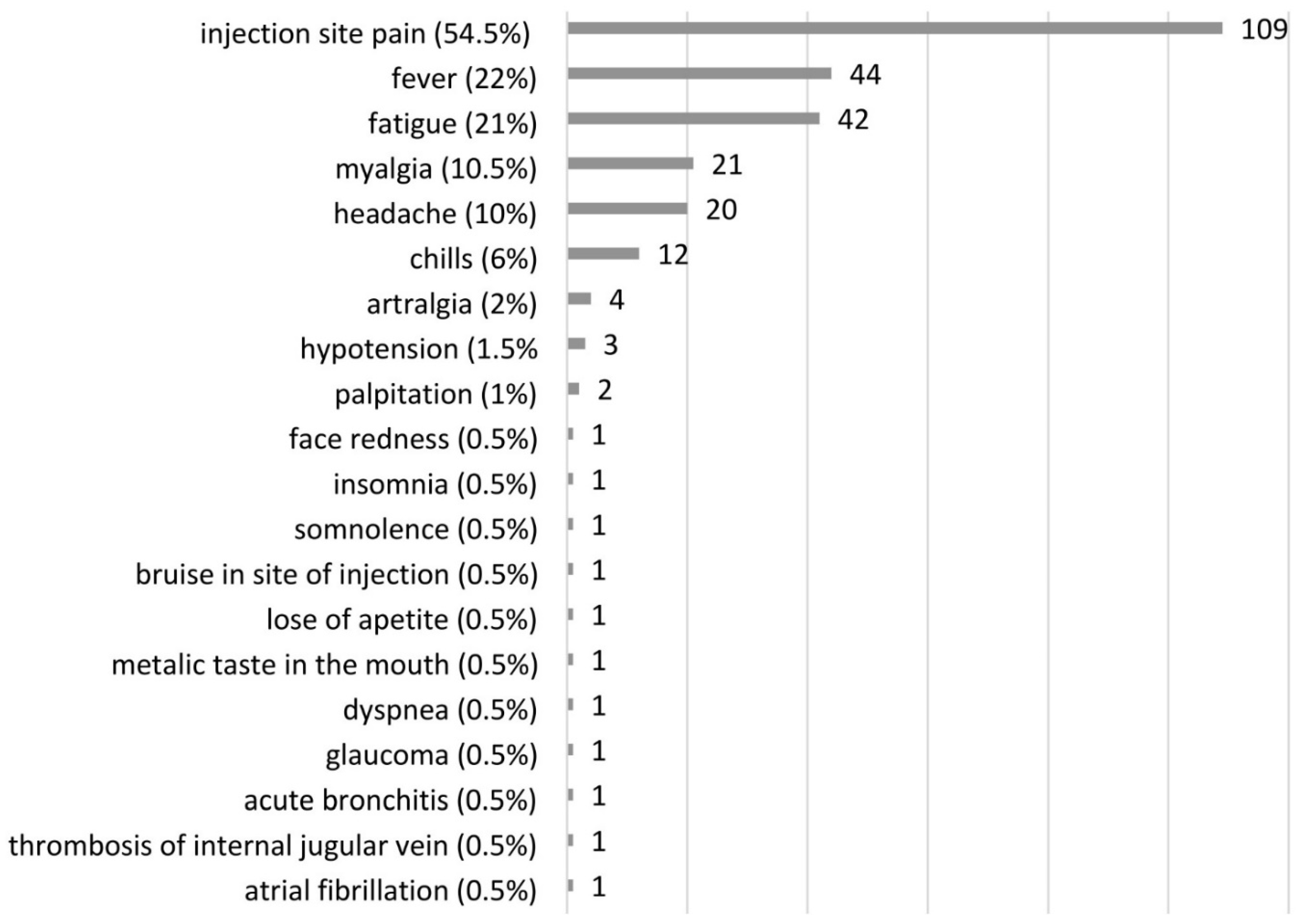
3.3. Adverse Events after COVID-19 Vaccination in PAH and CTEPH Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Kopec, G.; Kurzyna, M.; Mroczek, E.; Chrzanowski, L.; Mularek-Kubzdela, T.; Skoczylas, I.; Kusmierczyk, B.; Pruszczyk, P.; Blaszczak, P.; Lewicka, E.; et al. Characterization of Patients with Pulmonary Arterial Hypertension: Data from the Polish Registry of Pulmonary Hypertension (BNP-PL). J. Clin. Med. 2020, 9, 173. [Google Scholar] [CrossRef] [Green Version]
- Wieteska, M.; Biederman, A.; Kurzyna, M.; Dyk, W.; Burakowski, J.; Wawrzynska, L.; Szturmowicz, M.; Fijalkowska, A.; Szatkowski, P.; Torbicki, A. Outcome of Medically Versus Surgically Treated Patients With Chronic Thromboembolic Pulmonary Hypertension. Clin. Appl. Thromb. Hemost. 2016, 22, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopec, G.; Dzikowska-Diduch, O.; Mroczek, E.; Mularek-Kubzdela, T.; Chrzanowski, L.; Skoczylas, I.; Tomaszewski, M.; Peregud-Pogorzelska, M.; Karasek, D.; Lewicka, E.; et al. Characteristics and outcomes of patients with chronic thromboembolic pulmonary hypertension in the era of modern therapeutic approaches: Data from the Polish multicenter registry (BNP-PL). Ther. Adv. Chronic Dis. 2021, 12, 20406223211002961. [Google Scholar] [CrossRef]
- Godinas, L.; Iyer, K.; Meszaros, G.; Quarck, R.; Escribano-Subias, P.; Vonk Noordegraaf, A.; Jansa, P.; D’Alto, M.; Luknar, M.; Milutinov Ilic, S.; et al. PH CARE COVID survey: An international patient survey on the care for pulmonary hypertension patients during the early phase of the COVID-19 pandemic. Orphanet J. Rare Dis. 2021, 16, 196. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, Y.V.; Gillies, C.L.; Zaccardi, F.; Coles, B.; Davies, M.J.; Seidu, S.; Khunti, K. Impact of COVID-19 on routine care for chronic diseases: A global survey of views from healthcare professionals. Diabetes Metab. Syndr. 2020, 14, 965–967. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Burger, C.D.; Delossantos, G.B.; Grinnan, D.; Ralph, D.D.; Rayner, S.G.; Ryan, J.J.; Safdar, Z.; Ventetuolo, C.E.; Zamanian, R.T.; et al. A Survey-based Estimate of COVID-19 Incidence and Outcomes among Patients with Pulmonary Arterial Hypertension or Chronic Thromboembolic Pulmonary Hypertension and Impact on the Process of Care. Ann. Am. Thorac. Soc. 2020, 17, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Belge, C.; Quarck, R.; Godinas, L.; Montani, D.; Escribano Subias, P.; Vachiery, J.L.; Nashat, H.; Pepke-Zaba, J.; Humbert, M.; Delcroix, M. COVID-19 in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: A reference centre survey. ERJ Open Res. 2020, 6, 00520–2020. [Google Scholar] [CrossRef]
- Ahorsu, D.K.; Lin, C.Y.; Imani, V.; Saffari, M.; Griffiths, M.D.; Pakpour, A.H. The Fear of COVID-19 Scale: Development and Initial Validation. Int J. Ment. Health Addict. 2020, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pilch, I.; Kurasz, Z.; Turska-Kawa, A. Experiencing fear during the pandemic: Validation of the fear of COVID-19 scale in Polish. PeerJ 2021, 9, e11263. [Google Scholar] [CrossRef]
- Open Science Framework. Available online: https://osf.io/39jr8/ (accessed on 21 November 2020).
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Wichowicz, H.M.; Wieczorek, D. Screening post-stroke depression using the Hospital Anxiety and Depression Scale. Psychiatr. Pol. 2011, 45, 505–514. [Google Scholar]
- Wieteska-Miłek, M.S.S.; Florczyk, M.; Kuśmierczyk-Droszcz, B.; Ryczek, R.; Dzienisiewicz, M.; Torbicki, A.; Kurzyna, M. Fear of COVID-19, Anxiety and Depression in Patients with Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension during the Pandemic. J. Clin. Med. 2021, 10, 15. [Google Scholar] [CrossRef]
- Bhatia, R.T.; Gati, S.; Papadakis, M.; Sharma, S. The Impact of COVID-19 on the Continuity of Cardiovascular Care. Eur. Heart J. 2021, 42, 215–217. [Google Scholar] [CrossRef]
- Roncalli, J.; Roubille, F.; Lamblin, N.; Girerd, N.; Mouquet, F.; Chapet, N.; Roubille, C.; Berthelot, E.; Galois, K.; Battistella, P.; et al. Coronavirus disease vaccination in heart failure: No time to waste. Arch. Cardiovasc. Dis. 2021, 114, 434–438. [Google Scholar] [CrossRef]
- Girerd, N.; Chapet, N.; Roubille, C.; Roncalli, J.; Salvat, M.; Mouquet, F.; Lamblin, N.; Gueffet, J.P.; Damy, T.; Galinier, M.; et al. Vaccination for Respiratory Infections in Patients with Heart Failure. J. Clin. Med. 2021, 10, 4311. [Google Scholar] [CrossRef] [PubMed]
- Driggin, E.; Maddox, T.M.; Ferdinand, K.C.; Kirkpatrick, J.N.; Ky, B.; Morris, A.A.; Mullen, J.B.; Parikh, S.A.; Philbin, D.M., Jr.; Vaduganathan, M. ACC Health Policy Statement on Cardiovascular Disease Considerations for COVID-19 Vaccine Prioritization: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 77, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Szarowska, A.; Zaczynski, A.; Szymanski, P.; Borawska, B.; Szarek, I.; Szoszkiewicz, I.; Butkiewicz, S.; Szydlarska, D.; Gil, R.; Sliwczynski, A.; et al. Initiation of the COVID-19 vaccination program in Poland: Vaccination of the patient “zero” and first experience from the Central Clinical Hospital of the Ministry of Internal Affairs and Administration. Pol. Arch. Intern. Med. 2021, 131, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Website of the Republic of Poland. Narodowy Program Szczepień Przeciw COVID-19. Available online: https://www.gov.pl/web/szczepimysie/narodowy-program-szczepien-przeciw-covid-19 (accessed on 19 October 2021).
- Statistics and Research. Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations?country=POL (accessed on 19 October 2021).
- Oehler, D.; Bruno, R.R.; Boeken, U.; Westenfeld, R. Moderate acceptance of COVID-19 vaccination in patients pre- and post-heart transplantation: Experiences from a German Transplant Centre. Transpl. Infect. Dis. 2021, 23, e13681. [Google Scholar] [CrossRef]
- Brodziak, A.; Sigorski, D.; Osmola, M.; Wilk, M.; Gawlik-Urban, A.; Kiszka, J.; Machulska-Ciuraj, K.; Sobczuk, P. Attitudes of Patients with Cancer towards Vaccinations-Results of Online Survey with Special Focus on the Vaccination against COVID-19. Vaccines 2021, 9, 411. [Google Scholar] [CrossRef]
- Babicki, M.; Mastalerz-Migas, A. Attitudes toward Vaccination against COVID-19 in Poland. A Longitudinal Study Performed before and Two Months after the Commencement of the Population Vaccination Programme in Poland. Vaccines 2021, 9, 503. [Google Scholar] [CrossRef] [PubMed]
- Hanrath, A.T.; Payne, B.A.I.; Duncan, C.J.A. Prior SARS-CoV-2 infection is associated with protection against symptomatic reinfection. J. Infect. 2021, 82, e29–e30. [Google Scholar] [CrossRef] [PubMed]
- Breathnach, A.S.; Riley, P.A.; Cotter, M.P.; Houston, A.C.; Habibi, M.S.; Planche, T.D. Prior COVID-19 significantly reduces the risk of subsequent infection, but reinfections are seen after eight months. J. Infect. 2021, 82, e11–e12. [Google Scholar] [CrossRef]
- Sigorski, D.; Sobczuk, P.; Osmola, M.; Kuc, K.; Walerzak, A.; Wilk, M.; Ciszewski, T.; Kopec, S.; Hryn, K.; Rutkowski, P.; et al. Impact of COVID-19 on anxiety levels among patients with cancer actively treated with systemic therapy. ESMO Open 2020, 5, e000970. [Google Scholar] [CrossRef]
- Raciborski, F.; Samel-Kowalik, P.; Gujski, M.; Pinkas, J.; Arcimowicz, M.; Jankowski, M. Factors Associated with a Lack of Willingness to Vaccinate against COVID-19 in Poland: A 2021 Nationwide Cross-Sectional Survey. Vaccines 2021, 9, 1000. [Google Scholar] [CrossRef]
- Yuan, P.; Ai, P.; Liu, Y.; Ai, Z.; Wang, Y.; Cao, W.; Xia, X.; Zheng, J.C. Safety, Tolerability, and Immunogenicity of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. medRxiv 2020. [Google Scholar] [CrossRef]
- Kaur, R.J.; Dutta, S.; Bhardwaj, P.; Charan, J.; Dhingra, S.; Mitra, P.; Singh, K.; Yadav, D.; Sharma, P.; Misra, S. Adverse Events Reported From COVID-19 Vaccine Trials: A Systematic Review. Indian J. Clin. Biochem. 2021, 1–13. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Hatmal, M.M.; Al-Hatamleh, M.A.I.; Olaimat, A.N.; Hatmal, M.; Alhaj-Qasem, D.M.; Olaimat, T.M.; Mohamud, R. Side Effects and Perceptions Following COVID-19 Vaccination in Jordan: A Randomized, Cross-Sectional Study Implementing Machine Learning for Predicting Severity of Side Effects. Vaccines 2021, 9, 556. [Google Scholar] [CrossRef]
- Wise, J. COVID-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ 2021, 372, n699. [Google Scholar] [CrossRef]
- Wall, C.; Moore, J.; Thachil, J. Catheter-related thrombosis: A practical approach. J. Intensive Care Soc. 2016, 17, 160–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, G.A.; Hargroves, D.; Lowe, D.; Hicks, N.; Lip, G.Y.H.; Rooney, G.; Oatley, H. Targeted atrial fibrillation (AF) detection in COVID-19 vaccination clinics. Eur. Heart J. Qual. Care Clin. Outcomes 2021, 7, 526–528. [Google Scholar] [CrossRef] [PubMed]
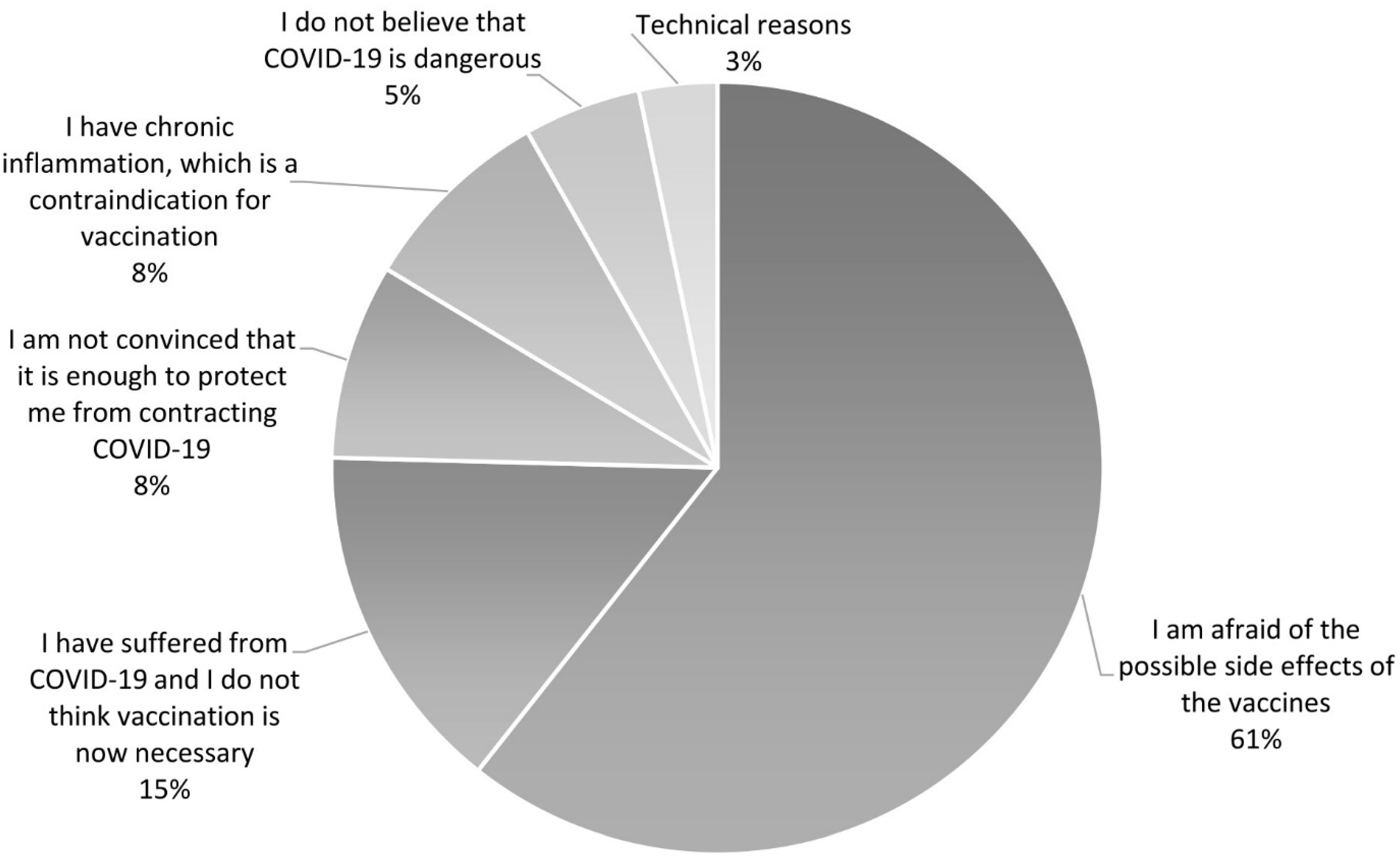
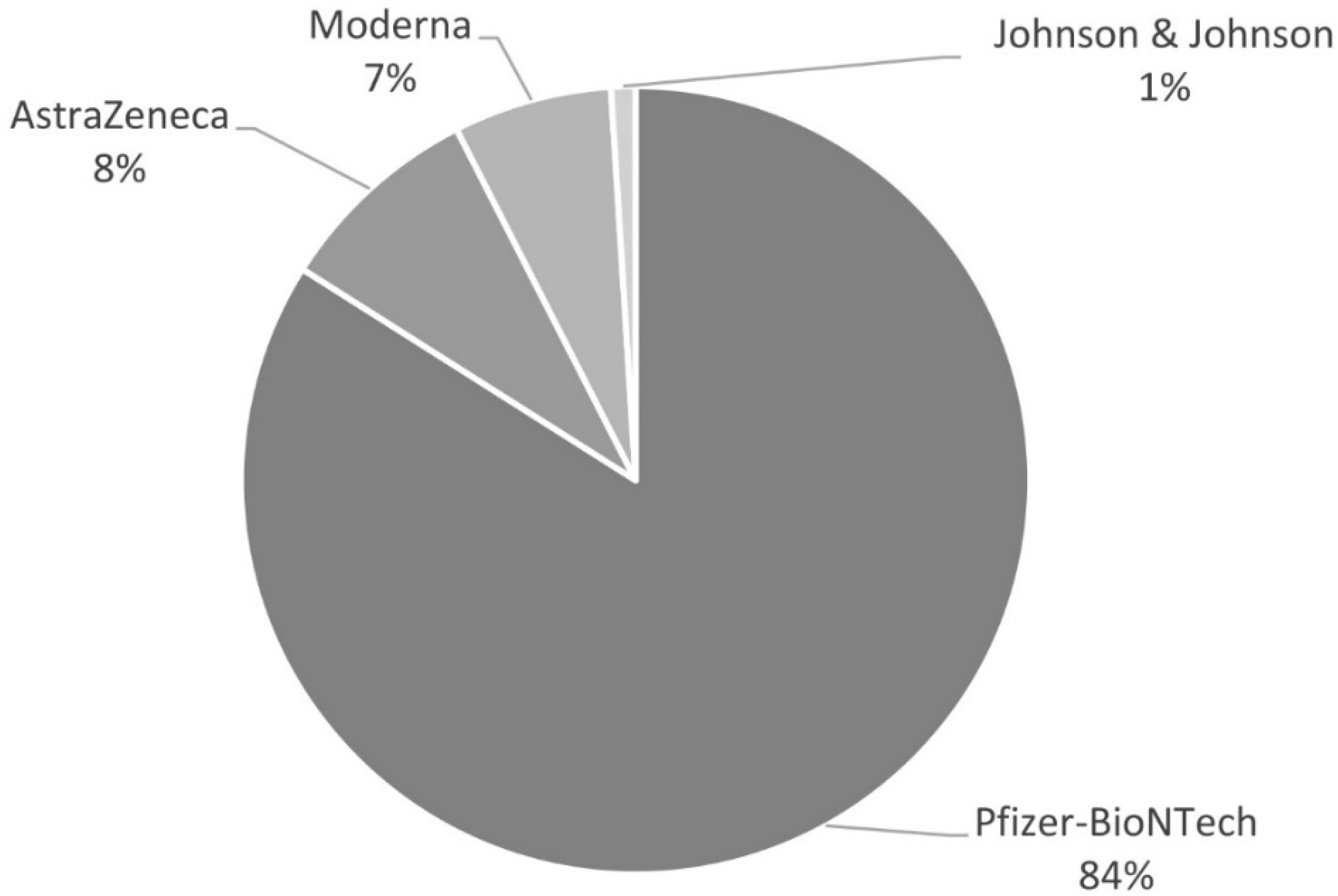
| Total Study Group n (%) or Mean (SD) | Vaccinated n (%) or Mean (SD) | Unvaccinated n (%) or Mean (SD) | p-Value Vaccinated vs. Unvaccinated | |
|---|---|---|---|---|
| Number of patients | 261 (100%) | 200 (77%) | 61 (23%) | |
| Age, years | 60 (18–92) | 62.5 (18–92) | 52.3 (23–87) | 0.005 * |
| Sex, female | 163 (62%) | 126 (63%) | 37 (61%) | 0.78 |
| Duration of disease, years | 7.3 ± 7.1 | 7.5 ± 7.1 | 6.3 ± 7.1 | 0.15 |
| PAH patients | 164 (63%) | 125 (63%) | 39 (64%) | 0.86 |
| Idiopathic PAH | 85 (52%) | 69 (55%) | 24 (41%) | |
| Heritable PAH | 5 (3%) | 3 (2%) | 2 (5%) | |
| PAH associated with CHD | 36 (22%) | 27 (22%) | 9 (23%) | |
| PAH associated with CTD | 30 (18%) | 22 (18%) | 8 (20%) | |
| PAH porto-pulmonary | 6 (4%) | 3 (2%) | 3 (8%) | |
| Drug-induced PAH | 1 (0.5%) | 1 (1%) | 0 | |
| PAH associated with HIV | 1 (0.5%) | 0 | 1 (3%) | |
| PAH monotherapy | 48 (29%) | 38 (30%) | 10 (26%) | 0.76 |
| PAH two drugs | 59 (36%) | 41 (33%) | 18 (46%) | |
| PAH three drugs | 57 (35%) | 46 (37%) | 11 (28%) | |
| CTEPH patients | 97 (37%) | 75 (37%) | 22 (36%) | 0.86 |
| CTEPH-BPA | 73 (75%) | 57 (76%) | 16 (73%) | 0.82 |
| CTEPH-PEA | 22 (23%) | 16 (21%) | 6 (27%) | 0.68 |
| CTEPH monotherapy (riociguat or sildenafil) | 75 (77%) | 56 (75%) | 19 (86%) | 0.41 |
| WHO functional class | 2.4 ± 0.7 | 2.4 ± 0.7 | 2.4 ± 0.8 | 0.92 |
| 1 | 22 (8%) | 15 (8%) | 7 (11%) | |
| 2 | 119 (46%) | 94 (47%) | 25 (41%) | |
| 3 | 108 (41%) | 83 (41%) | 25 (41%) | |
| 4 | 12 (5%) | 8 (4%) | 4 (7%) | |
| COVID-19 disease | 44 (17%) | 31 (16%) | 13 (21%) | 0.49 |
| Anticoagulation | 126 (48.3%) | 99 (49.5%) | 27 (44.3%) | 0.54 |
| Concomitant disease | 165 (63%) | 130 (65%) | 57 (75%) | 0.36 |
| Arterial hypertension | 117 (45%) | 96 (48%) | 40 (53%) | 0.10 |
| Diabetes | 43 (16%) | 35 (18%) | 12 (16%) | 0.60 |
| COPD | 23 (9%) | 19 (10%) | 9 (12%) | 0.73 |
| Coronary artery disease | 44 (17%) | 37 (19%) | 10 (13%) | 0.41 |
| Neoplasm | 29 (11%) | 18 (9%) | 10 (13%) | 0.29 |
| Obesity, BMI ≥ 30 kg/m2 | 70 (27%) | 54 (27%) | 23 (30%) | 0.92 |
| Fear of COVID-19 | 19 (7–35) | 19 (7–35) | 16 (7–35) | 0.037 ** |
| HADS-A ≥ 8 | 78 (30%) | 61 (31%) | 17 (28%) | 0.70 |
| HADS-D ≥ 8 | 51 (20%) | 36 (18%) | 15 (25%) | 0.48 |
| Univariate Analysis OR (95%CI) | p-Value (LR) | Multivariate Analysis OR (95%CI) | p-Value (Wald) | |
|---|---|---|---|---|
| Age ≥ 60 years | 2.7 (1.5–4.9) | 0.0015 * | 2.5 (1.3–4.6) | 0.005 * |
| Female gender | 1.1 (0.6–2.0) | 0.7 | ||
| CTEPH | 1.0 (0.6–1.9) | 0.8 | ||
| WHO functional class 3–4 | 0.9 (0.5–1.6) | 0.7 | ||
| History of COVID-19 | 0.7 (0.3–1.4) | 0.3 | ||
| Presence of concomitant disease | 1.4 (0.8–2.5) | 0.3 | ||
| Fear of COVID-19 ≥ median | 1.8 (1.0–3.3) | 0.049 * | 1.7 (0.9–3.1) | 0.09 |
| HADS-A ≥8 | 1.1 (0.6–2.2) | 0.6 | ||
| HADS-D ≥8 | 0.7 (0.4–1.4) | 0.3 |
| AEs | After First Dose (n = 200) n (%) | After Second Dose (n = 197) n (%) |
|---|---|---|
| Pain at the site of injection | 101 (50.5) | 56 (28.5) |
| fever | 35 (17.5) | 21 (10.5) |
| fatique | 29 (14.5) | 26 (13) |
| myalgia | 16 (8) | 12 (6) |
| chills | 16 (8) | 8 (4) |
| headache | 12 (6) | 11 (6) |
| other | 11 (5.5) | 10 (5) |
| Number of Side Effects | After First Dose (n = 200) n (%) | After Second Dose (n = 197) n (%) | p-Value |
|---|---|---|---|
| 0 | 78 (39) | 119 (59.5) | 0.001 * |
| 1 | 73 (36.5) | 43 (21.5) | |
| 2 | 24 (12) | 22 (11) | |
| 3 | 13 (6.5) | 10 (5) | |
| 4 | 9 (4.5) | 4 (2) | |
| ≥5 | 3 (1.5) | 2 (1) |
| Univariate Analysis OR (95%CI) | p-Value (LR) | Multivariate Analysis OR (95%CI) | p-Value (Wald) | |
|---|---|---|---|---|
| Age ≥ 60 years | 0.2 (0.1–0.5) | 0.0000 * | 0.3 (0.1–0.5) | 0.001 * |
| Female gender | 1.2 (0.7–2.2) | 0.7 | ||
| CTEPH | 0.6 (0.4–1.2) | 0.2 | ||
| WHO functional classes 3 and 4 | 0.5 (0.2–0.9) | 0.03 * | 0.8 (0.4–1.5) | 0.5 |
| Presence of concomitant disease | 0.4 (0.2–0.8) | 0.02 * | 0.7 (0.4–1.5) | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieteska-Miłek, M.; Szmit, S.; Florczyk, M.; Kuśmierczyk-Droszcz, B.; Ryczek, R.; Kurzyna, M. COVID-19 Vaccination in Patients with Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension: Safety Profile and Reasons for Opting against Vaccination. Vaccines 2021, 9, 1395. https://doi.org/10.3390/vaccines9121395
Wieteska-Miłek M, Szmit S, Florczyk M, Kuśmierczyk-Droszcz B, Ryczek R, Kurzyna M. COVID-19 Vaccination in Patients with Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension: Safety Profile and Reasons for Opting against Vaccination. Vaccines. 2021; 9(12):1395. https://doi.org/10.3390/vaccines9121395
Chicago/Turabian StyleWieteska-Miłek, Maria, Sebastian Szmit, Michał Florczyk, Beata Kuśmierczyk-Droszcz, Robert Ryczek, and Marcin Kurzyna. 2021. "COVID-19 Vaccination in Patients with Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension: Safety Profile and Reasons for Opting against Vaccination" Vaccines 9, no. 12: 1395. https://doi.org/10.3390/vaccines9121395
APA StyleWieteska-Miłek, M., Szmit, S., Florczyk, M., Kuśmierczyk-Droszcz, B., Ryczek, R., & Kurzyna, M. (2021). COVID-19 Vaccination in Patients with Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension: Safety Profile and Reasons for Opting against Vaccination. Vaccines, 9(12), 1395. https://doi.org/10.3390/vaccines9121395






