Identification of Promiscuous African Swine Fever Virus T-Cell Determinants Using a Multiple Technical Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Multiparametric In Silico Predictions of ASFV-CD8+ T-Cell Epitopes
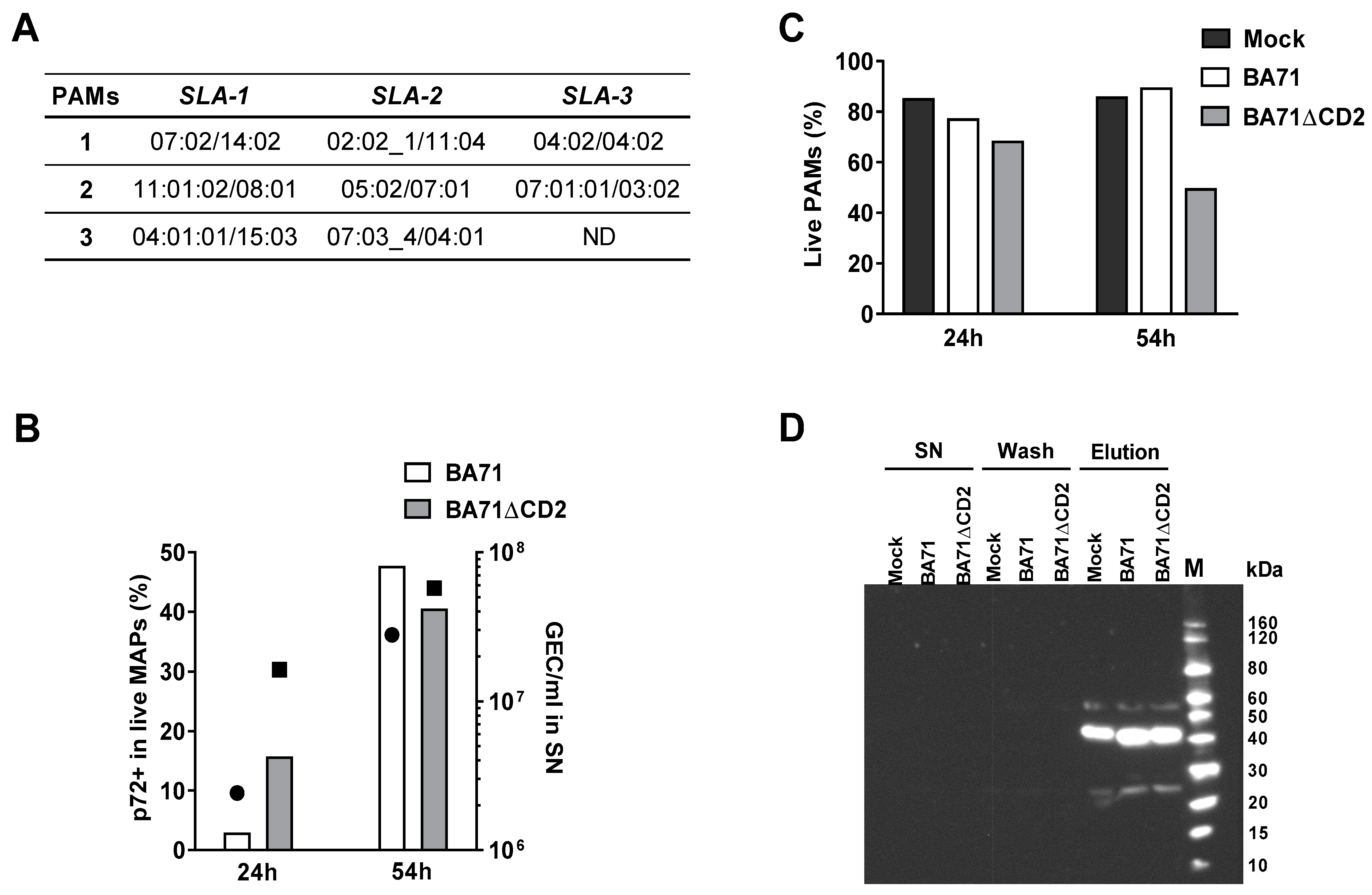
2.3. Typing of SLA I Genes
2.4. In Vitro Infection of PAMs with ASFV
2.5. Determination of Cell Viability and Percentage of Infected Cells by Flow Cytometry
2.6. Affinity Purification of SLA I Molecules
2.7. Western Blot to Detect Immunoprecipitated SLA I-Peptide Complexes
2.8. On-Tip Desalting and LC-MS/MS Analysis
2.9. Database Search and Peptide Identification
2.10. PBMC Purification
2.11. Porcine IFNγ ELISpot
3. Results
3.1. In Silico Prediction of CD8+ T-Cell Epitopes Using the Georgia2007/1 Proteome and In Vitro Validation as ASFV-Specific T-Cell Epitopes
3.2. Identification of SLA I-Restricted Peptides by Mass Spectrometry-Based Immunopeptidomics and In Vitro Validation as ASFV-Specific T-Cell Epitopes
3.3. Identification of ASFV Full-Length Proteins Promiscuously Recognized by ASFV-Specific T-Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arias, M.; Sánchez-Vizcaíno, J.M. African Swine Fever Eradication: The Spanish Model. In Trends in Emerging Viral Infections of Swine; Iowa State Press: Ames, IA, USA, 2002; pp. 133–139. [Google Scholar] [CrossRef]
- Alonso, C.; Borca, M.; Dixon, L.; Revilla, Y.; Rodríguez, F.; Escribano, J.M. ICTV Virus Taxonomy Profile: Asfarviridae. J. Gen. Virol. 2018, 99, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.E. On a Form of Swine Fever Occurring in British East Africa (Kenya Colony). J. Comp. Pathol. Ther. 1921, 34, 159–191. [Google Scholar] [CrossRef]
- Costard, S.; Mur, L.; Lubroth, J.; Sanchez-Vizcaino, J.M.; Pfeiffer, D.U. Epidemiology of African Swine Fever Virus. Virus Res. 2013, 173, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Alcrudo, D.; Lubroth, J.; Depner, K.; La Rocque, S. African Swine Fever in the Caucasus; FAO EMPRES Watch, April 2008; pp. 1–8. Available online: https://www.researchgate.net/publication/280559339_African_swine_fever_in_the_Caucasus (accessed on 6 January 2021).
- Kim, S.H.; Kim, J.; Son, K.; Choi, Y.; Jeong, H.S.; Kim, Y.K.; Park, J.E.; Hong, Y.J.; Lee, S.I.; Wang, S.J.; et al. Wild Boar Harbouring African Swine Fever Virus in the Demilitarized Zone in South Korea, 2019. In Emerging Microbes and Infections; Taylor and Francis Ltd.: Abingdon, UK, 2020; pp. 628–630. [Google Scholar] [CrossRef]
- Li, X.; Tian, K. African Swine Fever in China. Vet. Rec. 2018, 183, 300–301. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Li, J.; Fan, X.; Liu, F.; Li, L.; Wang, Q.; Ren, W.; Bao, J.; Liu, C.; Wang, H.; et al. Molecular Characterization of African Swine Fever Virus, China, 2018. Emerg. Infect. Dis. 2018, 24, 2131–2133. [Google Scholar] [CrossRef]
- Bosch-Camós, L.; López, E.; Rodriguez, F. African Swine Fever Vaccines: A Promising Work Still in Progress. Porc. Heal. Manag. 2020, 6, 17. [Google Scholar] [CrossRef]
- O’Donnell, V.; Holinka, L.G.; Gladue, D.P.; Sanford, B.; Krug, P.W.; Lu, X.; Arzt, J.; Reese, B.; Carrillo, C.; Risatti, G.R.; et al. African Swine Fever Virus Georgia Isolate Harboring Deletions of MGF360 and MGF505 Genes Is Attenuated in Swine and Confers Protection against Challenge with Virulent Parental Virus. J. Virol. 2015, 89, 6048–6056. [Google Scholar] [CrossRef]
- O’Donnell, V.; Risatti, G.R.; Holinka, L.G.; Krug, P.W.; Carlson, J.; Velazquez-Salinas, L.; Azzinaro, P.A.; Gladue, D.P.; Borca, M.V. Simultaneous Deletion of the 9GL and UK Genes from the African Swine Fever Virus Georgia 2007 Isolate Offers Increased Safety and Protection against Homologous Challenge. J. Virol. 2017, 91, e01760-16. [Google Scholar] [CrossRef]
- Monteagudo, P.L.; Lacasta, A.; López, E.; Bosch, L.; Collado, J.; Pina-Pedrero, S.; Correa-Fiz, F.; Accensi, F.; Navas, M.J.; Vidal, E.; et al. BA71ΔCD2: A New Recombinant Live Attenuated African Swine Fever Virus with Cross-Protective Capabilities. J. Virol. 2017, 91, e01058-17. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, D.; He, X.; Liu, R.; Wang, Z.; Zhang, X.; Li, F.; Shan, D.; Chen, H.; Zhang, J.; et al. A Seven-Gene-Deleted African Swine Fever Virus Is Safe and Effective as a Live Attenuated Vaccine in Pigs. Sci. China Life Sci. 2020, 63, 623–634. [Google Scholar] [CrossRef]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Holinka, L.G.; Velazquez-Salinas, L.; Zhu, J.; Gladue, D.P. Development of a Highly Effective African Swine Fever Virus Vaccine by Deletion of the I177L Gene Results in Sterile Immunity against the Current Epidemic Eurasia Strain. J. Virol. 2020, 94, e02017-19. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, G.; Dei Giudici, S.; Oggiano, A. Infection, Modulation and Responses of Antigen-Presenting Cells to African Swine Fever Viruses. Virus Res. 2018, 258, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Gil, S.; Sepúlveda, N.; Albina, E.; Leitão, A.; Martins, C. The Low-Virulent African Swine Fever Virus (ASFV/NH/P68) Induces Enhanced Expression and Production of Relevant Regulatory Cytokines (IFNalpha, TNFalpha and IL12p40) on Porcine Macrophages in Comparison to the Highly Virulent ASFV/L60. Arch. Virol. 2008, 153, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, G.; Graham, S.P.; Giudici, S.D.; Bonelli, P.; Pilo, G.; Anfossi, A.G.; Pittau, M.; Nicolussi, P.S.; Laddomada, A.; Oggiano, A. Characterization of the Interaction of African Swine Fever Virus with Monocytes and Derived Macrophage Subsets. Vet. Microbiol. 2017, 198, 88–98. [Google Scholar] [CrossRef]
- Franzoni, G.; Graham, S.P.; Sanna, G.; Angioi, P.; Fiori, M.S.; Anfossi, A.; Amadori, M.; Dei Giudici, S.; Oggiano, A. Interaction of Porcine Monocyte-Derived Dendritic Cells with African Swine Fever Viruses of Diverse Virulence. Vet. Microbiol. 2018, 216, 190–197. [Google Scholar] [CrossRef]
- Onisk, D.V.; Borca, M.V.; Kutish, S.; Kramer, E.; Irusta, P.; Rock, D.L. Passively Transferred African Swine Fever Virus Antibodies Protect Swine against Lethal Infection. Virology 1994, 198, 350–354. [Google Scholar] [CrossRef]
- Ruiz Gonzalvo, F.; Carnero, M.E.; Caballero, C.; Martínez, J. Inhibition of African Swine Fever Infection in the Presence of Immune Sera in Vivo and in Vitro. Am. J. Vet. Res. 1986, 47, 1249–1252. [Google Scholar]
- Oura, C.A.; Denyer, M.S.; Takamatsu, H.; Parkhouse, R.M. In Vivo Depletion of CD8+ T Lymphocytes Abrogates Protective Immunity to African Swine Fever Virus. J. Gen. Virol. 2005, 86, 2445–2450. [Google Scholar] [CrossRef]
- Argilaguet, J.M.; Pérez-Martín, E.; Nofrarías, M.; Gallardo, C.; Accensi, F.; Lacasta, A.; Mora, M.; Ballester, M.; Galindo-Cardiel, I.; López-Soria, S.; et al. DNA Vaccination Partially Protects against African Swine Fever Virus Lethal Challenge in the Absence of Antibodies. PLoS ONE 2012, 7, e40942. [Google Scholar] [CrossRef]
- Lacasta, A.; Ballester, M.; Monteagudo, P.L.; Rodríguez, J.M.; Salas, M.L.; Accensi, F.; Pina-Pedrero, S.; Bensaid, A.; Argilaguet, J.; López-Soria, S.; et al. Expression Library Immunization Can Confer Protection against Lethal Challenge with African Swine Fever Virus. J. Virol. 2014, 88, 13322–13332. [Google Scholar] [CrossRef]
- Bosch-Camós, L. Unmasking African Swine Fever Virus Antigens Inducing CD8+ T-Cell Responses with Protective Potential; Universitat Autònoma de Barcelona: Barcelona, Spain, 2019. [Google Scholar]
- Andrés, G.; Charro, D.; Matamoros, T.; Dillard, R.S.; Abrescia, N.G.A. The Cryo-EM Structure of African Swine Fever Virus Unravels a Unique Architecture Comprising Two Icosahedral Protein Capsids and Two Lipoprotein Membranes. J. Biol. Chem. 2020, 295, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Luo, Y.; Wang, Y.; Li, S.; Zhao, Z.; Bi, Y.; Sun, J.; Peng, R.; Song, H.; Zhu, D.; et al. Cryo-EM Structure of the African Swine Fever Virus. Cell Host Microbe 2019, 26, 836–843.e3. [Google Scholar] [CrossRef] [PubMed]
- Cackett, G.; Matelska, D.; Sýkora, M.; Portugal, R.; Malecki, M.; Bähler, J.; Dixon, L.; Werner, F. The African Swine Fever Virus Transcriptome. J. Virol. 2020, 94, e00119-20. [Google Scholar] [CrossRef] [PubMed]
- Alejo, A.; Matamoros, T.; Guerra, M.; Andrés, G. A Proteomic Atlas of the African Swine Fever Virus Particle. J. Virol. 2018, 92, e01293-18. [Google Scholar] [CrossRef]
- Rodriguez, F.; Slifka, M.K.; Harkins, S.; Whitton, J.L. Two Overlapping Subdominant Epitopes Identified by DNA Immunization Induce Protective CD8(+) T-Cell Populations with Differing Cytolytic Activities. J. Virol. 2001, 75, 7399–7409. [Google Scholar] [CrossRef]
- Rodriguez, F.; Whitton, J.L. Enhancing DNA Immunization. Virology 2000, 268, 233–238. [Google Scholar] [CrossRef]
- Takashima, A. Establishment of Fibroblast Cultures. Curr. Protoc. Cell Biol. 1998, 2.1.1–2.1.12. [Google Scholar] [CrossRef]
- Nielsen, M.; Andreatta, M. NetMHCpan-3.0; Improved Prediction of Binding to MHC Class I Molecules Integrating Information from Multiple Receptor and Peptide Length Datasets. Genome Med. 2016, 8, 33. [Google Scholar] [CrossRef]
- Calis, J.J.A.; Maybeno, M.; Greenbaum, J.A.; Weiskopf, D.; De Silva, A.D.; Sette, A.; Keşmir, C.; Peters, B. Properties of MHC Class I Presented Peptides That Enhance Immunogenicity. PLoS Comput. Biol. 2013, 9, e1003266. [Google Scholar] [CrossRef]
- Diez-Rivero, C.M.; Chenlo, B.; Zuluaga, P.; Reche, P.A. Quantitative Modeling of Peptide Binding to TAP Using Support Vector Machine. Proteins 2010, 78, 63–72. [Google Scholar] [CrossRef]
- Choi, H.; Le, M.T.; Lee, H.; Choi, M.-K.; Cho, H.-S.; Nagasundarapandian, S.; Kwon, O.-J.; Kim, J.-H.; Seo, K.; Park, J.-K.; et al. Sequence Variations of the Locus-Specific 5′ Untranslated Regions of SLA Class I Genes and the Development of a Comprehensive Genomic DNA-Based High-Resolution Typing Method for SLA-2. Tissue Antigens 2015, 86, 255–266. [Google Scholar] [CrossRef]
- Le, M.T.; Choi, H.; Lee, H.; Le, V.C.Q.; Ahn, B.; Ho, C.S.; Hong, K.; Song, H.; Kim, J.H.; Park, C. SLA-1 Genetic Diversity in Pigs: Extensive Analysis of Copy Number Variation, Heterozygosity, Expression, and Breed Specificity. Sci. Rep. 2020, 10, 743. [Google Scholar] [CrossRef]
- Lee, J.; Le, M.T.; Choi, M.K.; Quy Le, V.C.; Choi, H.; Lee, H.; Song, H.; Kim, J.H.; Park, C. Development of a Simple SLA-1 Copy-Number-Variation Typing and the Comparison of Typing Accuracy between Real-Time Quantitative and Droplet Digital PCR. In Animal Genetics; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2019; pp. 315–316. [Google Scholar] [CrossRef]
- Bullido, R.; Ezquerra, A.; Alonso, F.; Gómez del Moral, M.D.J. Characterization of a New Monoclonal Antibody (4B7) Specific for Porcine MHC (SLA) Class I Antigens. Investig. Agrar. Prod. Sanid. Anim. 1996, 11, 29–37. [Google Scholar]
- Yáñez, R.J.; Rodríguez, J.M.; Boursnell, M.; Rodríguez, J.F.; Viñuela, E. Two Putative African Swine Fever Virus Helicases Similar to Yeast “DEAH” Pre-MRNA Processing Proteins and Vaccinia Virus ATPases D11L and D6R. Gene 1993, 134, 161–174. [Google Scholar] [CrossRef]
- Simón-Mateo, C.; Andrés, G.; Viñuela, E. Polyprotein Processing in African Swine Fever Virus: A Novel Gene Expression Strategy for a DNA Virus. EMBO J. 1993, 12, 2977–2987. [Google Scholar] [CrossRef]
- Piriou-Guzylack, L.; Salmon, H. Membrane Markers of the Immune Cells in Swine: An Update. Vet. Res. 2008, 39, 54. [Google Scholar] [CrossRef]
- Takamatsu, H.-H.; Denyer, M.S.; Lacasta, A.; Stirling, C.M.A.; Argilaguet, J.M.; Netherton, C.L.; Oura, C.A.L.; Martins, C.; Rodríguez, F. Cellular Immunity in ASFV Responses. Virus Res. 2013, 173, 110–121. [Google Scholar] [CrossRef]
- Powell, P.P.; Dixon, L.K.; Parkhouse, R.M. An IkappaB Homolog Encoded by African Swine Fever Virus Provides a Novel Mechanism for Downregulation of Proinflammatory Cytokine Responses in Host Macrophages. J. Virol. 1996, 70, 8527–8533. [Google Scholar] [CrossRef] [PubMed]
- Sang, H.; Miller, G.; Lokhandwala, S.; Sangewar, N.; Waghela, S.D.; Bishop, R.P.; Mwangi, W. Progress toward Development of Effective and Safe African Swine Fever Virus Vaccines. Front. Vet. Sci. 2020, 7, 84. [Google Scholar] [CrossRef]
- Arias, M.; de la Torre, A.; Dixon, L.; Gallardo, C.; Jori, F.; Laddomada, A.; Martins, C.; Parkhouse, R.M.; Revilla, Y.; Rodriguez, F.A.J.-M.; et al. Approaches and Perspectives for Development of African Swine Fever Virus Vaccines. Vaccines 2017, 5, 35. [Google Scholar] [CrossRef]
- Ivanov, V.; Efremov, E.E.; Novikov, B.V.; Balyshev, V.M.; Tsibanov, S.Z.; Kalinovsky, T.; Kolbasov, D.V.; Niedzwiecki, A.; Rath, M. Vaccination with Viral Protein-Mimicking Peptides Postpones Mortality in Domestic Pigs Infected by African Swine Fever Virus. Mol. Med. Rep. 2011, 4, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Jancovich, J.K.; Chapman, D.; Hansen, D.T.; Robida, M.D.; Loskutov, A.; Craciunescu, F.; Borovkov, A.; Kibler, K.; Goatley, L.; King, K.; et al. Immunization of Pigs by DNA Prime and Recombinant Vaccinia Virus Boost To Identify and Rank African Swine Fever Virus Immunogenic and Protective Proteins. J. Virol. 2018, 92, e02219-17. [Google Scholar] [CrossRef]
- Netherton, C.L.; Goatley, L.C.; Reis, A.L.; Portugal, R.; Nash, R.H.; Morgan, S.B.; Gault, L.; Nieto, R.; Norlin, V.; Gallardo, C.; et al. Identification and Immunogenicity of African Swine Fever Virus Antigens. Front. Immunol. 2019, 10, 1318. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, S.-Y.; Pérez-Núñez, D.; Morozov, I.; Sánchez, E.; Gaudreault, N.; Trujillo, J.; Mur, L.; Nogal, M.; Madden, D.; Urbaniak, K.; et al. DNA-Protein Vaccination Strategy Does Not Protect from Challenge with African Swine Fever Virus Armenia 2007 Strain. Vaccines 2019, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Barderas, M.G.; Rodríguez, F.; Gómez-Puertas, P.; Avilés, M.; Beitia, F.; Alonso, C.; Escribano, J.M. Antigenic and Immunogenic Properties of a Chimera of Two Immunodominant African Swine Fever Virus Proteins. Arch. Virol. 2001, 146, 1681–1691. [Google Scholar] [CrossRef]
- Ruiz-Gonzalvo, F.; Rodríguez, F.; Escribano, J.M. Functional and Immunological Properties of the Baculovirus-Expressed Hemagglutinin of African Swine Fever Virus. Virology 1996, 218, 285–289. [Google Scholar] [CrossRef]
- Zsak, L.; Onisk, D.V.; Afonso, C.L.; Rock, D.L. Virulent African Swine Fever Virus Isolates Are Neutralized by Swine Immune Serum and by Monoclonal Antibodies Recognizing a 72-KDa Viral Protein. Virology 1993, 196, 596–602. [Google Scholar] [CrossRef]
- Neilan, J.G.; Zsak, L.; Lu, Z.; Burrage, T.G.; Kutish, G.F.; Rock, D.L. Neutralizing Antibodies to African Swine Fever Virus Proteins P30, P54, and P72 Are Not Sufficient for Antibody-Mediated Protection. Virology 2004, 319, 337–342. [Google Scholar] [CrossRef]
- Lokhandwala, S.; Petrovan, V.; Popescu, L.; Sangewar, N.; Elijah, C.; Stoian, A.; Olcha, M.; Ennen, L.; Bray, J.; Bishop, R.P.; et al. Adenovirus-Vectored African Swine Fever Virus Antigen Cocktails Are Immunogenic but Not Protective against Intranasal Challenge with Georgia 2007/1 Isolate. Vet. Microbiol. 2019, 235, 10–20. [Google Scholar] [CrossRef]
- Chapman, D.A.G.; Darby, A.C.; Da Silva, M.; Upton, C.; Radford, A.D.; Dixon, L.K. Genomic Analysis of Highly Virulent Georgia 2007/1 Isolate of African Swine Fever Virus. Emerg. Infect. Dis. 2011, 17, 599–605. [Google Scholar] [CrossRef]
- Goatley, L.C.; Reis, A.L.; Portugal, R.; Goldswain, H.; Shimmon, G.L.; Hargreaves, Z.; Ho, C.-S.; Montoya, M.; Sánchez-Cordón, P.J.; Taylor, G.; et al. A Pool of Eight Virally Vectored African Swine Fever Antigens Protect Pigs against Fatal Disease. Vaccines 2020, 8, 234. [Google Scholar] [CrossRef] [PubMed]
- Correa-Fiz, F. Identification of Potential Vaccine Candidates against the African Swine Fever Virus through Reverse Vaccinology; Universitat Autònoma de Barcelona: Barcelona, Spain, 2014. [Google Scholar]
- Moise, L.; Gutiérrez, A.H.; Khan, S.; Tan, S.; Ardito, M.; Martin, W.D.; De Groot, A.S. New Immunoinformatics Tools for Swine: Designing Epitope-Driven Vaccines, Predicting Vaccine Efficacy, and Making Vaccines on Demand. Front. Immunol. 2020, 11, 563362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Qi, J.; Feng, S.; Gao, F.; Liu, J.; Pan, X.; Chen, R.; Li, Q.; Chen, Z.; Li, X.; et al. Crystal Structure of Swine Major Histocompatibility Complex Class I SLA-1*0401 and Identification of 2009 Pandemic Swine-Origin Influenza A H1N1 Virus Cytotoxic T Lymphocyte Epitope Peptides. J. Virol. 2011, 85, 11709–11724. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Wu, Y.; Wang, S.; Wang, Z.; Jiang, B.; Liu, Y.; Liang, R.; Zhou, W.; Zhang, N.; Xia, C. Structural and Biochemical Analyses of Swine Major Histocompatibility Complex Class I Complexes and Prediction of the Epitope Map of Important Influenza A Virus Strains. J. Virol. 2016, 90, 6625–6641. [Google Scholar] [CrossRef]
- Gao, C.; He, X.; Quan, J.; Jiang, Q.; Lin, H.; Chen, H.; Qu, L. Specificity Characterization of SLA Class I Molecules Binding to Swine-Origin Viral Cytotoxic T Lymphocyte Epitope Peptides in Vitro. Front. Microbiol. 2017, 8, 2524. [Google Scholar] [CrossRef]
- Maccari, G.; Robinson, J.; Ballingall, K.; Guethlein, L.A.; Grimholt, U.; Kaufman, J.; Ho, C.-S.; de Groot, N.G.; Flicek, P.; Bontrop, R.E.; et al. IPD-MHC 2.0: An Improved Inter-Species Database for the Study of the Major Histocompatibility Complex. Nucleic Acids Res. 2017, 45, D860–D864. [Google Scholar] [CrossRef]
- Ros-Lucas, A.; Correa-Fiz, F.; Bosch-Camós, L.; Rodriguez, F.; Alonso-Padilla, J. Computational Analysis of African Swine Fever Virus Protein Space for the Design of an Epitope-Based Vaccine Ensemble. Pathogens 2020, 9, 1078. [Google Scholar] [CrossRef]
- Probst-Kepper, M.; Hecht, H.-J.; Herrmann, H.; Janke, V.; Ocklenburg, F.; Klempnauer, J.; van den Eynde, B.J.; Weiss, S. Conformational Restraints and Flexibility of 14-Meric Peptides in Complex with HLA-B*3501. J. Immunol. 2004, 173, 5610–5616. [Google Scholar] [CrossRef]
- Tynan, F.E.; Borg, N.A.; Miles, J.J.; Beddoe, T.; El-Hassen, D.; Silins, S.L.; van Zuylen, W.J.M.; Purcell, A.W.; Kjer-Nielsen, L.; McCluskey, J.; et al. High Resolution Structures of Highly Bulged Viral Epitopes Bound to Major Histocompatibility Complex Class I. J. Biol. Chem. 2005, 280, 23900–23909. [Google Scholar] [CrossRef]
- Schellens, I.M.M.; Hoof, I.; Meiring, H.D.; Spijkers, S.N.M.; Poelen, M.C.M.; van Gaans-van den Brink, J.A.M.; van der Poel, K.; Costa, A.I.; van Els, C.A.C.M.; van Baarle, D.; et al. Comprehensive Analysis of the Naturally Processed Peptide Repertoire: Differences between HLA-A and B in the Immunopeptidome. PLoS ONE 2015, 10, e0136417. [Google Scholar] [CrossRef]
- Pymm, P.; Illing, P.T.; Ramarathinam, S.H.; O’Connor, G.M.; Hughes, V.A.; Hitchen, C.; Price, D.A.; Ho, B.K.; McVicar, D.W.; Brooks, A.G.; et al. MHC-I Peptides Get out of the Groove and Enable a Novel Mechanism of HIV-1 Escape. Nat. Struct. Mol. Biol. 2017, 24, 387–394. [Google Scholar] [CrossRef]
- Ramarathinam, S.H.; Gras, S.; Alcantara, S.; Yeung, A.W.S.; Mifsud, N.A.; Sonza, S.; Illing, P.T.; Glaros, E.N.; Center, R.J.; Thomas, S.R.; et al. Identification of Native and Posttranslationally Modified HLA-B*57:01-Restricted HIV Envelope Derived Epitopes Using Immunoproteomics. Proteomics 2018, 18, e1700253. [Google Scholar] [CrossRef]
- Trujillo, J.A.; Croft, N.P.; Dudek, N.L.; Channappanavar, R.; Theodossis, A.; Webb, A.I.; Dunstone, M.A.; Illing, P.T.; Butler, N.S.; Fett, C.; et al. The Cellular Redox Environment Alters Antigen Presentation. J. Biol. Chem. 2014, 289, 27979–27991. [Google Scholar] [CrossRef]
- Sercarz, E.E.; Lehmann, P.V.; Ametani, A.; Benichou, G.; Miller, A.; Moudgil, K. Dominance and Crypticity of T Cell Antigenic Determinants. Annu. Rev. Immunol. 1993, 11, 729–766. [Google Scholar] [CrossRef]
- Oura, C.A.; Powell, P.P.; Parkhouse, R.M. Detection of African Swine Fever Virus in Infected Pig Tissues by Immunocytochemistry and in Situ Hybridisation. J. Virol. Methods 1998, 72, 205–217. [Google Scholar] [CrossRef]
- Pan, I.-C. Spontaneously Susceptible Cells and Cell Culture Methodologies for African Swine Fever Virus. In African Swine Fever; Becker, Y., Ed.; Developments in Veterinary Virology; Springer: Boston, MA, USA, 1987; pp. 81–126. [Google Scholar]
- Gregg, D.A.; Mebus, C.A.; Schlafer, D.H. Early Infection of Interdigitating Dendritic Cells in the Pig Lymph Node with African Swine Fever Viruses of High and Low Virulence: Immunohistochemical and Ultrastructural Studies. J. Vet. Diagn. Investig. 1995, 7, 23–30. [Google Scholar] [CrossRef]
- Gregg, D.A.; Schlafer, D.H.; Mebus, C.A. African Swine Fever Virus Infection of Skin-Derived Dendritic Cells in Vitro Causes Interference with Subsequent Foot-and-Mouth Disease Virus Infection. J. Vet. Diagn. Investig. 1995, 7, 44–51. [Google Scholar] [CrossRef]
- Golding, J.P.; Goatley, L.; Goodbourn, S.; Dixon, L.K.; Taylor, G.; Netherton, C.L. Sensitivity of African Swine Fever Virus to Type I Interferon Is Linked to Genes within Multigene Families 360 and 505. Virology 2016, 493, 154–161. [Google Scholar] [CrossRef]
- Gómez-Villamandos, J.C.; Hervás, J.; Méndez, A.; Carrasco, L.; Martín de las Mulas, J.; Villeda, C.J.; Wilkinson, P.J.; Sierra, M.A. Experimental African Swine Fever: Apoptosis of Lymphocytes and Virus Replication in Other Cells. J. Gen. Virol. 1995, 76, 2399–2405. [Google Scholar] [CrossRef]
- Dinter, J.; Gourdain, P.; Lai, N.Y.; Duong, E.; Bracho-Sanchez, E.; Rucevic, M.; Liebesny, P.H.; Xu, Y.; Shimada, M.; Ghebremichael, M.; et al. Different Antigen-Processing Activities in Dendritic Cells, Macrophages, and Monocytes Lead to Uneven Production of HIV Epitopes and Affect CTL Recognition. J. Immunol. 2014, 193, 4322–4334. [Google Scholar] [CrossRef]
- Robbins, P.F.; El-Gamil, M.; Li, Y.F.; Fitzgerald, E.B.; Kawakami, Y.; Rosenberg, S.A. The Intronic Region of an Incompletely Spliced Gp100 Gene Transcript Encodes an Epitope Recognized by Melanoma-Reactive Tumor-Infiltrating Lymphocytes. J. Immunol. 1997, 159, 303–308. [Google Scholar] [PubMed]
- Chalick, M.; Jacobi, O.; Pichinuk, E.; Garbar, C.; Bensussan, A.; Meeker, A.; Ziv, R.; Zehavi, T.; Smorodinsky, N.I.; Hilkens, J.; et al. MUC1-ARF—A Novel MUC1 Protein That Resides in the Nucleus and Is Expressed by Alternate Reading Frame Translation of MUC1 MRNA. PLoS ONE 2016, 11, e0165031. [Google Scholar] [CrossRef] [PubMed]
- Probst-Kepper, M.; Stroobant, V.; Kridel, R.; Gaugler, B.; Landry, C.; Brasseur, F.; Cosyns, J.P.; Weynand, B.; Boon, T.; Van Den Eynde, B.J. An Alternative Open Reading Frame of the Human Macrophage Colony-Stimulating Factor Gene Is Independently Translated and Codes for an Antigenic Peptide of 14 Amino Acids Recognized by Tumor-Infiltrating CD8 T Lymphocytes. J. Exp. Med. 2001, 193, 1189–1198. [Google Scholar] [CrossRef]
- Mayrand, S.M.; Schwarz, D.A.; Green, W.R. An Alternative Translational Reading Frame Encodes an Immunodominant Retroviral CTL Determinant Expressed by an Immunodeficiency-Causing Retrovirus. J. Immunol. 1998, 160, 39–50. [Google Scholar] [PubMed]
- Zhu, D.Y.; Deng, X.Z.; Jiang, L.F.; Xiao, W.; Pei, J.P.; Li, B.J.; Wang, C.J.; Zhang, J.H.; Zhang, Q.; Zhou, Z.X.; et al. Potential Role of Hepatitis C Virus Alternate Reading Frame Protein in Negative Regulation of T-Bet Gene Expression. Inflammation 2015, 38, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Cardinaud, S.; Moris, A.; Février, M.; Rohrlich, P.-S.; Weiss, L.; Langlade-Demoyen, P.; Lemonnier, F.A.; Schwartz, O.; Habel, A. Identification of Cryptic MHC I-Restricted Epitopes Encoded by HIV-1 Alternative Reading Frames. J. Exp. Med. 2004, 199, 1053–1063. [Google Scholar] [CrossRef]
- Jenson, J.S.; Childerstone, A.; Takamatsu, H.-H.; Dixon, L.K.; Parkhouse, R.M.E. The Cellular Immune Recognition of Proteins Expressed by an African Swine Fever Virus Random Genomic Library. J. Immunol. Methods 2000, 242, 33–42. [Google Scholar] [CrossRef]
- Borca, M.V.; Carrillo, C.; Zsak, L.; Laegreid, W.W.; Kutish, G.F.; Neilan, J.G.; Burrage, T.G.; Rock, D.L. Deletion of a CD2-Like Gene, 8-DR, from African Swine Fever Virus Affects Viral Infection in Domestic Swine. J. Virol. 1998, 72, 2881–2889. [Google Scholar] [CrossRef]
- Han, J.; Shui, J.-W.; Zhang, X.; Zheng, B.; Han, S.; Tan, T.-H. HIP-55 Is Important for T-Cell Proliferation, Cytokine Production, and Immune Responses. Mol. Cell. Biol. 2005, 25, 6869–6878. [Google Scholar] [CrossRef]
- Pérez-Núñez, D.; García-Urdiales, E.; Martínez-Bonet, M.; Nogal, M.L.; Barroso, S.; Revilla, Y.; Madrid, R. CD2v Interacts with Adaptor Protein AP-1 during African Swine Fever Infection. PLoS ONE 2015, 10, e0123714. [Google Scholar] [CrossRef]
- Kulpa, D.A.; Del Cid, N.; Peterson, K.A.; Collins, K.L. Adaptor Protein 1 Promotes Cross-Presentation through the Same Tyrosine Signal in Major Histocompatibility Complex Class I as That Targeted by HIV-1. J. Virol. 2013, 87, 8085–8098. [Google Scholar] [CrossRef][Green Version]
- Wonderlich, E.R.; Williams, M.; Collins, K.L. The Tyrosine Binding Pocket in the Adaptor Protein 1 (AP-1) Μ1 Subunit Is Necessary for Nef to Recruit AP-1 to the Major Histocompatibility Complex Class I Cytoplasmic Tail. J. Biol. Chem. 2008, 283, 3011–3022. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.; An, L.L.; Harkins, S.; Zhang, J.; Yokoyama, M.; Widera, G.; Fuller, J.T.; Kincaid, C.; Campbell, I.L.; Whitton, J.L. DNA Immunization with Minigenes: Low Frequency of Memory Cytotoxic T Lymphocytes and Inefficient Antiviral Protection Are Rectified by Ubiquitination. J. Virol. 1998, 72, 5174–5181. [Google Scholar] [CrossRef]
- Graham, S.P.; Pellé, R.; Honda, Y.; Mwangi, D.M.; Tonukari, N.J.; Yamage, M.; Glew, E.J.; de Villiers, E.P.; Shah, T.; Bishop, R.; et al. Theileria Parva Candidate Vaccine Antigens Recognized by Immune Bovine Cytotoxic T Lymphocytes. Proc. Natl. Acad. Sci. USA 2006, 103, 3286–3291. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.; Ngugi, D.; Lizundia, R.; Hostettler, I.; Woods, K.; Ballingall, K.; MacHugh, N.D.; Morrison, W.I.; Weir, W.; Shiels, B.; et al. Identification of Theileria Lestoquardi Antigens Recognized by CD8+ T Cells. PLoS ONE 2016, 11, e0162571. [Google Scholar] [CrossRef]
- Balmelli, C.; Ruggli, N.; McCullough, K.; Summerfield, A. Fibrocytes Are Potent Stimulators of Anti-Virus Cytotoxic T Cells. J. Leukoc. Biol. 2005, 77, 923–933. [Google Scholar] [CrossRef]
- Mulumba-Mfumu, L.K.; Achenbach, J.E.; Mauldin, M.R.; Dixon, L.K.; Tshilenge, C.G.; Thiry, E.; Moreno, N.; Blanco, E.; Saegerman, C.; Lamien, C.E.; et al. Genetic Assessment of African Swine Fever Isolates Involved in Outbreaks in the Democratic Republic of Congo between 2005 and 2012 Reveals Co-Circulation of P72 Genotypes I, IX and XIV, Including 19 Variants. Viruses 2017, 9, 31. [Google Scholar] [CrossRef]
- Simulundu, E.; Chambaro, H.M.; Sinkala, Y.; Kajihara, M.; Ogawa, H.; Mori, A.; Ndebe, J.; Dautu, G.; Mataa, L.; Lubaba, C.H.; et al. Co-Circulation of Multiple Genotypes of African Swine Fever Viruses among Domestic Pigs in Zambia (2013–2015). Transbound. Emerg. Dis. 2018, 65, 114–122. [Google Scholar] [CrossRef]
| 68 | 69 | 70 | 71 | 72 | 73 | 74 | 75 | 76 | 77 | 78 | 79 | 80 | 81 | 82 | 83 | 84 | 85 | 86 | Overlapping Peptides |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | Q | M | A | P | G | G | S | Y | 8 | ||||||||||
| SLA-1*0801, SLA-2*1001 | |||||||||||||||||||
| L | Q | M | A | P | G | G | S | Y | F | 9 | |||||||||
| SLA-1*0801, SLA-2*0601, SLA-2*1001 | |||||||||||||||||||
| L | Q | M | A | P | G | G | S | Y | F | I | 12 | ||||||||
| SLA-2*0601, SLA-2*1001 | |||||||||||||||||||
| Q | M | A | P | G | G | S | Y | 8 | |||||||||||
| SLA-1*0201, SLA-1*0202, SLA-1*0401, SLA-1*0701, SLA-1*0702, SLA-1*0801, SLA-1*1301, SLA-2*1001 | |||||||||||||||||||
| Q | M | A | P | G | G | S | Y | F | 9 | ||||||||||
| SLA-1*0201, SLA-1*0202, SLA-1*0401, SLA-1*0801, SLA-1*1301, SLA-2*1001 | |||||||||||||||||||
| M | A | P | G | G | S | Y | F | I | 12 | ||||||||||
| SLA-2*0502 | |||||||||||||||||||
| Y | F | I | T | D | N | M | T | E | E | F | 12 | ||||||||
| SLA-1*1301 | |||||||||||||||||||
| F | I | T | D | N | M | T | E | E | F | 9 | |||||||||
| SLA-1*1301 | |||||||||||||||||||
| I | T | D | N | M | T | E | E | F | 6 | ||||||||||
| SLA-1*0201, SLA-1*0401, SLA-1*0601, SLA-1*1301 | |||||||||||||||||||
| Function/Protein | Total Peptides | BA71 | BA71ΔCD2 | Activity/Similarity | Temporal Expression |
|---|---|---|---|---|---|
| Multigene Families | 14 | 5 | 9 | ||
| MGF110-6L | 2 | 1 | 1 | Early | |
| MGF360-10L | 2 | 1 | 1 | Unknown | |
| MGF360-8L | 2 | 1 | 1 | Early | |
| MGF505-1R | 3 | 1 | 2 | Early | |
| MGF505-2R | 1 | 0 | 1 | Late | |
| MGF505-3R | 1 | 0 | 1 | Early | |
| MGF505-5R | 1 | 0 | 1 | Early | |
| MGF505-7R | 1 | 0 | 1 | Early | |
| MGF505-9R | 1 | 1 | 0 | Early | |
| Transcription, Replication and Repair | 41 | 13 | 28 | ||
| C315R | 1 | 0 | 1 | Transcription factor IIB-like | Early/Late |
| C475L | 4 | 1 | 3 | Poly(A) polymerase large subunit | Late |
| D1133L | 7 | 3 | 4 | Helicase superfamily II | Late |
| D205R | 3 | 0 | 3 | RNA polymerase subunit 5 | Early |
| D250R | 1 | 0 | 1 | Ribonucleotide reductase (small subunit) | Early |
| D339L | 1 | 0 | 1 | RNA polymerase subunit 7 | Early |
| E301R | 2 | 1 | 1 | Proliferating cell nuclear antigen-like protein | Late |
| EP1242L | 2 | 2 | 0 | RNA polymerase subunit 2 | Early/Late |
| EP364R | 1 | 0 | 1 | ERCC nuclease domain | Late |
| EP424R | 3 | 2 | 1 | FtsJ-like methyl transferase domain | Early |
| F334L | 1 | 0 | 1 | Ribonucleotide reductase (small subunit) | Early |
| G1211R | 4 | 1 | 3 | DNA polymerase family B | Early/Late |
| G1340L | 1 | 0 | 1 | VV A8L-like transcription factor | Late |
| H359L | 1 | 1 | 0 | RNA polymerase subunit 3 | Early |
| I243L | 1 | 1 | 0 | Transcription factor SII | Early/Late |
| M448R | 1 | 0 | 1 | RNA ligase | Late |
| NP1450L | 1 | 0 | 1 | RNA polymerase subunit 1 | Early/Late |
| NP419L | 1 | 0 | 1 | DNA ligase | Early/Late |
| P1192R | 4 | 1 | 3 | DNA topoisomerase type II | Early/Late |
| Q706L | 1 | 0 | 1 | Helicase superfamily II | Late |
| Morphogenesis | 24 | 12 | 12 | ||
| A137R | 1 | 0 | 1 | Protein p11.5 | Late |
| A151R | 1 | 0 | 1 | Protein oxidation pathway | Early/Late |
| B602L | 3 | 1 | 2 | Chaperone | Late |
| B646L | 5 | 2 | 3 | Major capsid protein p72 | Late |
| CP2475L | 10 | 7 | 3 | Polyprotein pp220 | Late |
| E120R | 1 | 0 | 1 | Structural protein p14.5, DNA-binding protein | Late |
| E183L | 2 | 1 | 1 | Structural protein p54 | Late |
| E248R | 1 | 1 | 0 | Structural protein | Early/Late |
| Host Cell Interaction | 6 | 1 | 5 | ||
| A179L | 2 | 1 | 1 | Bcl-2 apoptosis inhibitor | Late |
| A238L | 1 | 0 | 1 | IkB-like protein, inhibitor of host gene transcription | Early |
| QP383R | 3 | 0 | 3 | Nif-S like | Late |
| Uncharacterized | 47 | 19 | 28 | ||
| B117L | 1 | 0 | 1 | Late | |
| B125R | 2 | 0 | 2 | Late | |
| B475L | 9 | 5 | 4 | Late | |
| C129R | 3 | 1 | 2 | Late | |
| C257L | 1 | 0 | 1 | Late | |
| CP123L | 2 | 0 | 2 | Late | |
| CP312R | 1 | 0 | 1 | Early/Late | |
| DP238L | 2 | 1 | 1 | Early | |
| E111R | 1 | 0 | 1 | Early/Late | |
| F317L | 3 | 1 | 2 | Late | |
| H233R | 3 | 1 | 2 | Late | |
| H339R | 3 | 1 | 2 | Late | |
| I226R | 2 | 2 | 0 | Early/Late | |
| I73R | 2 | 1 | 1 | Early/Late | |
| I9R | 1 | 0 | 1 | Unknown | |
| K145R | 6 | 3 | 3 | Late | |
| M1249L | 5 | 3 | 2 | Late | |
| TOTAL | 132 | 50 | 82 |
| Peptide Sequence | Protein | Responding Animals | Sample | Georgia2007/1 Identity |
|---|---|---|---|---|
| NPTIIMEQY | H339R | 1/10 (10%) | BA71 | 100% |
| KNILNTLMF | I226R | 1/10 (10%) | BA71 | 100% |
| DKDGNSALHYL | A238L | 6/20 (30%) | BA71ΔCD2 | 100% |
| AKIVEEGGEES | K145R | 4/20 (20%) | BA71/BA71ΔCD2 | 100% |
| NSTLVIRI | MGF505-7R | 4/20 (20%) | BA71ΔCD2 | 87.5% (NSTLVIRL) |
| Plasmid Mix | E75 Locus | Protein Name | Plasmid Mix | E75 Locus | Protein Name |
|---|---|---|---|---|---|
| Mix 1 | 2 | DP93R | Mix 5 | 92 | B407L |
| 3 | MGF360-2L | 93 | B175L | ||
| 4 | KP177R | 94 | B263R | ||
| 6 | L60L | 104 | O174L | ||
| 7 | MGF360-3L | 109 | D250R | ||
| 8 | MGF110-1L | 110 | D129L | ||
| 10 | MGF110-13L | 111 | D79L | ||
| 13 | MGF110-12L | 116 | D345L | ||
| 14 | MGF110-14L | 117 | S183 | ||
| 15 | MGF360-4L | 118 | S273R | ||
| Mix 2 | 16 | MGF360-6L | 120 | H359L | |
| 17 | X69R | Mix 6 | 121 | H171R | |
| 18 | MGF300-1L | 122 | H124R | ||
| 20 | MGF300-2R | 132 | E184L | ||
| 22 | MGF300-4L | 133 | E183L | ||
| 23 | MGF360-8L | 135 | E301R | ||
| 25 | MGF360-10L | 137 | E199L | ||
| 26 | MGF360-11L | 138 | E165R | ||
| 28 | MGF360-12L | 139 | E248R | ||
| 30 | MGF360-14L | 140 | E120R | ||
| Mix 3 | 31 | MGF505-2R | 141 | E296R | |
| 33 | MGF505-4R | 142 | E111R | ||
| 35 | MGF505-6R | Mix 7 | 143 | E66L | |
| 36 | MGF505-7R | 144 | I267L | ||
| 39 | A224L | 151 | I215L | ||
| 40 | A104R | 152 | I177L | ||
| 41 | A118R | 153 | I196L | ||
| 45 | MGF360-15R | 158 | MGF100-2L | ||
| 46 | A238L | 161 | I8L | ||
| 47 | A859L | 163 | I10L | ||
| Mix 4 | 48 | A179L | 164 | L11L | |
| 49 | A137R | 167 | DP96R | ||
| 50 | F317L | 168 | MGF360-19R | ||
| 53 | F165R | ||||
| 55 | K205R | ||||
| 56 | K78R | ||||
| 63 | EP152R | ||||
| 66 | EP364R | ||||
| 77 | C62L | ||||
| 83 | B438L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosch-Camós, L.; López, E.; Navas, M.J.; Pina-Pedrero, S.; Accensi, F.; Correa-Fiz, F.; Park, C.; Carrascal, M.; Domínguez, J.; Salas, M.L.; et al. Identification of Promiscuous African Swine Fever Virus T-Cell Determinants Using a Multiple Technical Approach. Vaccines 2021, 9, 29. https://doi.org/10.3390/vaccines9010029
Bosch-Camós L, López E, Navas MJ, Pina-Pedrero S, Accensi F, Correa-Fiz F, Park C, Carrascal M, Domínguez J, Salas ML, et al. Identification of Promiscuous African Swine Fever Virus T-Cell Determinants Using a Multiple Technical Approach. Vaccines. 2021; 9(1):29. https://doi.org/10.3390/vaccines9010029
Chicago/Turabian StyleBosch-Camós, Laia, Elisabet López, María Jesús Navas, Sonia Pina-Pedrero, Francesc Accensi, Florencia Correa-Fiz, Chankyu Park, Montserrat Carrascal, Javier Domínguez, Maria Luisa Salas, and et al. 2021. "Identification of Promiscuous African Swine Fever Virus T-Cell Determinants Using a Multiple Technical Approach" Vaccines 9, no. 1: 29. https://doi.org/10.3390/vaccines9010029
APA StyleBosch-Camós, L., López, E., Navas, M. J., Pina-Pedrero, S., Accensi, F., Correa-Fiz, F., Park, C., Carrascal, M., Domínguez, J., Salas, M. L., Nikolin, V., Collado, J., & Rodríguez, F. (2021). Identification of Promiscuous African Swine Fever Virus T-Cell Determinants Using a Multiple Technical Approach. Vaccines, 9(1), 29. https://doi.org/10.3390/vaccines9010029








