New-Onset and Relapsed Kidney Histopathology Following COVID-19 Vaccination: A Systematic Review
Abstract
:1. Introduction
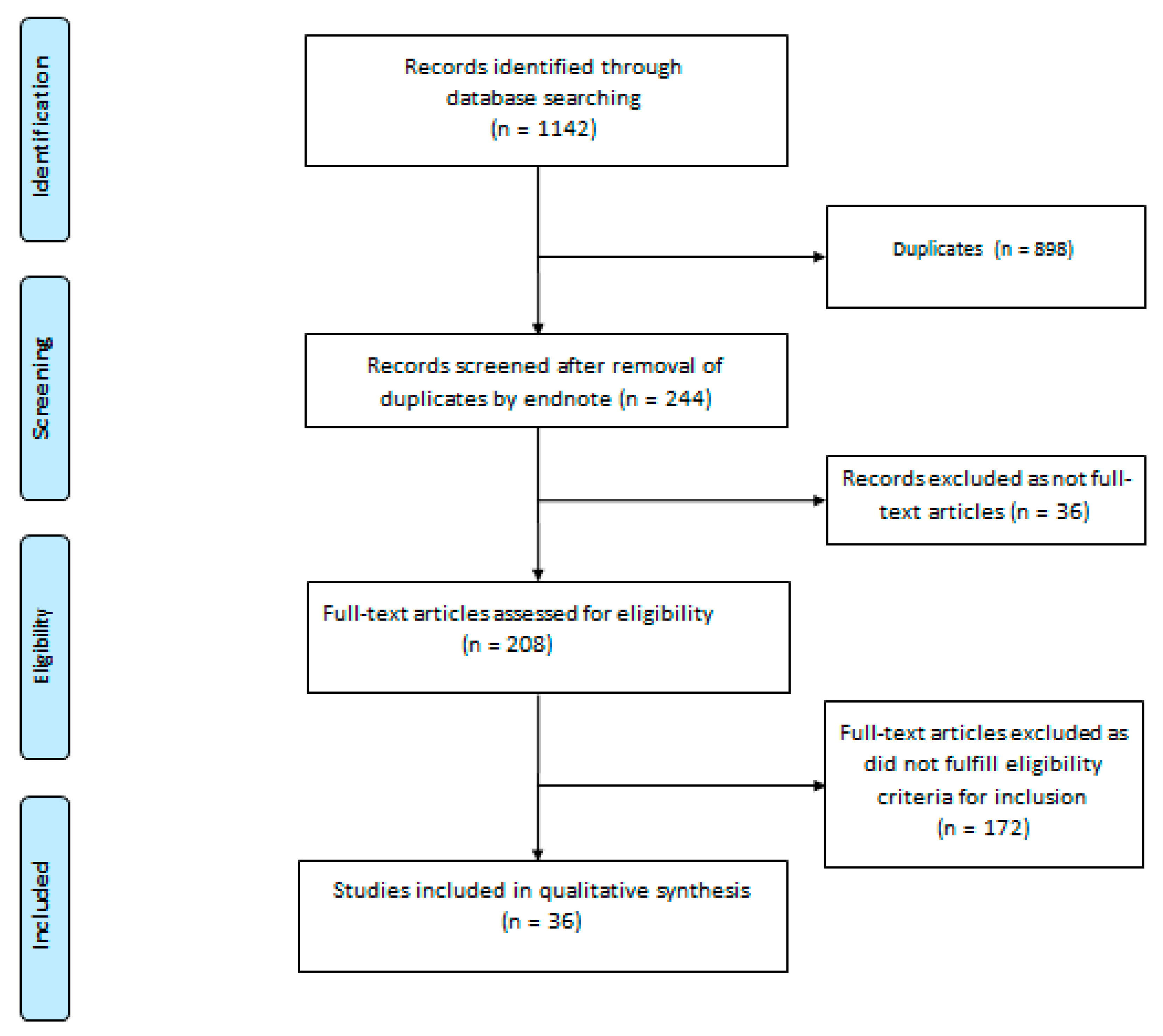
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy and Study Selection
2.3. Data Extraction
2.4. Study Registration
3. Results
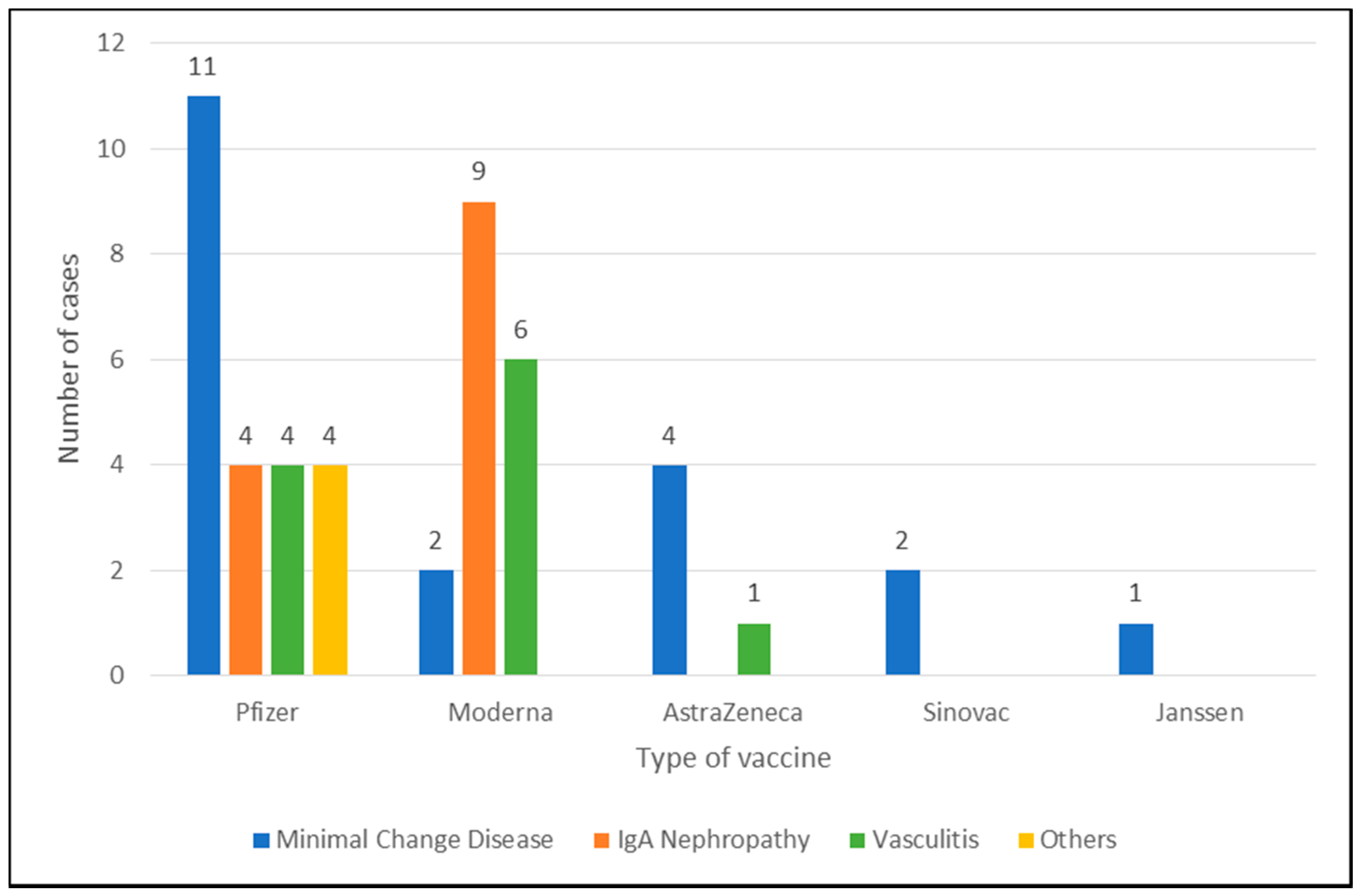
3.1. Minimal Change Disease
3.2. IgA Nephropathy
3.3. Vasculitis
3.4. Other Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.H.; Hirsch, J.S.; Wanchoo, R.; Sachdeva, M.; Sakhiya, V.; Hong, S.; Jhaveri, K.D.; Fishbane, S.; Abate, M.; Andrade, H.P.; et al. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020, 98, 1530–1539. [Google Scholar] [CrossRef]
- Henry, B.M.; Lippi, G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int. Urol. Nephrol. 2020, 52, 1193–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Windpessl, M.; Bruchfeld, A.; Anders, H.-J.; Kramer, H.; Waldman, M.; Renia, L.; Ng, L.F.P.; Xing, Z.; Kronbichler, A. COVID-19 vaccines and kidney disease. Nat. Rev. Nephrol. 2021, 17, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Chitturi, C.; Yee, J. Vaccination in chronic kidney disease. Adv. Chronic Kidney Dis. 2019, 26, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T-cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Ewer, K.J.; Barrett, J.R.; Belij-Rammerstorfer, S.; Sharpe, H.; Makinson, R.; Morter, R.; Flaxman, A.; Wright, D.; Bellamy, D.; Bittaye, M.; et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 2021, 27, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, L.; Sapojnikov, M.; Wechsler, A.; Varadi-Levi, R.; Zamir, D.; Tobar, A.; Levin-Iaina, N.; Fytlovich, S.; Yagil, Y. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am. J. Kidney Dis. 2021, 78, 142–145. [Google Scholar] [CrossRef]
- Maas, R.J.; Gianotten, S.; van der Meijden, W.A.G. An additional case of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am. J. Kidney Dis. 2021, 78, 312. [Google Scholar] [CrossRef]
- D’Agati, V.D.; Kudose, S.; Bomback, A.S.; Adamidis, A.; Tartini, A. Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int. 2021, 100, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Weijers, J.; Alvarez, C.; Hermans, M.M. Post-vaccinal minimal change disease. Kidney Int. 2021, 100, 459–461. [Google Scholar] [CrossRef]
- Salem, F.; Rein, J.L.; Yu, S.M.W.; Abramson, M.; Cravedi, P.; Chung, M. Report of three cases of minimal change disease following the second dose of mRNA SARS-CoV-2 COVID-19 vaccine. Kidney Int. Rep. 2021, 6, 2523–2524. [Google Scholar] [CrossRef]
- Kobayashi, S.; Fugo, K.; Yamazaki, K.; Terawaki, H. Minimal change disease soon after Pfizer-BioNTech COVID-19 vaccination. Clin. Kidney J. 2021. in print. [Google Scholar] [CrossRef]
- Leclerc, S.; Royal, V.; Lamarche, C.; Laurin, L.P. Minimal change disease with severe acute kidney injury following the Oxford-AstraZeneca COVID-19 Vaccine: A case report. Am. J. Kidney Dis. 2021. in print. [Google Scholar] [CrossRef] [PubMed]
- Anupama, Y.; Patel, R.G.; Vankalakunti, M. Nephrotic syndrome following ChAdOx1 nCoV-19 vaccine against SARScoV-2. Kidney Int. Rep. 2021, 6, 2248. [Google Scholar] [CrossRef]
- Holzworth, A.; Couchot, P.; Cruz-Knight, W.; Brucculeri, M. Minimal change disease following the Moderna mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021, 100, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-H.; Han, M.-H.; Kim, Y.-J.; Kim, M.-S.; Jung, H.-Y.; Choi, J.-Y.; Cho, J.-H.; Kim, C.-D.; Park, S.-H. New-onset Nephrotic Syndrome after Janssen COVID-19 vaccination: A case report and literature review. J. Korean Med. Sci. 2021, 36, 218. [Google Scholar] [CrossRef] [PubMed]
- Dirim, A.B.; Safak, S.; Andac, B.; Garayeva, N.; Demir, E.; Artan, A.S.; Ozluk, Y.; Kilicaslan, I.; Oto, O.A.; Ozturk, S.; et al. Minimal change disease following vaccination with CoronaVac. Clin. Kidney J. 2021, 14, 2268–2269. [Google Scholar] [CrossRef]
- Komaba, H.; Wada, T.; Fukagawa, M. Relapse of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am. J. Kidney Dis. 2021, 78, 469–470. [Google Scholar] [CrossRef]
- Mancianti, N.; Guarnieri, A.; Tripodi, S.; Salvo, D.P.; Garosi, G. Minimal change disease following vaccination for SARS-CoV-2. J. Nephrol. 2021, 34, 1039–1040. [Google Scholar] [CrossRef] [PubMed]
- Kervella, D.; Jacquemont, L.; Chapelet-Debout, A.; Deltombe, C.; Ville, S. Minimal change disease relapse following SARS-CoV-2 mRNA vaccine. Kidney Int. 2021, 100, 457–458. [Google Scholar] [CrossRef]
- Schwotzer, N.; Kissling, S.; Fakhouri, F. Letter regarding “Minimal change disease relapse following SARS-CoV-2 mRNA vaccine.”. Kidney Int. 2021, 100, 458–459. [Google Scholar] [CrossRef]
- Morlidge, C.; El-Kateb, S.; Jeevaratnam, P.; Thompson, B. Relapse of minimal change disease following the AstraZeneca COVID-19 vaccine. Kidney Int. 2021, 100, 459. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Z.; Tan, R.Y.; Choo, J.C.; Lim, C.C.; Tan, C.S.; Loh, A.H.; Tien, C.S.; Tan, P.H.; Woo, K.T. Is COVID-19 vaccination unmasking glomerulonephritis? Kidney Int. 2021, 100, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, M.A.; Liu, M.; Saganas, C.; Montani, M.; Vogt, B.; Huynh-Do, U.; Fuster, D.G. De novo vasculitis after mRNA-1273 (Moderna) vaccination. Kidney Int. 2021, 100, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Abramson, M.; Yu, S.M.; Campbell, K.N.; Chung, M.; Salem, F. IgA nephropathy after SARS-CoV-2 vaccination. Kidney Med. 2021. in print. [Google Scholar] [CrossRef] [PubMed]
- Kudose, S.; Friedmann, P.; Albajrami, O.; D’Agati, V.D. Histologic correlates of gross hematuria following Moderna COVID-19 vaccine in patients with IgA nephropathy. Kidney Int. 2021, 100, 468–469. [Google Scholar] [CrossRef]
- Park, K.; Miyake, S.; Tai, C.; Tseng, M.; Andeen, N.K.; Kung, V.L. Letter regarding: “A case of gross hematuria and IgA nephropathy flare-up following SARS-CoV-2 vaccination”. Kidney Int. Rep. 2021, 6, 2246–2247. [Google Scholar] [CrossRef]
- Rahim, S.E.; Lin, J.T.; Wang, J.C. A case of gross hematuria and IgA nephropathy flare-up following SARS-CoV-2 vaccination. Kidney Int. 2021, 100, 238. [Google Scholar] [CrossRef]
- Perrin, P.; Bassand, X.; Benotmane, I.; Bouvier, N. Gross hematuria following SARS-CoV-2 vaccination in patients with IgA nephropathy. Kidney Int. 2021, 100, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Negrea, L.; Rovin, B.H. Gross hematuria following vaccination for severe acute respiratory syndrome coronavirus 2 in 2 patients with IgA nephropathy. Kidney Int. 2021, 99, 1487. [Google Scholar] [CrossRef]
- Shakoor, M.T.; Birkenbach, M.P.; Lynch, M. ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 vaccine. Am. J. Kidney Dis. 2021. in print. [Google Scholar] [CrossRef]
- Dube, G.K.; Benvenuto, L.J.; Batal, I. Antineutrophil cytoplasmic autoantibody–Associated glomerulonephritis following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int. Rep. 2021. in print. [Google Scholar] [CrossRef]
- Sekar, A.; Campbell, R.; Tabbara, J.; Rastogi, P. ANCA glomerulonephritis post Moderna COVID-19 vaccination. Kidney Int. 2021, 100, 473–474. [Google Scholar] [CrossRef]
- Gillion, V.; Jadoul, M.; Demoulin, N.; Aydin, S.; Devresse, A. Granulomatous vasculitis after the AstraZeneca anti–SARS-CoV-2 vaccine. Kidney Int. 2021, 100, 706–707. [Google Scholar] [CrossRef]
- Obeid, M.; Fenwick, C.; Pantaleo, G. Reactivation of IgA vasculitis after COVID-19 vaccination. Lancet Rheumatol. 2021, 3, e617. [Google Scholar] [CrossRef]
- Sacker, A.; Kung, V.; Andeen, N. Anti-GBM nephritis with mesangial IgA deposits after SARS-CoV-2 mRNA vaccination. Kidney Int. 2021, 100, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Tuschen, K.; Bräsen, J.H.; Schmitz, J.; Vischedyk, M.; Weidemann, A. Relapse of class V Lupus Nephritis after vaccination with COVID-19 mRNA vaccine. Kidney Int. 2021, 100, 941–944. [Google Scholar] [CrossRef]
- Aydın, M.F.; Yıldız, A.; Oruç, A.; Sezen, M.; Dilek, K.; Güllülü, M.; Yavuz, M.; Ersoy, A. Relapse of primary membranous nephropathy after inactivated SARS-CoV-2 virus vaccination. Kidney Int. 2021, 100, 464–465. [Google Scholar] [CrossRef] [PubMed]
- Del Bello, A.; Marion, O.; Delas, A.; Congy-Jolivet, N.; Colombat, M.; Kamar, N. Acute rejection after anti–SARS-CoV-2 mRNA vaccination in a patient who underwent a kidney transplant. Kidney Int. 2021, 100, 238–239. [Google Scholar] [CrossRef]
- Masset, C.; Kervella, D.; Kandel-Aznar, C.; Fantou, A.; Blancho, G.; Hamidou, M. Relapse of IgG4-related nephritis following mRNA COVID-19 vaccine. Kidney Int. 2021, 100, 465–466. [Google Scholar] [CrossRef]
- De Fabritiis, M.; Angelini, M.L.; Fabbrizio, B.; Cenacchi, G.; Americo, C.; Cristino, S.; Lifrieri, M.F.; Cappuccilli, M.; Spazzoli, A.; Zambianchi, L.; et al. Renal thrombotic microangiopathy in concurrent COVID-19 vaccination and infection. Pathogens 2021, 10, 1045. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, J.; Pagot, E.; Limal, N.; Hüe, S.; Audard, V.; Moktefi, A.; El Karoui, K. Scleroderma renal crisis following mRNA vaccination against SARS-CoV-2. Kidney Int. 2021. in print. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, M.; Massella, L.; Ruggiero, B.; Emma, F. Minimal change disease. Clin. J. Am. Soc. Nephrol 2017, 12, 332–345. [Google Scholar] [CrossRef] [Green Version]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Patel, C.; Shah, H.H. Vaccine-associated kidney diseases: A narrative review of the literature. Saudi J. Kidney Dis. Transpl. 2019, 30, 1002–1009. [Google Scholar] [CrossRef]
- Abdel-Hafez, M.; Shimada, M.; Lee, P.Y.; Johnson, R.J.; Garin, E.H. Idiopathic nephrotic syndrome and atopy: Is there a common link? Am. J. Kidney Dis. 2009, 54, 945–953. [Google Scholar] [CrossRef] [Green Version]
- Schena, F.P.; Nistor, I. Epidemiology of IgA nephropathy: A global perspective. Semin. Nephrol. 2018, 38, 435–442. [Google Scholar] [CrossRef]
- Hiki, Y.; Odani, H.; Takahashi, M.; Yasuda, Y.; Nishimoto, A.; Iwase, H.; Shinzato, T.; Kobayashi, Y.; Maeda, K. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001, 59, 1077–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, J.; Julian, B.A.; Tomana, M.; Mestecky, J. IgA Glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin. Nephrol. 2008, 28, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Lai, K.N. Pathogenesis of IgA nephropathy. Nat. Rev. Nephrol. 2012, 8, 275–283. [Google Scholar] [CrossRef]
- Van den Wall Bake, A.W.; Beyer, W.E.; Evers-Schouten, J.H.; Hermans, J.; Daha, M.R.; Masurel, N.; Van Es, L.A. Humoral immune response to influenza vaccination in patients with primary immunoglobulin A nephropathy. An analysis of isotype distribution and size of the influenza-specific antibodies. J. Clin. Investig. 1989, 84, 1070–1075. [Google Scholar] [CrossRef] [Green Version]
- Wisnewski, A.V.; Luna, J.C.; Redlich, C.A. Human IgG and IgA responses to COVID-19 mRNA vaccines. PLoS ONE 2021, 16, e0249499. [Google Scholar] [CrossRef] [PubMed]
- Jeffs, L.S.; Nitschke, J.; Tervaert, J.W.; Peh, C.A.; Hurtado, P.R. Viral RNA in the influenza vaccine may have contributed to the development of ANCA-associated vasculitis in a patient following immunisation. Clin. Rheumatol. 2016, 35, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Prendecki, M.; Clarke, C.; Cairns, T.; Cook, T.; Roufosse, C.; Thomas, D.; Willicombe, M.; Pusey, C.D.; McAdoo, S.P. Anti–glomerular basement membrane disease during the COVID-19 pandemic. Kidney Int. 2020, 98, 780–781. [Google Scholar] [CrossRef]
- de la Flor, J.; Linares, T.; Alonso-Riaño, M.; Segura, P.; Albarracin, C.; Ruiz, E.; Gallegos, G.; Pozo, M.R. A case of acute interstitial nephritis following the Pfizer-BioNTech COVID-19 vaccine. Nefrologia 2021. in print. [Google Scholar] [CrossRef]
- Hanna, C.; Hernandez, L.P.H.; Bu, L.; Kizilbash, S.; Najera, L.; Rheault, M.N.; Czyzyk, J.; Kouri, A.M. IgA nephropathy presenting as macroscopic hematuria in 2 pediatric patients after receiving the Pfizer COVID-19 vaccine. Kidney Int. 2021, 100, 705–706. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Moreira, E.D.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N. Engl. J. Med. 2021. in print. [Google Scholar] [CrossRef] [PubMed]
- Soiza, R.L.; Scicluna, C.; Thomson, E.C. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing 2021, 50, 279–283. [Google Scholar] [CrossRef] [PubMed]
| Author and Country of Case Report (Ref.) | Age (Years) | Sex | Time to Presentation from Day of Vaccination (Days) | Comorbidities | New Onset or Relapse | Vaccine Brand | Vaccine Dose | Baseline Creatinine (mg/dL) | Presentation Creatinine (mg/dL) | Presentation Proteinuria (g/Day) | Presentation Albumin (g/dL) | Hematuria | Treatment Received | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimal Change Disease | ||||||||||||||
| Lebedev et al., Israel [9] | 50 | M | 10 | Nil | N | Pfizer-BioNTech | 1 | 0.78 | 2.31 | 6.9 | 1.93 | Yes | Prednisolone | RP, DI |
| Maas et al., Netherlands [10] | Early 80s | M | 7 | Venous thromboembolisms | N | Pfizer-BioNTech | 1 | - | 1.43 | 15.3 | 1.03 | No | Prednisolone | RP, DI |
| D’Agati et al., United States [11] | 77 | M | 7 | T2DM, obesity, smoking, and CAD | N | Pfizer-BioNTech | 1 | 1.0–1.3 | 2.33 | 23.2 | 3.0 | No | Pulsed MP, prednisolone, and diuresis | RP, DI |
| Weijers et al., Netherlands [12] | 61 | F | 8 | Autoimmune hepatitis and hypothyroidism | N | Pfizer-BioNTech | 1 | - | 1.47 | 12 | 2.1 | No | Steroids | RP, HD received, DI on discharge |
| Salem et al., United States [13] | 41 | F | 5 | Asthma | N | Pfizer-BioNTech | 2 | - | - | 14.4 | 2.6 | Yes | - | - |
| Kobayashi et al., Japan [14] | 75 | M | 7 | Nil | N | Pfizer-BioNTech | 2 | 0.96 | 1.24 | 7.72 | 1.1 | No | Intravenous MP and prednisolone | RP, DI |
| Leclerc et al., Canada [15] | 71 | M | 13 | Dyslipidemia | N | Oxford- AstraZeneca | 1 | 0.7 | 10.6 | 23.2 | 2.8 | Yes | Pulsed MP and prednisolone | RP, HD received, DI on discharge |
| Anupama et al., India [16] | 19 | F | 1 | - | N | Oxford- AstraZeneca | 1 | - | 1.09 | 31.8 | 2.15 | No | Prednisolone | RP, DI |
| Holzworth et al., United States [17] | 63 | F | Less than 1 week | Hypertension and tobacco dependence | N | Moderna | 1 | 0.7 | 1.48 | 13.4 | 0.7 | No | Pulsed MP, prednisolone, ARB, and diuresis | RP, DI |
| Lim et al., South Korea [18] | 51 | M | 28 | Nil | N | Janssen | 1 | - | 1.54 | 8.6 | 1.6 | Yes | Parenteral MP and oral steroids | RP, DI |
| Dirim et al., Turkey [19] | 65 | M | 17 | T2DM, Hashimoto’s thyroiditis | N | Sinovac | 1 | - | 1 | 11.9 | 1.1 | No | MP and diuresis | RP, DI |
| Komaba et al., Japan [20] | Mid 60s | M | 19 | MCD | R | Pfizer-BioNTech | 1 | - | 0.99 | 11.48 | 2.8 | No | Prednisolone and cyclosporine | RP, DI |
| Mancianti et al., Italy [21] | 39 | M | 3 | MCD | R | Pfizer-BioNTech | 1 | 0.9 | 1.8 | 8 | 2.7 | No | Prednisolone | RP, DI |
| Kervella et al., France [22] | 34 | F | 10 (first dose) 37 (second dose) | MCD | R | Pfizer-BioNTech | 2 | - | - | 24 (first dose) 30 (second dose) | - | No | Steroid increased from regular dose | RP, DI |
| Salem et al., United States [13] | 34 | F | 28 | Steroid-sensitive MCD | R | Pfizer-BioNTech | 2 | - | - | 12.9 | 2.8 | No | - | - |
| Schwotzer et al., Switzerland [23] | 22 | M | 3 | MCD | R | Pfizer-BioNTech | 1 | - | 0.80 | - | 2.3 | No | Prednisolone, remained on tacrolimus | RP, DI |
| Morlidge et al., United Kingdom [24] | 30 | M | 2 | MCD | R | Oxford- AstraZeneca | 1 | - | 0.93 | 24.1 | 4.7 | No | Prednisolone | RP, DI |
| Morlidge et al., United Kingdom [24] | 40 | F | 1 | MCD | R | Oxford- AstraZeneca | 1 | - | - | - | - | No | Regular prednisolone, increased dose | RP, DI |
| Salem et al., United States [13] | 33 | F | 21 | MCD | R | Moderna | 2 | - | - | 6.4 | 2.3 | No | - | - |
| Author and Country of Case Report | Age | Sex | Time to Presentation from Day of Vaccination (Days) | Comorbidities | New Onset or Known Case | Vaccine Brand | Vaccine Dose | Baseline Creatinine (mg/dL) | Presentation Creatinine (mg/dL) | Presentation Proteinuria (g/Day) | Presentation Albumin (g/dL) | RBC per High-powered Field | Treatment Received | Hematuria Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tan et al., Singapore [25] | 41 | F | 1 | Gestational DM | N | Pfizer- BioNTech | 2 | - | 1.73 | 2.03 | - | >200 | Pulsed MP, prednisolone, and cyclophosphamide | - |
| Andregg et al., Switzerland [26] | 39 | M | Immediately after second dose | HTN | N | Moderna | 2 | - | - | - | - | Macroscopic hematuria | Glucocorticoids and cyclophosphamide | RH |
| Abramson et al., United States [27] | 30 | M | 1 | Nil | N | Moderna | 2 | - | 1.02 | 0.8 | - | >30 | ARB | RH |
| Kudose et al., United States [28] | 50 | F | 2 | HTN, obesity, and antiphospholipid syndrome | N | Moderna | 2 | 1.3 | 1.7 | 2 | - | >50 | Supportive | RH |
| Kudose et al., United States [28] | 19 | M | 2 | Undiagnosed microhematuria for 6 months prior | N | Moderna | 2 | - | 1.2 | - | - | Gross hematuria | Supportive | RH |
| Park et al., United States [29] | 50 | M | 1 | CKD and HTN | N | Moderna | 2 | 1.17 | 1.54 | 3.56 | - | >50 | RAASi | RH |
| Rahim et al., United States [30] | 52 | F | 1 | IgA nephropathy | KC | Pfizer- BioNTech | 2 | 0.7–0.8 | - | 4.2 | - | Gross hematuria | RAASi | RH |
| Perrin et al., France [31] | 41 | F | 2 | IgA nephropathy, KT patient | KC | Pfizer- BioNTech | 1 | - | - | 0.47 | - | Gross hematuria | Supportive | RH |
| Perrin et al., France [31] | 27 | F | 2 | IgA nephropathy, HD patient | KC | Pfizer- BioNTech | 2 | - | - | 1.9 | - | Nil gross hematuria | Supportive | RH |
| Perrin et al., France [31] | 22 | M | 2 and 25 after first dose, 2 after second dose | IgA vasculitis | KC | Moderna | 2 | - | - | 0.34 | - | Nil gross hematuria | Supportive | RH |
| Negrea et al., United States [32] | 38 | F | - | IgA nephropathy | KC | Moderna | 2 | - | - | 1.40 | - | Microscopic hematuria | - | - |
| Negrea et al., United States [32] | 38 | F | - | IgA nephropathy | KC | Moderna | 2 | - | - | 0.40 | - | Microscopic hematuria | - | - |
| Cases with no kidney biopsy | ||||||||||||||
| Park et al., United States [29] | 22 | F | 2 | Nil | N | Moderna | 2 | 0.80 | 0.80 | 0.40 | - | >50 | Supportive | RH |
| Park et al., United States [29] | 39 | F | 2 | Nil | N | Moderna | 2 | - | 0.80 | 0.90 | - | >50 | Supportive | RH |
| Author and Country of Case Report | Age | Sex | Time to Presentation from Day of Vaccination (Days) | Comorbidities | Vaccine Brand | Number of Vaccine Doses | Baseline Creatinine (mg/dL) | Presentation Creatinine (mg/dL) | Presentation Proteinuria (g/Day) | Presentation Albumin (g/dL) | RBC with High-Powered Field | Treatment Received | Hematuria Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-neutrophil Cytoplasmic Autoantibody (ANCA)-associated vasculitis | |||||||||||||
| Shakoor et al., United States [33] | 78 | F | 28 (after first dose) and 6 (after second dose) | T2DM, HTN, and paroxysmal AF | Pfizer- BioNTech | 2 | 0.77 | 1.31 (fist dose) 3.54 (second dose) | 0.1 | - | 99 (fist dose) and 56 (second dose) | Intravenous MP and prednisolone | DI |
| Dube et al., United States [34] | 29 | F | 49 | Congenital diffuse cystic lung disease, awaiting lung transplant | Pfizer- BioNTech | 2 | 0.8 | 1.91 | 0.633 | 4.4 | 12 | MP, prednisolone, rituximab, and intravenous cyclophosphamide | DI |
| Sekar et al., United States [35] | 52 | M | 14 | HTN | Moderna | 2 | 1.11 | 8.41 | - | - | Microscopic hematuria | Prednisolone, rituximab, and cyclophosphamide | DD |
| Andregg et al., Switzerland [26] | 81 | M | - | Nil | Moderna | 1 | - | - | - | - | - | High-dose steroids, cyclophosphamide, and plasma exchange | DI |
| Granulomatous Vasculitis | |||||||||||||
| Gillion et al., Belgium [36] | 77 | M | 28 | Nil significant | Oxford- AstraZeneca | 1 | 1.2 | 2.7 | 0.07 | - | No hematuria | MP | DI |
| IgA Vasculitis (Henoch–Schonlein Purpura) | |||||||||||||
| Obeid et al., Switzerland [37] | 78 | F | 7 | IgA vasculitis with leukocytoclastic vasculitis | Moderna | 1 | 1.08 | 1.18 | - | - | 150 | MP | DI |
| Park et al., United States [29] | 67 | M | 28 | CKD and HTN | Moderna | 1 | 1.20 | 2.90 | 2.10 | - | >50 | Steroid | DI |
| Anti-glomerular Basement Membrane (Anti-GBM) Disease | |||||||||||||
| Tan et al., Singapore [25] | 60 | F | 1 | Hyperlipidemia | Pfizer- BioNTech | 2 | - | 6.11 | 7.58 | - | >200 | Pulsed MP, prednisolone, cyclophosphamide, and plasma exchange | - |
| Sacker et al., United States [38] | ‘Older Woman’ | F | 14 | Nil significant | Moderna | 2 | - | 7.8 | 1.9 | - | Gross hematuria | MP, cyclophosphamide, plasma exchange, and HD | DD |
| Lupus Nephritis | |||||||||||||
| Tuschen et al., Germany [39] | 42 | F | 7 | SLE with lupus nephritis class V | Pfizer- BioNTech | 1 | - | - | 6 | - | No hematuria | MMFand prednisolone | DI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.H.L.; Kalra, P.A.; Chinnadurai, R. New-Onset and Relapsed Kidney Histopathology Following COVID-19 Vaccination: A Systematic Review. Vaccines 2021, 9, 1252. https://doi.org/10.3390/vaccines9111252
Wu HHL, Kalra PA, Chinnadurai R. New-Onset and Relapsed Kidney Histopathology Following COVID-19 Vaccination: A Systematic Review. Vaccines. 2021; 9(11):1252. https://doi.org/10.3390/vaccines9111252
Chicago/Turabian StyleWu, Henry H. L., Philip A. Kalra, and Rajkumar Chinnadurai. 2021. "New-Onset and Relapsed Kidney Histopathology Following COVID-19 Vaccination: A Systematic Review" Vaccines 9, no. 11: 1252. https://doi.org/10.3390/vaccines9111252
APA StyleWu, H. H. L., Kalra, P. A., & Chinnadurai, R. (2021). New-Onset and Relapsed Kidney Histopathology Following COVID-19 Vaccination: A Systematic Review. Vaccines, 9(11), 1252. https://doi.org/10.3390/vaccines9111252







