Resources, Production Scales and Time Required for Producing RNA Vaccines for the Global Pandemic Demand
Abstract
:1. Introduction
2. Methods
2.1. Data Sources
2.2. Vaccine Production Process Modelling
3. Results and Discussion
3.1. How Can We Produce RNA Vaccines and What Are the Key Manufacturing Uncertainties and Challenges?
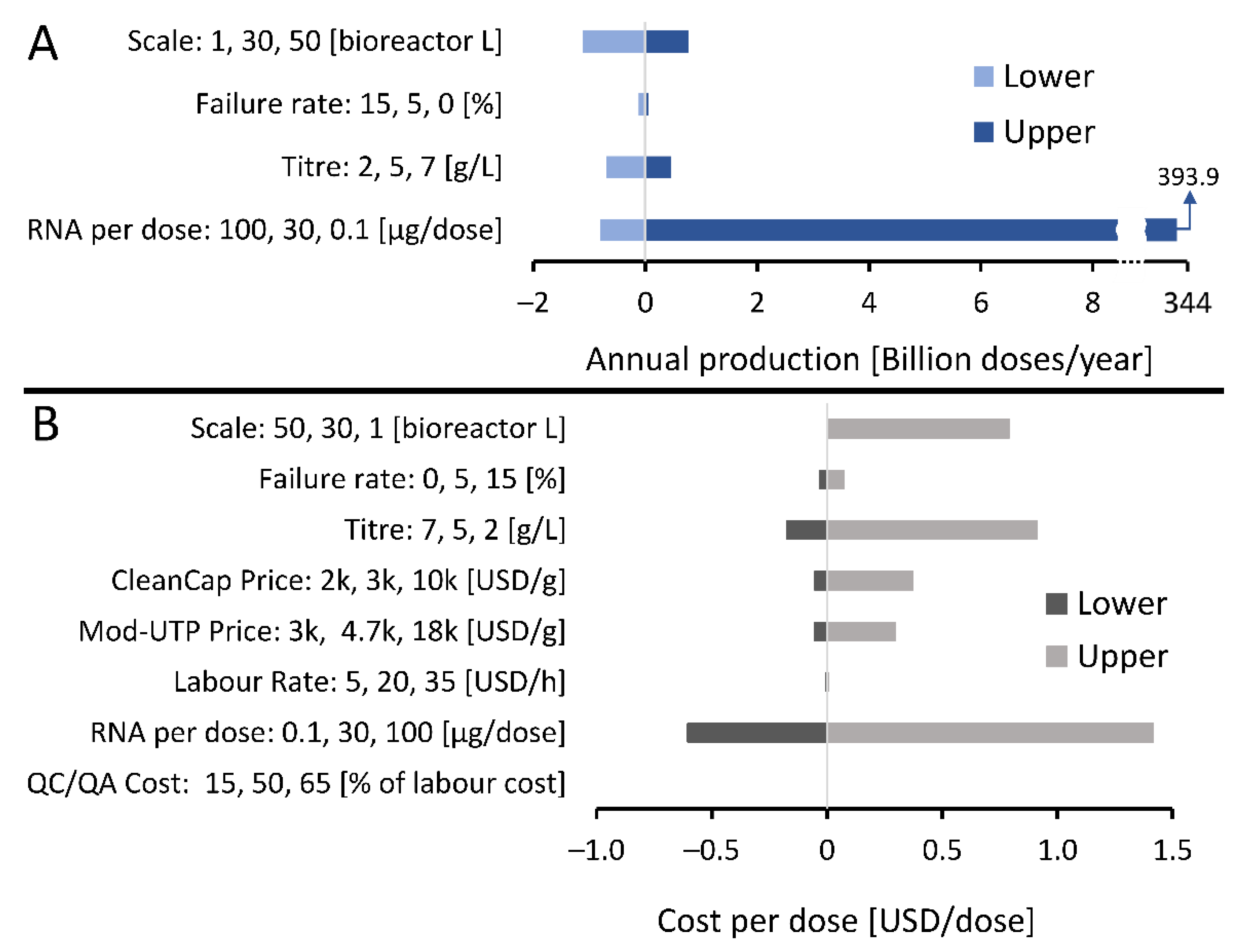
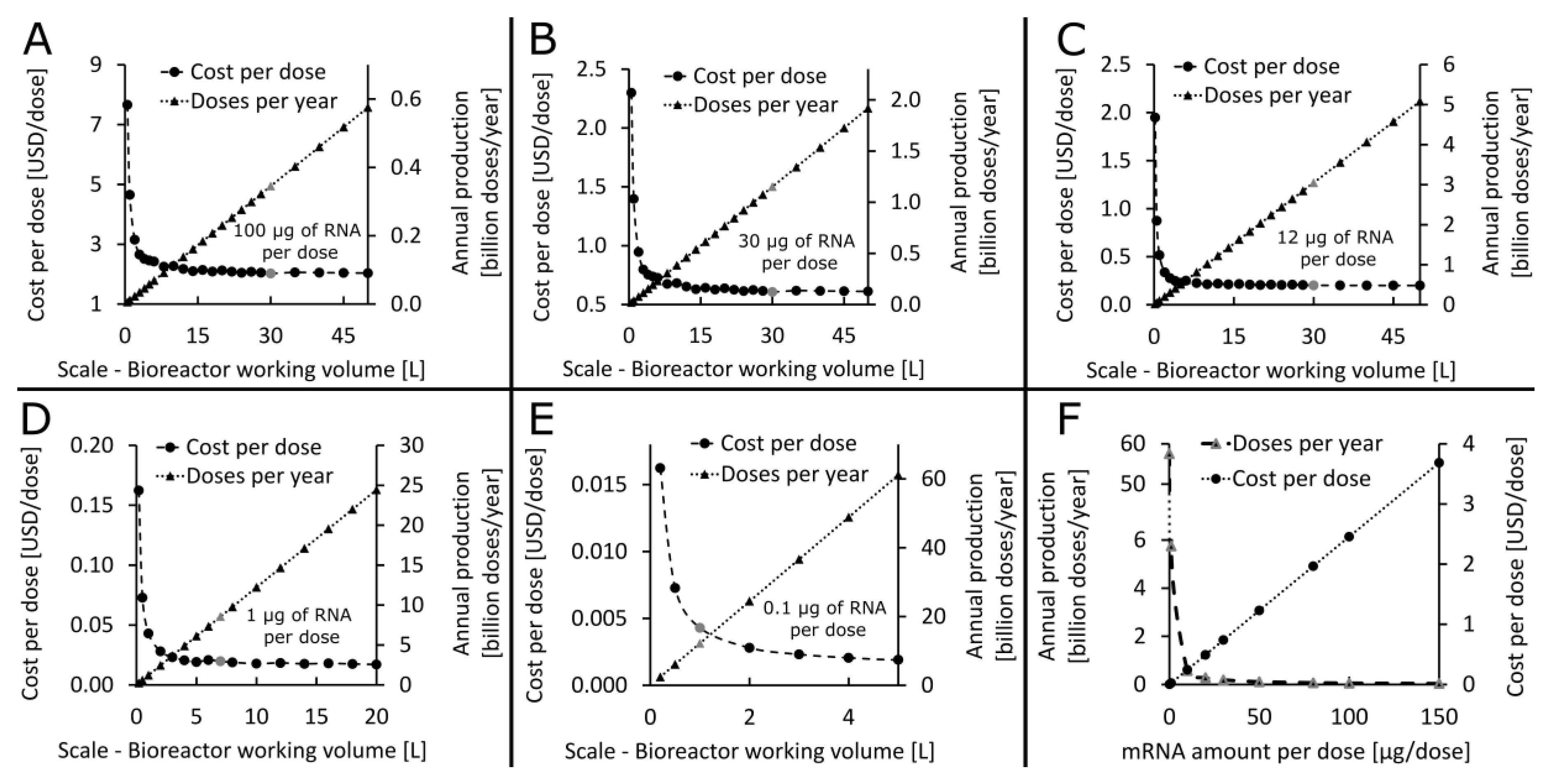
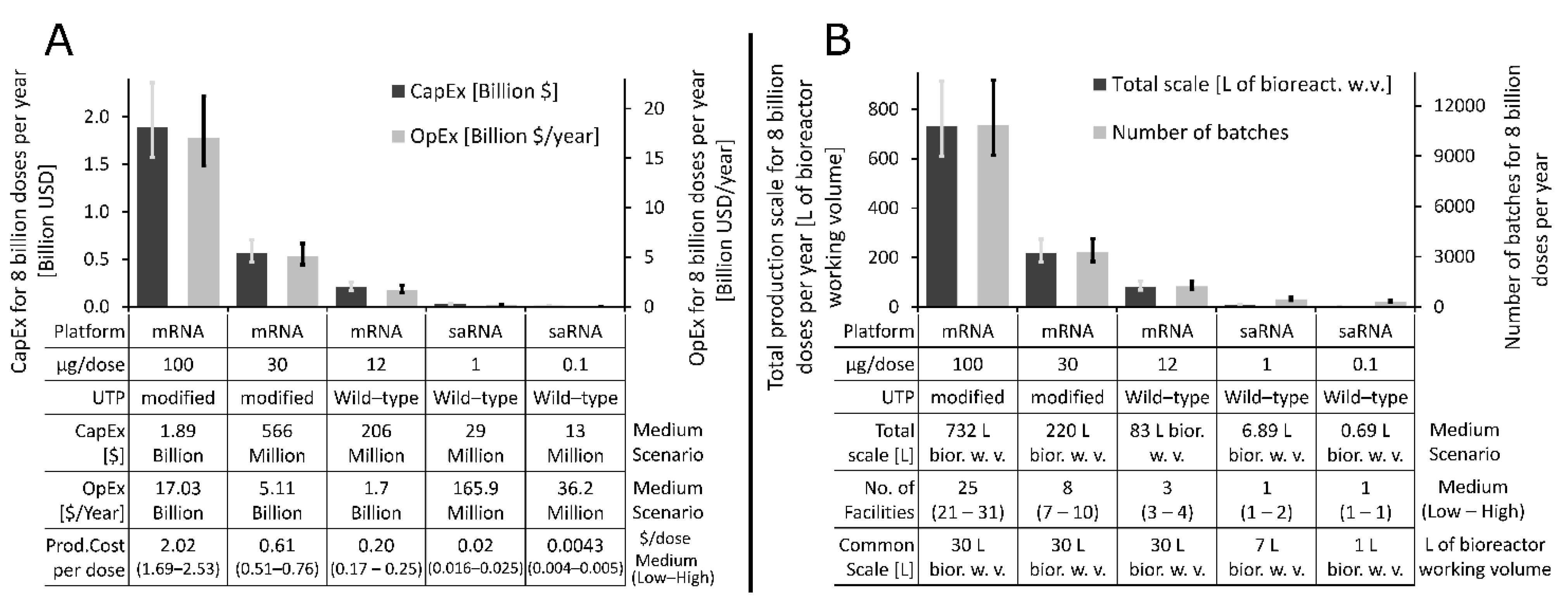
3.2. What Production Scales and Resources Are Needed to Manufacture RNA Vaccines for Immunising the World’s Population against COVID-19?
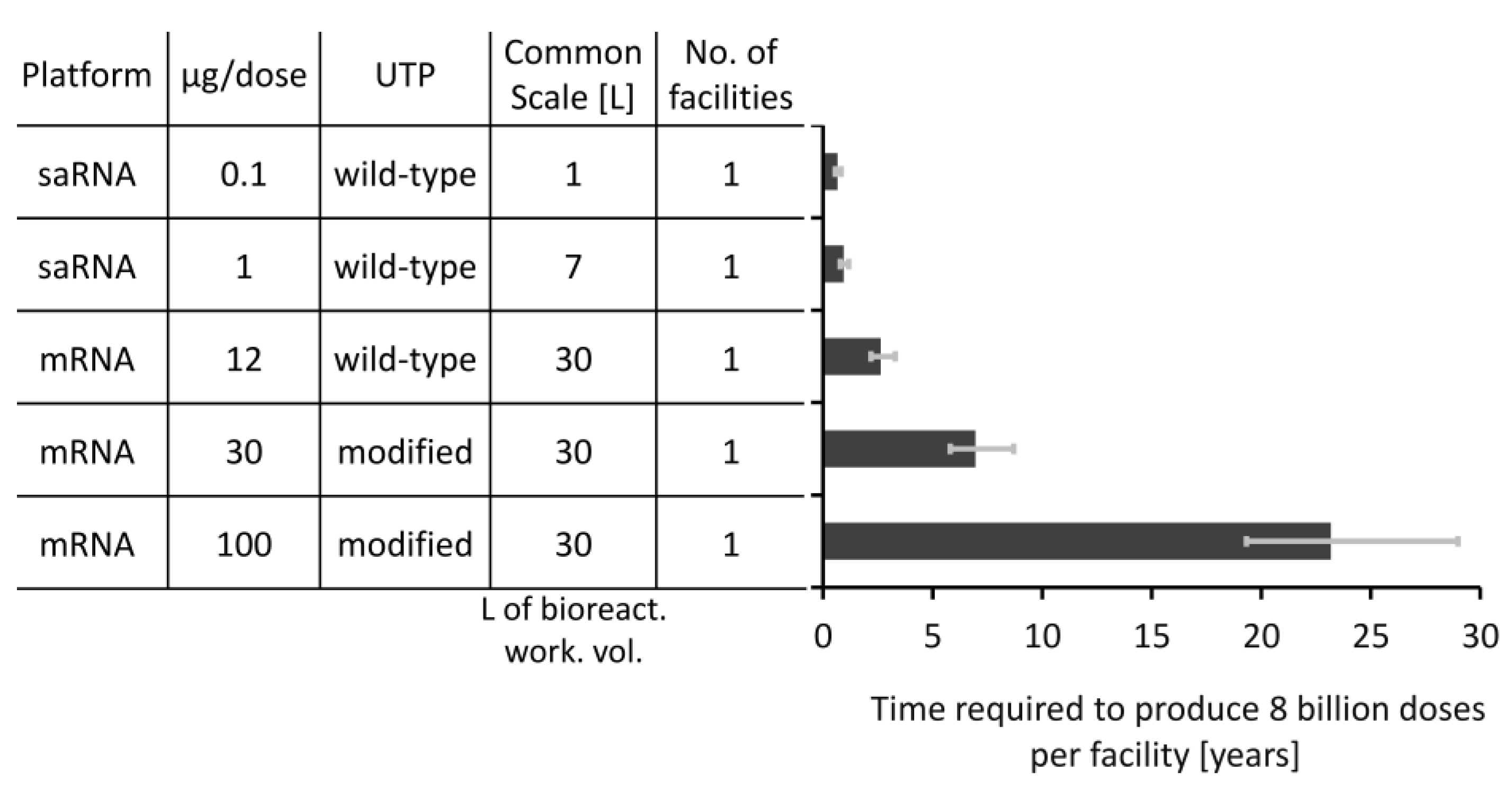
3.3. How Long Will It Take to Manufacture Vaccines to Immunise the World’s Population?
3.4. How to Be better Prepared for Rapid-Response Manufacturing for Future Pandemics?
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. DRAFT Landscape of COVID-19 Candidate Vaccines. 2020. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 12 November 2020).
- Dolgin, E. COVID-19 Vaccines Poised for Launch, but Impact on Pandemic Unclear. Nat. Biotechnol. 2020. Available online: https://www.nature.com/articles/d41587-020-00022-y (accessed on 26 November 2020). [CrossRef] [PubMed]
- McKay, P.F.; Hu, K.; Blakney, A.K.; Samnuan, K.; Brown, J.C.; Penn, R.; Zhou, J.; Bouton, C.R.; Rogers, P.; Polra, K.; et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 2020, 11, 3523. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.B.; Lambert, L.; Kinnear, E.; Busse, D.; Erbar, S.; Reuter, K.C.; Wicke, L.; Perkovic, M.; Beissert, T.; Haas, H.; et al. Self-Amplifying RNA Vaccines Give Equivalent Protection against Influenza to mRNA Vaccines but at Much Lower Doses. Mol. Ther. 2018, 26, 26446–26455. [Google Scholar] [CrossRef] [Green Version]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geall, A.J.; Verma, A.; Otten, G.R.; Shaw, C.A.; Hekele, A.; Banerjee, K.; Cu, Y.; Beard, C.W.; Brito, L.A.; Krucker, T.; et al. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl. Acad. Sci. USA 2012, 109, 14604–14609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Ye, T.; Zhong, Z.; García-Sastre, A.; Schotsaert, M.; De Geest, B.G. Current Status of COVID-19 (Pre)Clinical Vaccine Development. Angew. Chem. Int. Ed. 2020, 59, 18885–18897. [Google Scholar] [CrossRef]
- Servick, K. This mysterious $2 billion biotech is revealing the secrets behind its new drugs and vaccines. Am. Assoc. Adv. Sci. 2020. Available online: https://www.sciencemag.org/news/2017/02/mysterious-2-billion-biotech-revealing-secrets-behind-its-new-drugs-and-vaccines (accessed on 12 November 2020).
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Kremsner, P.; Mann, P.; Bosch, J.; Fendel, R.; Gabor, J.J.; Kreidenweiss, A.; Schunk, M.; Schindler, C.; Bosch, J.; Fendel, R.; et al. Phase 1 Assessment of the Safety and Immunogenicity of an mRNA- Lipid Nanoparticle Vaccine Candidate Against SARS-CoV-2 in Human Volunteers. MEDRXIV 2020. Available online: http://medrxiv.org/content/early/2020/11/09/2020.11.09.20228551.abstract (accessed on 12 December 2020).
- Fletcher, J. Clinical Trial to Assess the Safety of a Coronavirus Vaccine in Healthy Men and Women. ISRCTN Regist. 2020. Available online: http://www.isrctn.com/ISRCTN17072692 (accessed on 9 October 2020).
- Schlake, T.; Thess, A.; Fotin-Mleczek, M.; Kallen, K.-J. Developing mRNA-vaccine technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef] [Green Version]
- Ljungberg, K.; Liljeström, P. Self-replicating alphavirus RNA vaccines. Expert Rev. Vaccines 2015, 14, 177–194. [Google Scholar] [CrossRef]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef] [Green Version]
- Brito, L.A.; Kommareddy, S.; Maione, D.; Uematsu, Y.; Giovani, C.; Berlanda Scorza, F.; Otten, G.R.; Yu, D.; Mandl, C.W.; Mason, P.W.; et al. Chapter Seven-Self-Amplifying mRNA Vaccines. Adv. Genet. 2015, 89, 179–233. [Google Scholar]
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E.; Lee, A.; Woods, A.; Yuzhakov, O.; Himansu, S.; Deterling, J.; Geilich, B.M.; Ketova, T.; et al. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Mol. Ther. Nucleic Acids 2019, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bancel, S.; Issa William, J.; Aunins John, G.; Chakraborty, T. Manufacturing Methods for Production of RNA Transcripts. WO/2014/152027; PCT/US2014/026835; US20160024547A1. United States Patent and Trademark Office, 2014. Available online: https://patentimages.storage.googleapis.com/7a/bb/8f/5ce58cdaa18a0d/US20160024547A1.pdf (accessed on 10 November 2020).
- Berlanda, S.F.; Wen, Y.; Geall, A.; Porter, F. RNA Purification Methods. 20160024139, EP2970948A1; WO2014140211A1. 2016. Available online: https://patents.google.com/patent/EP2970948A1/no (accessed on 1 May 2018).
- Funkner, A.; Dorner, S.; Sewing, S.; Kamm, J.; Broghammer, N.; Ketterer, T.; Mutzke, T. A Method for Producing and Purifying RNA, Comprising at Least One Step of Tangential Flow Filtration. PCT/EP2016/062152; WO/2016/193206. World Intellectual Property Organization, 2016. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2016193206 (accessed on 10 October 2020).
- Wochner, A.; Roos, T.; Ketterer, T. Methods and Means for Enhancing RNA Production. U.S. Patent 20170114378A1; United States Patent and Trademark Office, 27 April 2017. Available online: https://patents.google.com/patent/US20170114378A1/de (accessed on 10 October 2020).
- Heartlein, M.; Derosa, F.; Dias, A.; Karve, S. Methods for Purification of Messenger RNA. DK14714150.1T; PCT/US2014/028441. 2014. Available online: https://patents.google.com/patent/DK2970955T3/en (accessed on 15 December 2019).
- Kis, Z.; Shattock, R.; Shah, N.; Kontoravdi, C. Emerging Technologies for Low-Cost, Rapid Vaccine Manufacture. Biotechnol. J. 2019, 14, 1800376. [Google Scholar] [CrossRef] [Green Version]
- Kis, Z.; Kontoravdi, C.; Dey, A.K.; Shattock, R.; Shah, N. cRapid development and deployment of high-volume vaccines for pandemic response. J. Adv. Manuf. Process. 2020, 2, e10060. [Google Scholar] [CrossRef]
- Blakney, A.K.; McKay, P.F.; Yus, B.I.; Aldon, Y.; Shattock, R.J. Inside out: Optimization of lipid nanoparticle formulations for exterior complexation and in vivo delivery of saRNA. Gene Ther. 2019, 26, 363–372. [Google Scholar] [CrossRef]
- ModernaTX. A Phase 2a, Randomized, Observer-Blind, Placebo Controlled, Dose-Confirmation Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of mRNA-1273 SARS-COV-2 Vaccine in Adults Aged 18 Years and Older. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04405076 (accessed on 1 October 2020).
- ModernaTX. A Phase 3, Randomized, Stratified, Observer-Blind, Placebo-Controlled Study to Evaluate the Efficacy, Safety, and Immunogenicity of mRNA-1273 SARS-CoV-2 Vaccine in Adults Aged 18 Years and Older. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04470427 (accessed on 1 October 2020).
- CureVac AG. COVID-19: A Phase 2a, Partially Observer-blind, Multicenter, Controlled, Dose-confirmation Clinical Trial to Evaluate the Safety, Reactogenicity and Immunogenicity of the Investigational SARS-CoV-2 mRNA Vaccine CVnCoV in Adults >60 Years of Age and 18 to 60 Years of Age. ClinicalTrials.gov, 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04515147 (accessed on 9 October 2020).
- CureVac AG. A Phase 1, Partially Blind, Placebo-controlled, Dose-escalation, First-in-human, Clinical Trial to Evaluate the Safety, Reactogenicity and Immunogenicity After 1 and 2 Doses of the Investigational SARS-CoV-2 mRNA Vaccine CVnCoV Administered Intramuscularl. ClinicalTrials.gov, 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04449276 (accessed on 9 October 2020).
- Petrides, D. SuperPro Designer User Guide—A Comprehensive Simulation Tool for the Design, Retrofit & Evaluation of Specialty Chemical, Biochemical, Pharmaceutical, Consumer Product, Food, Agricultural, Mineral Processing, Packaging AND Water Purification, Wastewater. Scotch Plains, NJ, USA. 2013. Available online: http://www.intelligen.com/downloads/SuperPro_ManualForPrinting_v10.pdf (accessed on 22 March 2020).
- Petrides, D. Bioprocess Design and Economics. In Bioseparations Science and Engineering, 2nd ed.; Oxford University Press: Oxford, UK, 2015; pp. 1–83. Available online: http://www.intelligen.com/downloads/BioProcessDesignAndEconomics_March_2015.pdf (accessed on 12 May 2020).
- Petrides, D.; Carmichael, D.; Siletti, C.; Koulouris, A. Biopharmaceutical Process Optimization with Simulation and Scheduling Tools. Bioengineering. MDPI: Basel, Switzerland; pp. 154–187. Available online: https://www.mdpi.com/2306-5354/1/4/154 (accessed on 10 June 2020).
- Roces, C.B.; Lou, G.; Jain, N.; Abraham, S.; Thomas, A.; Halbert, G.W.; Perrie, Y. Manufacturing considerations for the development of lipid nanoparticles using microfluidics. Pharmaceutics 2020, 12, 1095. [Google Scholar] [CrossRef]
- Maier, M.A.; Jayaraman, M.; Matsuda, S.; Liu, J.; Barros, S.; Querbes, W.; Tam, Y.K.; Ansell, S.M.; Kumar, V.; Qin, J.; et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 2013, 21, 1570–1578. Available online: https://pubmed.ncbi.nlm.nih.gov/23799535 (accessed on 5 June 2013). [CrossRef] [Green Version]
- Payne, J.E.; Chivukula, P. Ionizable Cationic Lipid for Rna Delivery. USA: WIPO (PCT); WO2015074085A1; US9593077B2; EP3071547A1. 2014. Available online: https://patents.google.com/patent/WO2015074085A1/ (accessed on 15 August 2020).
- Dammes, N.; Peer, D. Paving the Road for RNA Therapeutics. Trends Pharmacol. Sci. 2020, 41, 755–775. Available online: https://pubmed.ncbi.nlm.nih.gov/32893005 (accessed on 3 September 2020). [CrossRef]
- Maeki, M.; Kimura, N.; Sato, Y.; Harashima, H.; Tokeshi, M. Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv. Drug Deliv. Rev. 2018, 128, 84–100. [Google Scholar] [CrossRef]
- Webb, C.; Forbes, N.; Roces, C.B.; Anderluzzi, G.; Lou, G.; Abraham, S.; Ingalls, L.; Marshall, K.; Leaver, T.J.; Watts, J.A.; et al. Using microfluidics for scalable manufacturing of nanomedicines from bench to GMP: A case study using protein-loaded liposomes. Int. J. Pharm. 2020, 582, 119266. [Google Scholar] [CrossRef]
- O’Hare, R.; Lynch, P. First Novel COVID-19 Vaccine Candidate Commences Animal Testing. 2020. Available online: https://www.univadis.co.uk/viewarticle/first-novel-covid-19-vaccine-candidate-commences-animal-testing-712575 (accessed on 28 February 2020).
- Hodgson, J. The Pandemic Pipeline. Nature Biotechnology. United States. 2020. Available online: https://www.nature.com/articles/d41587-020-00005-z (accessed on 15 December 2020).
- Prazeres, D.M.F.; Ferreira, G.N.M.; Monteiro, G.A.; Cooney, C.L.; Cabral, J.M.S. Large-scale production of pharmaceutical-grade plasmid DNA for gene therapy: Problems and bottlenecks. Trends Biotechnol. 1999, 17, 169–174. [Google Scholar] [CrossRef]
- Schmeer, M.; Buchholz, T.; Schleef, M. Plasmid DNA Manufacturing for Indirect and Direct Clinical Applications. Hum Gene Ther. 2017, 28, 856–861. [Google Scholar] [CrossRef]
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics—Developing a new class of drugs. Nat Rev Drug Discov. 2014, 3, 759–780. [Google Scholar] [CrossRef]
- Karda, R.; Counsell, J.R.; Karbowniczek, K.; Caproni, L.J.; Tite, J.P.; Waddington, S.N. Production of lentiviral vectors using novel, enzymatically produced, linear DNA. Gene Ther. 2019, 26, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Karbowniczek, K.; Rothwell, P.; Extance, J.; Milsom, S.; Lukashchuk, V.; Bowes, K.; Smith, D.; Caproni, L. DoggyboneTM DNA: an advanced platform for AAV production. Cell Gene Ther. Insights 2017, 3, 731–738. [Google Scholar] [CrossRef] [Green Version]
- Touchlight Genetics Ltd. Our Unique Synthetic DNA Vectors Advance Medicine and Manufacturing; Touchlight: London, UK, 2020; Available online: https://www.touchlight.com/technology/ (accessed on 15 December 2020).
- Wen, E.P.; Ellis, R.J.; Pujar, N.S. Vaccine Development and Manufacturing; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; Available online: https://books.google.co.uk/books?id=GXqZAQAACAAJ (accessed on 12 February 2019).
- Pfizer Inc. Pfizer and BioNTech Conclude Phase 3 Study of Covid-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints. 2020. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine (accessed on 16 December 2020).
- Reuters. Moderna to Supply up to 125 Million COVID-19 Vaccine Doses Globally in First Quarter. Reuters Health News. Available online: https://www.reuters.com/article/uk-health-coronavirus-moderna/moderna-to-supply-up-to-125-million-covid-19-vaccine-doses-globally-in-first-quarter-idUKKBN28D3FC (accessed on 16 December 2020).
- Reuters. Lonza aims to make ingredients for 400 million doses of Moderna’s COVID vaccine annually. Reuters Healthc. Pharma 2020. Available online: https://www.reuters.com/article/us-health-coronavirus-lonza-moderna-idUSKBN27W1N0 (accessed on 16 December 2020).
- MEDInstill. INTACTTM Modular Filler (IMF). 2020. Available online: http://www.medinstill.com/intact_modular_filler_imf.php (accessed on 20 April 2020).
- MEDInstill. Email and Teleconference Correspondence with Experts from MEDInstill; MEDInstill: New Milford, CT, USA, 2020. [Google Scholar]
- Kroll, P.; Hofer, A.; Ulonska, S.; Kager, J.; Herwig, C. Model-Based Methods in the Biopharmaceutical Process Lifecycle. Pharm. Res. 2017, 34, 2596–2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommeregger, W.; Sissolak, B.; Kandra, K.; von Stosch, M.; Mayer, M.; Striedner, G. Quality by control: Towards model predictive control of mammalian cell culture bioprocesses. Biotechnol. J. 2017, 12, 1600546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesbah, A.; Paulson, J.A.; Lakerveld, R.; Braatz, R.D. Model Predictive Control of an Integrated Continuous Pharmaceutical Manufacturing Pilot Plant. Org. Process Res. Dev. 2017, 21, 844–854. [Google Scholar] [CrossRef] [Green Version]
| No. | Vaccine Type | Developer | Vaccine Name | RNA Per Dose (µg/Dose) | Probable Doses Per Person | Type of UTP * | Manufacturing Location | Clinical Development Phase | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | mRNA | Moderna Inc. NIAID | mRNA-1273 | 100 | 2 | Modified | USA, Spain, Switzerland, | Phase 3 clinical trial | [1,7,8,9] |
| 2 | mRNA | BioNTech SE; Pfizer Inc. | BNT162b2 | 30 | 2 | Modified | USA, Germany, Belgium | Phase 3 clinical trial | [1,8,10,11,12] |
| 3 | mRNA | CureVac N.V. | CVnCoV | 12 | 2 | Wild-type | Germany | Phase 2 clinical trial | [1,8,13] |
| 4 | saRNA | Imperial College London | LNP-nCoVsaRNA | 1 | 2 | Wild-type | UK | Phase 1/2 clinical trial | [1,3,14] |
| 5 | saRNA | T.B.D. ** | T.B.D. ** | 0.1 | 1 | Wild-type | T.B.D. ** | N.A. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kis, Z.; Kontoravdi, C.; Shattock, R.; Shah, N. Resources, Production Scales and Time Required for Producing RNA Vaccines for the Global Pandemic Demand. Vaccines 2021, 9, 3. https://doi.org/10.3390/vaccines9010003
Kis Z, Kontoravdi C, Shattock R, Shah N. Resources, Production Scales and Time Required for Producing RNA Vaccines for the Global Pandemic Demand. Vaccines. 2021; 9(1):3. https://doi.org/10.3390/vaccines9010003
Chicago/Turabian StyleKis, Zoltán, Cleo Kontoravdi, Robin Shattock, and Nilay Shah. 2021. "Resources, Production Scales and Time Required for Producing RNA Vaccines for the Global Pandemic Demand" Vaccines 9, no. 1: 3. https://doi.org/10.3390/vaccines9010003






