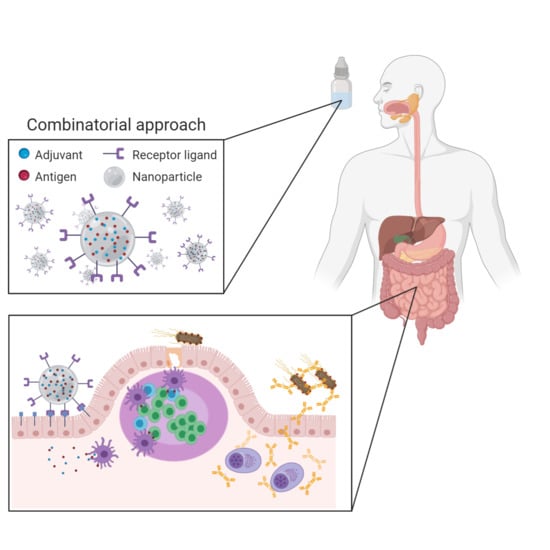
Advances in Oral Subunit Vaccine Design
Abstract
:1. Introduction
2. Oral Vaccination Strategies
2.1. Oral Adjuvants
2.1.1. Toxin Derivates
2.1.2. PRR Ligands
2.1.3. Other Immune Modulating Molecules
2.1.4. Use of Adjuvants for the Induction of SIgA after Parenteral Administration
2.2. Delivery Systems
2.2.1. Living Delivery Systems
Recombinant Bacteria
Viral Vectors
2.2.2. Non-Living Delivery Systems
Virus-Like Particles
Micro- and Nanoparticles
Lipid-Based Delivery Systems
Nanogels
2.3. Antigen Delivery to the Intestinal Immune System
2.3.1. Microfold Cells
2.3.2. Enterocytes
2.3.3. Antigen-Presenting Cells
2.4. The Effect of Microbiota and Other Factors on Oral Vaccination
3. Expert Opinion
Author Contributions
Funding
Conflicts of Interest
References
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwood, B. The contribution of vaccination to global health: Past, present and future. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, S.A.; Ahmed, J.; Datta, S.D.; Burns, C.C.; Quddus, A.; Vertefeuille, J.F.; Wassilak, S.G.F. Progress toward Polio Eradication—Worldwide, January 2017-March 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 458–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roeder, P.L. Rinderpest: The end of cattle plague. Prev. Vet. Med. 2011, 102, 98–106. [Google Scholar] [CrossRef]
- Hoelzer, K.; Bielke, L.; Blake, D.P.; Cox, E.; Cutting, S.M.; Devriendt, B.; Erlacher-Vindel, E.; Goossens, E.; Karaca, K.; Lemiere, S.; et al. Vaccines as alternatives to antibiotics for food producing animals. Part 2: New approaches and potential solutions. Vet. Res. 2018, 49, 70. [Google Scholar] [CrossRef] [Green Version]
- Hoelzer, K.; Bielke, L.; Blake, D.P.; Cox, E.; Cutting, S.M.; Devriendt, B.; Erlacher-Vindel, E.; Goossens, E.; Karaca, K.; Lemiere, S.; et al. Vaccines as alternatives to antibiotics for food producing animals. Part 1: Challenges and needs. Vet. Res. 2018, 49, 64. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Jin, L.; Chen, T. The Effects of Secretory IgA in the Mucosal Immune System. Biomed Res. Int. 2020, 2020, 2032057. [Google Scholar] [CrossRef]
- Mantis, N.J.; Rol, N.; Corthesy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]
- Vela Ramirez, J.E.; Sharpe, L.A.; Peppas, N.A. Current state and challenges in developing oral vaccines. Adv Drug Deliv. Rev 2017, 114, 116–131. [Google Scholar] [CrossRef]
- Hutton, G.; Tediosi, F. The costs of introducing a malaria vaccine through the expanded program on immunization in Tanzania. Am. J. Trop. Med. Hyg. 2006, 75, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Tordesillas, L.; Berin, M.C. Mechanisms of Oral Tolerance. Clin. Rev. Allergy Immunol. 2018, 55, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Ebbo, M.; Crinier, A.; Vely, F.; Vivier, E. Innate lymphoid cells: Major players in inflammatory diseases. Nat. Rev. Immunol. 2017, 17, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Weiner, H.L.; da Cunha, A.P.; Quintana, F.; Wu, H. Oral tolerance. Immunol. Rev. 2011, 241, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Mestecky, J.; Russell, M.W.; Elson, C.O. Perspectives on mucosal vaccines: Is mucosal tolerance a barrier? J. Immunol. 2007, 179, 5633–5638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavot, V.; Rochereau, N.; Genin, C.; Verrier, B.; Paul, S. New insights in mucosal vaccine development. Vaccine 2012, 30, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Subiza, J.L.; El-Qutob, D.; Fernandez-Caldas, E. New developments in oral vaccines and mucosal adjuvants. Recent Pat. Inflamm. Allergy Drug Discov. 2015, 9, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Mudie, D.M.; Amidon, G.L.; Amidon, G.E. Physiological parameters for oral delivery and in vitro testing. Mol. Pharm. 2010, 7, 1388–1405. [Google Scholar] [CrossRef] [Green Version]
- McDermott, A.J.; Huffnagle, G.B. The microbiome and regulation of mucosal immunity. Immunology 2014, 142, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Ciabattini, A.; Olivieri, R.; Lazzeri, E.; Medaglini, D. Role of the Microbiota in the Modulation of Vaccine Immune Responses. Front. Microbiol. 2019, 10, 1305. [Google Scholar] [CrossRef] [Green Version]
- Clements, J.D.; Norton, E.B. The Mucosal Vaccine Adjuvant LT(R192G/L211A) or dmLT. mSphere 2018, 3. [Google Scholar] [CrossRef] [Green Version]
- Harro, C.; Louis Bourgeois, A.; Sack, D.; Walker, R.; DeNearing, B.; Brubaker, J.; Maier, N.; Fix, A.; Dally, L.; Chakraborty, S.; et al. Live attenuated enterotoxigenic Escherichia coli (ETEC) vaccine with dmLT adjuvant protects human volunteers against virulent experimental ETEC challenge. Vaccine 2019, 37, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Qadri, F.; Akhtar, M.; Bhuiyan, T.R.; Chowdhury, M.I.; Ahmed, T.; Rafique, T.A.; Khan, A.; Rahman, S.I.A.; Khanam, F.; Lundgren, A.; et al. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: A double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect. Dis. 2020, 20, 208–219. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.; Chowdhury, M.I.; Bhuiyan, T.R.; Kaim, J.; Ahmed, T.; Rafique, T.A.; Khan, A.; Rahman, S.I.A.; Khanam, F.; Begum, Y.A.; et al. Evaluation of the safety and immunogenicity of the oral inactivated multivalent enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi adults in a double-blind, randomized, placebo-controlled Phase I trial using electrochemiluminescence and ELISA assays for immunogenicity analyses. Vaccine 2019, 37, 5645–5656. [Google Scholar] [CrossRef] [PubMed]
- Lebens, M.; Terrinoni, M.; Karlsson, S.L.; Larena, M.; Gustafsson-Hedberg, T.; Kallgard, S.; Nygren, E.; Holmgren, J. Construction and preclinical evaluation of mmCT, a novel mutant cholera toxin adjuvant that can be efficiently produced in genetically manipulated Vibrio cholerae. Vaccine 2016, 34, 2121–2128. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vannucci, L.; Sima, P. beta-glucan as a new tool in vaccine development. Scand. J. Immunol. 2020, 91, e12833. [Google Scholar] [CrossRef] [PubMed]
- Baert, K.; De Geest, B.G.; De Greve, H.; Cox, E.; Devriendt, B. Duality of beta-glucan microparticles: Antigen carrier and immunostimulants. Int. J. Nanomed. 2016, 11, 2463–2469. [Google Scholar] [CrossRef] [Green Version]
- Doherty, T.M.; Olsen, A.W.; van Pinxteren, L.; Andersen, P. Oral vaccination with subunit vaccines protects animals against aerosol infection with Mycobacterium tuberculosis. Infect. Immun. 2002, 70, 3111–3121. [Google Scholar] [CrossRef] [Green Version]
- Girard, A.; Saron, W.; Bergeron-Sandoval, L.P.; Sarhan, F.; Archambault, D. Flagellin produced in plants is a potent adjuvant for oral immunization. Vaccine 2011, 29, 6695–6703. [Google Scholar] [CrossRef]
- Salman, H.H.; Irache, J.M.; Gamazo, C. Immunoadjuvant capacity of flagellin and mannosamine-coated poly(anhydride) nanoparticles in oral vaccination. Vaccine 2009, 27, 4784–4790. [Google Scholar] [CrossRef]
- McCluskie, M.J.; Weeratna, R.D.; Krieg, A.M.; Davis, H.L. CpG DNA is an effective oral adjuvant to protein antigens in mice. Vaccine 2000, 19, 950–957. [Google Scholar] [CrossRef]
- Linghua, Z.; Xingshan, T.; Fengzhen, Z. In vivo oral administration effects of various oligodeoxynucleotides containing synthetic immunostimulatory motifs in the immune response to pseudorabies attenuated virus vaccine in newborn piglets. Vaccine 2008, 26, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Courtney, A.N.; Nehete, P.N.; Nehete, B.P.; Thapa, P.; Zhou, D.; Sastry, K.J. Alpha-galactosylceramide is an effective mucosal adjuvant for repeated intranasal or oral delivery of HIV peptide antigens. Vaccine 2009, 27, 3335–3341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davitt, C.J.H.; Longet, S.; Albutti, A.; Aversa, V.; Nordqvist, S.; Hackett, B.; McEntee, C.P.; Rosa, M.; Coulter, I.S.; Lebens, M.; et al. Alpha-galactosylceramide enhances mucosal immunity to oral whole-cell cholera vaccines. Mucosal Immunol. 2019, 12, 1055–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, K.; Chamberlain, L.M.; Schofield, K.M.; Wells, J.M.; Le Page, R.W. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat. Biotechnol. 1997, 15, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xing, Y.; Guo, L.; Lv, X.; Song, H.; Xi, T. Oral immunization with recombinant Lactococcus lactis delivering a multi-epitope antigen CTB-UE attenuates Helicobacter pylori infection in mice. Pathog. Dis. 2014, 72, 78–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, R.; Zhang, Y.; Long, B.; Li, Y.; Wu, Y.; Duan, S.; Zhu, B.; Wu, X.; Fan, H. Oral Immunization with Recombinant Lactobacillus acidophilus Expressing espA-Tir-M Confers Protection against Enterohemorrhagic Escherichia coli O157:H7 Challenge in Mice. Front. Microbiol. 2017, 8, 417. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, B.; Loos, M.; Vanrompay, D.; Cox, E. Oral immunization with Lactococcus lactis-expressing EspB induces protective immune responses against Escherichia coli O157:H7 in a murine model of colonization. Vaccine 2014, 32, 3909–3916. [Google Scholar] [CrossRef] [Green Version]
- Wen, L.J.; Hou, X.L.; Wang, G.H.; Yu, L.Y.; Wei, X.M.; Liu, J.K.; Liu, Q.; Wei, C.H. Immunization with recombinant Lactobacillus casei strains producing K99, K88 fimbrial protein protects mice against enterotoxigenic Escherichia coli. Vaccine 2012, 30, 3339–3349. [Google Scholar] [CrossRef]
- Kajikawa, A.; Satoh, E.; Leer, R.J.; Yamamoto, S.; Igimi, S. Intragastric immunization with recombinant Lactobacillus casei expressing flagellar antigen confers antibody-independent protective immunity against Salmonella enterica serovar Enteritidis. Vaccine 2007, 25, 3599–3605. [Google Scholar] [CrossRef]
- Marelli, B.; Perez, A.R.; Banchio, C.; de Mendoza, D.; Magni, C. Oral immunization with live Lactococcus lactis expressing rotavirus VP8 subunit induces specific immune response in mice. J. Virol. Methods 2011, 175, 28–37. [Google Scholar] [CrossRef]
- Yang, W.T.; Yang, G.L.; Yang, X.; Shonyela, S.M.; Zhao, L.; Jiang, Y.L.; Huang, H.B.; Shi, C.W.; Wang, J.Z.; Wang, G.; et al. Recombinant Lactobacillus plantarum expressing HA2 antigen elicits protective immunity against H9N2 avian influenza virus in chickens. Appl. Microbiol. Biotechnol. 2017, 101, 8475–8484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Jiang, P.H.; Li, N.J.; Shi, M.; Huang, W. Oral vaccination of mice against rodent malaria with recombinant Lactococcus lactis expressing MSP-1(19). World J. Gastroenterol. 2005, 11, 6975–6980. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Dai, Y.; Chen, J.; Wang, X.; Tang, B.; Zhu, Y.; Hua, Z. Oral delivery of the Sj23LHD-GST antigen by Salmonella typhimurium type III secretion system protects against Schistosoma japonicum infection in mice. PLoS Negl. Trop. Dis. 2011, 5, e1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tvinnereim, A.R.; Hamilton, S.E.; Harty, J.T. CD8(+)-T-cell response to secreted and nonsecreted antigens delivered by recombinant Listeria monocytogenes during secondary infection. Infect. Immun. 2002, 70, 153–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Premanand, B.; Prabakaran, M.; Kiener, T.K.; Kwang, J. Recombinant baculovirus associated with bilosomes as an oral vaccine candidate against HEV71 infection in mice. PLoS ONE 2013, 8, e55536. [Google Scholar] [CrossRef] [Green Version]
- Basak, S.; Chu, K.B.; Kang, H.J.; Kim, M.J.; Lee, S.H.; Yoon, K.W.; Jin, H.; Suh, J.W.; Moon, E.K.; Quan, F.S. Orally administered recombinant baculovirus vaccine elicits partial protection against avian influenza virus infection in mice. Microb. Pathog. 2020, 149, 104495. [Google Scholar] [CrossRef]
- Stephenson, K.E.; Keefer, M.C.; Bunce, C.A.; Frances, D.; Abbink, P.; Maxfield, L.F.; Neubauer, G.H.; Nkolola, J.; Peter, L.; Lane, C.; et al. First-in-human randomized controlled trial of an oral, replicating adenovirus 26 vector vaccine for HIV-1. PLoS ONE 2018, 13, e0205139. [Google Scholar] [CrossRef]
- Scallan, C.D.; Tingley, D.W.; Lindbloom, J.D.; Toomey, J.S.; Tucker, S.N. An adenovirus-based vaccine with a double-stranded RNA adjuvant protects mice and ferrets against H5N1 avian influenza in oral delivery models. Clin. Vaccine Immunol. 2013, 20, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Kim, L.; Martinez, C.J.; Hodgson, K.A.; Trager, G.R.; Brandl, J.R.; Sandefer, E.P.; Doll, W.J.; Liebowitz, D.; Tucker, S.N. Systemic and mucosal immune responses following oral adenoviral delivery of influenza vaccine to the human intestine by radio controlled capsule. Sci. Rep. 2016, 6, 37295. [Google Scholar] [CrossRef] [Green Version]
- Gurwith, M.; Lock, M.; Taylor, E.M.; Ishioka, G.; Alexander, J.; Mayall, T.; Ervin, J.E.; Greenberg, R.N.; Strout, C.; Treanor, J.J.; et al. Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: A randomised, double-blind, placebo-controlled, phase 1 study. Lancet Infect. Dis. 2013, 13, 238–250. [Google Scholar] [CrossRef] [Green Version]
- Liebowitz, D.; Gottlieb, K.; Kolhatkar, N.S.; Garg, S.J.; Asher, J.M.; Nazareno, J.; Kim, K.; McIlwain, D.R.; Tucker, S.N. Efficacy, immunogenicity, and safety of an oral influenza vaccine: A placebo-controlled and active-controlled phase 2 human challenge study. Lancet Infect. Dis. 2020, 20, 435–444. [Google Scholar] [CrossRef]
- Joyce, C.; Scallan, C.D.; Mateo, R.; Belshe, R.B.; Tucker, S.N.; Moore, A.C. Orally administered adenoviral-based vaccine induces respiratory mucosal memory and protection against RSV infection in cotton rats. Vaccine 2018, 36, 4265–4277. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Liebowitz, D.; Lin, K.; Kasparek, K.; Pasetti, M.F.; Garg, S.J.; Gottlieb, K.; Trager, G.; Tucker, S.N. Safety and immunogenicity of an oral tablet norovirus vaccine, a phase I randomized, placebo-controlled trial. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.G.; Adams, R.J.; Gambhira, R.; Siracusa, M.C.; Scott, A.L.; Roden, R.B.; Ketner, G. Immune responses in macaques to a prototype recombinant adenovirus live oral human papillomavirus 16 vaccine. Clin. Vaccine Immunol. 2014, 21, 1224–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, H.; Jackson, F.; Bean, K.; Panasuk, B.; Niezgoda, M.; Slate, D.; Li, J.; Dietzschold, B.; Mattis, J.; Rupprecht, C.E. Oral immunization of raccoons and skunks with a canine adenovirus recombinant rabies vaccine. Vaccine 2009, 27, 7194–7197. [Google Scholar] [CrossRef]
- Xiang, Z.Q.; Greenberg, L.; Ertl, H.C.; Rupprecht, C.E. Protection of non-human primates against rabies with an adenovirus recombinant vaccine. Virology 2014, 450–451, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Serradell, M.C.; Rupil, L.L.; Martino, R.A.; Prucca, C.G.; Carranza, P.G.; Saura, A.; Fernandez, E.A.; Gargantini, P.R.; Tenaglia, A.H.; Petiti, J.P.; et al. Efficient oral vaccination by bioengineering virus-like particles with protozoan surface proteins. Nat. Commun. 2019, 10, 361. [Google Scholar] [CrossRef]
- Marasini, N.; Skwarczynski, M.; Toth, I. Oral delivery of nanoparticle-based vaccines. Expert Rev. Vaccines 2014, 13, 1361–1376. [Google Scholar] [CrossRef]
- Kour, P.; Rath, G.; Sharma, G.; Goyal, A.K. Recent advancement in nanocarriers for oral vaccination. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1102–S1114. [Google Scholar] [CrossRef] [Green Version]
- Baert, K.; de Geest, B.G.; de Rycke, R.; da Fonseca Antunes, A.B.; de Greve, H.; Cox, E.; Devriendt, B. beta-glucan microparticles targeted to epithelial APN as oral antigen delivery system. J. Control. Release Off. J. Control. Release Soc. 2015, 220, 149–159. [Google Scholar] [CrossRef]
- Liu, J.; Wu, J.; Wang, B.; Zeng, S.; Qi, F.; Lu, C.; Kimura, Y.; Liu, B. Oral vaccination with a liposome-encapsulated influenza DNA vaccine protects mice against respiratory challenge infection. J. Med Virol. 2014, 86, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, J.; Feng, Y.; Liu, Y.; McHenga, S.S.; Shan, F.; Sasaki, J.; Lu, C. Liposomal oral DNA vaccine (mycobacterium DNA) elicits immune response. Vaccine 2010, 28, 3134–3142. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zhang, Y.; Wang, H.; Jin, J.; Piao, J.; Piao, J.; Liu, Q.; Li, W. Reduction of Salmonella enteritidis number after infections by immunization of liposome-associated recombinant SefA. Avian Dis. 2013, 57, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Marasini, N.; Giddam, A.K.; Ghaffar, K.A.; Batzloff, M.R.; Good, M.F.; Skwarczynski, M.; Toth, I. Multilayer engineered nanoliposomes as a novel tool for oral delivery of lipopeptide-based vaccines against group A Streptococcus. Nanomedicine 2016, 11, 1223–1236. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Katare, O.P.; Singh, B.; Vyas, S.P. M-cell targeted delivery of recombinant hepatitis B surface antigen using cholera toxin B subunit conjugated bilosomes. Int. J. Pharm. 2010, 385, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Prabakaran, D.; Jain, S.; Mishra, V.; Jaganathan, K.S.; Vyas, S.P. Cholera toxin B subunit conjugated bile salt stabilized vesicles (bilosomes) for oral immunization. Int. J. Pharm. 2004, 278, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Harde, H.; Indulkar, A.; Agrawal, A.K. Improved stability and immunological potential of tetanus toxoid containing surface engineered bilosomes following oral administration. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 431–440. [Google Scholar] [CrossRef]
- Mann, J.F.; Ferro, V.A.; Mullen, A.B.; Tetley, L.; Mullen, M.; Carter, K.C.; Alexander, J.; Stimson, W.H. Optimisation of a lipid based oral delivery system containing A/Panama influenza haemagglutinin. Vaccine 2004, 22, 2425–2429. [Google Scholar] [CrossRef]
- Mann, J.F.; Shakir, E.; Carter, K.C.; Mullen, A.B.; Alexander, J.; Ferro, V.A. Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection. Vaccine 2009, 27, 3643–3649. [Google Scholar] [CrossRef]
- Hernandez-Adame, L.; Angulo, C.; Garcia-Silva, I.; Palestino, G.; Rosales-Mendoza, S. An overview of nanogel-based vaccines. Expert Rev. Vaccines 2019, 18, 951–968. [Google Scholar] [CrossRef]
- Rochereau, N.; Drocourt, D.; Perouzel, E.; Pavot, V.; Redelinghuys, P.; Brown, G.D.; Tiraby, G.; Roblin, X.; Verrier, B.; Genin, C.; et al. Dectin-1 is essential for reverse transcytosis of glycosylated SIgA-antigen complexes by intestinal M cells. PLoS Biol. 2013, 11, e1001658. [Google Scholar] [CrossRef] [PubMed]
- Langermann, S.; Mollby, R.; Burlein, J.E.; Palaszynski, S.R.; Auguste, C.G.; DeFusco, A.; Strouse, R.; Schenerman, M.A.; Hultgren, S.J.; Pinkner, J.S.; et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J. Infect. Dis. 2000, 181, 774–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Yang, I.Y.; Jang, S.H.; Kim, J.; Truong, T.T.; Van Pham, T.; Truong, N.U.; Lee, K.Y.; Jang, Y.S. C5a receptor-targeting ligand-mediated delivery of dengue virus antigen to M cells evokes antigen-specific systemic and mucosal immune responses in oral immunization. Microbes Infect. 2013, 15, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Pridgen, E.M.; Alexis, F.; Kuo, T.T.; Levy-Nissenbaum, E.; Karnik, R.; Blumberg, R.S.; Langer, R.; Farokhzad, O.C. Transepithelial transport of Fc-targeted nanoparticles by the neonatal fc receptor for oral delivery. Sci. Transl. Med. 2013, 5, 213ra167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snoeck, V.; Van den Broeck, W.; De Colvenaer, V.; Verdonck, F.; Goddeeris, B.; Cox, E. Transcytosis of F4 fimbriae by villous and dome epithelia in F4-receptor positive pigs supports importance of receptor-dependent endocytosis in oral immunization strategies. Vet. Immunol. Immunopathol. 2008, 124, 29–40. [Google Scholar] [CrossRef]
- Melkebeek, V.; Rasschaert, K.; Bellot, P.; Tilleman, K.; Favoreel, H.; Deforce, D.; De Geest, B.G.; Goddeeris, B.M.; Cox, E. Targeting aminopeptidase N, a newly identified receptor for F4ac fimbriae, enhances the intestinal mucosal immune response. Mucosal Immunol. 2012, 5, 635–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Broeck, W.; Cox, E.; Goddeeris, B.M. Induction of immune responses in pigs following oral administration of purified F4 fimbriae. Vaccine 1999, 17, 2020–2029. [Google Scholar] [CrossRef]
- Verdonck, F.; De Hauwere, V.; Bouckaert, J.; Goddeeris, B.M.; Cox, E. Fimbriae of enterotoxigenic Escherichia coli function as a mucosal carrier for a coupled heterologous antigen. J. Control. Release Off. J. Control. Release Soc. 2005, 104, 243–258. [Google Scholar] [CrossRef]
- Bakshi, S.; Sanz Garcia, R.; Van der Weken, H.; Tharad, A.; Pandey, S.; Juarez, P.; Virdi, V.; Devriendt, B.; Cox, E.; Depicker, A. Evaluating single-domain antibodies as carriers for targeted vaccine delivery to the small intestinal epithelium. J. Control. Release Off. J. Control. Release Soc. 2020, 321, 416–429. [Google Scholar] [CrossRef]
- da Silva, A.J.; Zangirolami, T.C.; Novo-Mansur, M.T.; Giordano Rde, C.; Martins, E.A. Live bacterial vaccine vectors: An overview. Braz. J. Microbiol. 2014, 45, 1117–1129. [Google Scholar] [CrossRef]
- Perrie, Y.; Mohammed, A.R.; Kirby, D.J.; McNeil, S.E.; Bramwell, V.W. Vaccine adjuvant systems: Enhancing the efficacy of sub-unit protein antigens. Int. J. Pharm. 2008, 364, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Shah, R. Vaccine uptake in under 19s: NICE Quality Standard (QS 145) 2017. Arch. Dis. Child. Educ. Pract. Ed. 2018, 103, 109. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, E.C.; O’Hagan, D.T. Delivery systems and adjuvants for oral vaccines. Expert Opin. Drug Deliv. 2006, 3, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Naili, I.; Vinot, J.; Baudner, B.C.; Bernalier-Donadille, A.; Pizza, M.; Desvaux, M.; Jubelin, G.; D’Oro, U.; Buonsanti, C. Mixed mucosal-parenteral immunizations with the broadly conserved pathogenic Escherichia coli antigen SslE induce a robust mucosal and systemic immunity without affecting the murine intestinal microbiota. Vaccine 2019, 37, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Davitt, C.J.; Lavelle, E.C. Delivery strategies to enhance oral vaccination against enteric infections. Adv. Drug Deliv. Rev. 2015, 91, 52–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vajdy, M.; Lycke, N.Y. Cholera toxin adjuvant promotes long-term immunological memory in the gut mucosa to unrelated immunogens after oral immunization. Immunology 1992, 75, 488–492. [Google Scholar]
- Clements, J.D.; Hartzog, N.M.; Lyon, F.L. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine 1988, 6, 269–277. [Google Scholar] [CrossRef]
- Snider, D.P. The Mucosal Adjuvant Activities of ADP-Ribosylating Bacterial Enterotoxins. Crit. Rev. Immunol. 2017, 37, 499–530. [Google Scholar] [CrossRef]
- Lycke, N.; Lebrero-Fernandez, C. ADP-ribosylating enterotoxins as vaccine adjuvants. Curr. Opin. Pharmacol. 2018, 41, 42–51. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, H.A.; Seo, K.H.; Lee, H.K.; Kang, B.Y.; Im, S.Y. Cholera toxin breakdowns oral tolerance via activation of canonical NF-kappaB. Cell. Immunol. 2013, 285, 92–99. [Google Scholar] [CrossRef]
- Sanchez, J.; Holmgren, J. Virulence factors, pathogenesis and vaccine protection in cholera and ETEC diarrhea. Curr. Opin. Immunol. 2005, 17, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gusti, V.; Saraswati, A.; Lo, D.D. Convergent and divergent development among M cell lineages in mouse mucosal epithelium. J. Immunol. 2011, 187, 5277–5285. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Moxley, R.A.; Zhang, W. Application of a novel epitope and structure vaccinology-assisted fimbria-toxin multiepitope fusion antigen of enterotoxigenic Escherichia coli for multivalent vaccine development against porcine post-weaning diarrhea. Appl. Environ. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Pang, S.; Wu, W.; Jiang, B.; Zhang, W.; Liu, S.; Wang, X.; Pan, Z.; Zhu, G. A multivalent vaccine candidate targeting enterotoxigenic Escherichia coli fimbriae for broadly protecting against porcine post-weaning diarrhea. Vet. Res. 2020, 51, 93. [Google Scholar] [CrossRef] [PubMed]
- Nandre, R.; Ruan, X.; Lu, T.; Duan, Q.; Sack, D.; Zhang, W. Enterotoxigenic Escherichia coli Adhesin-Toxoid Multiepitope Fusion Antigen CFA/I/II/IV-3xSTaN12S-mnLTG192G/L211A-Derived Antibodies Inhibit Adherence of Seven Adhesins, Neutralize Enterotoxicity of LT and STa Toxins, and Protect Piglets against Diarrhea. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef] [Green Version]
- Ruan, X.; Sack, D.A.; Zhang, W. Genetic fusions of a CFA/I/II/IV MEFA (multiepitope fusion antigen) and a toxoid fusion of heat-stable toxin (STa) and heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC) retain broad anti-CFA and antitoxin antigenicity. PLoS ONE 2015, 10, e0121623. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.; Lu, T.; Mani, S.; Bourgeois, A.L.; Walker, R.; Sack, D.A.; Zhang, W. Adjuvant effect of enterotoxigenic Escherichia coli (ETEC) double-mutant heat-labile toxin (dmLT) on systemic immunogenicity induced by the CFA/I/II/IV MEFA ETEC vaccine: Dose-related enhancement of antibody responses to seven ETEC adhesins (CFA/I, CS1-CS6). Hum. Vaccines Immunother. 2020, 16, 419–425. [Google Scholar] [CrossRef]
- Duan, Q.; Lee, K.H.; Nandre, R.M.; Garcia, C.; Chen, J.; Zhang, W. MEFA (multiepitope fusion antigen)-Novel Technology for Structural Vaccinology, Proof from Computational and Empirical Immunogenicity Characterization of an Enterotoxigenic Escherichia coli (ETEC) Adhesin MEFA. J. Vaccines Vaccin. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Duverger, A.; Jackson, R.J.; van Ginkel, F.W.; Fischer, R.; Tafaro, A.; Leppla, S.H.; Fujihashi, K.; Kiyono, H.; McGhee, J.R.; Boyaka, P.N. Bacillus anthracis edema toxin acts as an adjuvant for mucosal immune responses to nasally administered vaccine antigens. J. Immunol. 2006, 176, 1776–1783. [Google Scholar] [CrossRef] [Green Version]
- Price, A.E.; Shamardani, K.; Lugo, K.A.; Deguine, J.; Roberts, A.W.; Lee, B.L.; Barton, G.M. A Map of Toll-like Receptor Expression in the Intestinal Epithelium Reveals Distinct Spatial, Cell Type-Specific, and Temporal Patterns. Immunity 2018, 49, 560–575.e566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, L.A. DNA makes RNA makes innate immunity. Cell 2009, 138, 428–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, K.; Akira, S. Toll-like receptors. Curr. Protoc. Immunol. 2015, 109, 14.12.1–14.12.10. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, L.; Fischer, D.D.; Kandasamy, S.; Saif, L.J.; Vlasova, A.N. Tissue-specific mRNA expression profiles of porcine Toll-like receptors at different ages in germ-free and conventional pigs. Vet. Immunol. Immunopathol. 2016, 171, 7–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrick, B.M.; Yao, X.D.; Zahoor, M.A.; Abimiku, A.; Osawe, S.; Rosenthal, K.L. TLR10 Senses HIV-1 Proteins and Significantly Enhances HIV-1 Infection. Front. Immunol. 2019, 10, 482. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Flavell, R.A.; Eisenbarth, S.C. The role of NOD-like Receptors in shaping adaptive immunity. Curr. Opin. Immunol. 2010, 22, 34–40. [Google Scholar] [CrossRef]
- Sahoo, M.; Ceballos-Olvera, I.; del Barrio, L.; Re, F. Role of the inflammasome, IL-1beta, and IL-18 in bacterial infections. Sci. World J. 2011, 11, 2037–2050. [Google Scholar] [CrossRef] [Green Version]
- Hardison, S.E.; Brown, G.D. C-type lectin receptors orchestrate antifungal immunity. Nat. Immunol. 2012, 13, 817–822. [Google Scholar] [CrossRef]
- Rehwinkel, J.; Reis e Sousa, C. RIGorous detection: Exposing virus through RNA sensing. Science 2010, 327, 284–286. [Google Scholar] [CrossRef]
- Saxena, M.; Yeretssian, G. NOD-Like Receptors: Master Regulators of Inflammation and Cancer. Front. Immunol. 2014, 5, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hjelm, B.E.; Kilbourne, J.; Herbst-Kralovetz, M.M. TLR7 and 9 agonists are highly effective mucosal adjuvants for norovirus virus-like particle vaccines. Hum. Vaccines Immunother. 2014, 10, 410–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldrick, P.; Richardson, D.; Elliott, G.; Wheeler, A.W. Safety evaluation of monophosphoryl lipid A (MPL): An immunostimulatory adjuvant. Regul. Toxicol. Pharmacol. 2002, 35, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Mizel, S.B.; Bates, J.T. Flagellin as an adjuvant: Cellular mechanisms and potential. J. Immunol. 2010, 185, 5677–5682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, B.; Liu, X.; Fang, Y.; Zhou, P.; Zhang, Y.; Wang, Y. Flagellin as a vaccine adjuvant. Expert Rev. Vaccines 2018, 17, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Treanor, J.J.; Taylor, D.N.; Tussey, L.; Hay, C.; Nolan, C.; Fitzgerald, T.; Liu, G.; Kavita, U.; Song, L.; Dark, I.; et al. Safety and immunogenicity of a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125) in healthy young adults. Vaccine 2010, 28, 8268–8274. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.N.; Treanor, J.J.; Strout, C.; Johnson, C.; Fitzgerald, T.; Kavita, U.; Ozer, K.; Tussey, L.; Shaw, A. Induction of a potent immune response in the elderly using the TLR-5 agonist, flagellin, with a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125, STF2.HA1 SI). Vaccine 2011, 29, 4897–4902. [Google Scholar] [CrossRef]
- Bode, C.; Yang, X.P.; Kiu, H.; Klinman, D.M. Suppressive oligodeoxynucleotides promote the development of Th17 cells. PLoS ONE 2013, 8, e67991. [Google Scholar] [CrossRef] [Green Version]
- Pirahmadi, S.; Zakeri, S.; Mehrizi, A.A.; Djadid, N.D.; Raz, A.A.; Sani, J.J. Combining Monophosphoryl Lipid A (MPL), CpG Oligodeoxynucleotide (ODN), and QS-21 Adjuvants Induces Strong and Persistent Functional Antibodies and T Cell Responses against Cell-Traversal Protein for Ookinetes and Sporozoites (CelTOS) of Plasmodium falciparum in BALB/c Mice. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef] [Green Version]
- Ugolini, M.; Gerhard, J.; Burkert, S.; Jensen, K.J.; Georg, P.; Ebner, F.; Volkers, S.M.; Thada, S.; Dietert, K.; Bauer, L.; et al. Recognition of microbial viability via TLR8 drives TFH cell differentiation and vaccine responses. Nat. Immunol. 2018, 19, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Carreno, L.J.; Kharkwal, S.S.; Porcelli, S.A. Optimizing NKT cell ligands as vaccine adjuvants. Immunotherapy 2014, 6, 309–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutjahr, A.; Papagno, L.; Nicoli, F.; Kanuma, T.; Kuse, N.; Cabral-Piccin, M.P.; Rochereau, N.; Gostick, E.; Lioux, T.; Perouzel, E.; et al. The STING ligand cGAMP potentiates the efficacy of vaccine-induced CD8+ T cells. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Kawane, K.; Motani, K.; Nagata, S. DNA degradation and its defects. Cold Spring Harb. Perspect. Biol. 2014, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picker, L.J.; Butcher, E.C. Physiological and molecular mechanisms of lymphocyte homing. Annu. Rev. Immunol. 1992, 10, 561–591. [Google Scholar] [CrossRef] [PubMed]
- Gregor, C.E.; Foeng, J.; Comerford, I.; McColl, S.R. Chemokine-Driven CD4(+) T Cell Homing: New Concepts and Recent Advances. Adv. Immunol. 2017, 135, 119–181. [Google Scholar] [CrossRef]
- Mwanza-Lisulo, M.; Kelly, P. Potential for use of retinoic acid as an oral vaccine adjuvant. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef]
- Sirisinha, S. The pleiotropic role of vitamin A in regulating mucosal immunity. Asian Pac. J. Allergy Immunol. 2015, 33, 71–89. [Google Scholar]
- Wang, S.; Wu, C.; Zhang, Y.; Zhong, Q.; Sun, H.; Cao, W.; Ge, G.; Li, G.; Zhang, X.F.; Chen, J. Integrin alpha4beta7 switches its ligand specificity via distinct conformer-specific activation. J. Cell Biol. 2018, 217, 2799–2812. [Google Scholar] [CrossRef]
- Kunkel, E.J.; Campbell, J.J.; Haraldsen, G.; Pan, J.; Boisvert, J.; Roberts, A.I.; Ebert, E.C.; Vierra, M.A.; Goodman, S.B.; Genovese, M.C.; et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 2000, 192, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Papadakis, K.A.; Prehn, J.; Nelson, V.; Cheng, L.; Binder, S.W.; Ponath, P.D.; Andrew, D.P.; Targan, S.R. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J. Immunol. 2000, 165, 5069–5076. [Google Scholar] [CrossRef] [PubMed]
- Parmo-Cabanas, M.; Garcia-Bernal, D.; Garcia-Verdugo, R.; Kremer, L.; Marquez, G.; Teixido, J. Intracellular signaling required for CCL25-stimulated T cell adhesion mediated by the integrin alpha4beta1. J. Leukoc. Biol. 2007, 82, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Gehad, A.; Al-Banna, N.A.; Vaci, M.; Issekutz, A.C.; Mohan, K.; Latta, M.; Issekutz, T.B. Differing requirements for CCR4, E-selectin, and alpha4beta1 for the migration of memory CD4 and activated T cells to dermal inflammation. J. Immunol. 2012, 189, 337–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habtezion, A.; Nguyen, L.P.; Hadeiba, H.; Butcher, E.C. Leukocyte Trafficking to the Small Intestine and Colon. Gastroenterology 2016, 150, 340–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerutti, A. The regulation of IgA class switching. Nat. Rev. Immunol. 2008, 8, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Chorny, A.; Puga, I.; Cerutti, A. Innate signaling networks in mucosal IgA class switching. Adv. Immunol. 2010, 107, 31–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marks, E.; Ortiz, C.; Pantazi, E.; Bailey, C.S.; Lord, G.M.; Waldschmidt, T.J.; Noelle, R.J.; Elgueta, R. Retinoic Acid Signaling in B Cells Is Required for the Generation of an Effective T-Independent Immune Response. Front. Immunol. 2016, 7, 643. [Google Scholar] [CrossRef] [Green Version]
- Upham, J.W.; Sehmi, R.; Hayes, L.M.; Howie, K.; Lundahl, J.; Denburg, J.A. Retinoic acid modulates IL-5 receptor expression and selectively inhibits eosinophil-basophil differentiation of hemopoietic progenitor cells. J. Allergy Clin. Immunol. 2002, 109, 307–313. [Google Scholar] [CrossRef]
- Diehl, S.A.; Schmidlin, H.; Nagasawa, M.; Blom, B.; Spits, H. IL-6 triggers IL-21 production by human CD4+ T cells to drive STAT3-dependent plasma cell differentiation in B cells. Immunol. Cell Biol. 2012, 90, 802–811. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Saba, J.D. Regulation of Immune Cell Migration by Sphingosine-1-Phosphate. Cell. Mol. Biol. (OMICS) 2015, 61, 121. [Google Scholar]
- Mora, J.R.; Iwata, M.; Eksteen, B.; Song, S.Y.; Junt, T.; Senman, B.; Otipoby, K.L.; Yokota, A.; Takeuchi, H.; Ricciardi-Castagnoli, P.; et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 2006, 314, 1157–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, A.T.; Yao, S.; Gong, B.; Nurieva, R.I.; Elson, C.O.; Cong, Y. Interleukin (IL)-21 promotes intestinal IgA response to microbiota. Mucosal Immunol. 2015, 8, 1072–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tezuka, H.; Ohteki, T. Regulation of IgA Production by Intestinal Dendritic Cells and Related Cells. Front. Immunol. 2019, 10, 1891. [Google Scholar] [CrossRef] [PubMed]
- Hatayama, T.; Segawa, R.; Mizuno, N.; Eguchi, S.; Akamatsu, H.; Fukuda, M.; Nakata, F.; Leonard, W.J.; Hiratsuka, M.; Hirasawa, N. All-Trans Retinoic Acid Enhances Antibody Production by Inducing the Expression of Thymic Stromal Lymphopoietin Protein. J. Immunol. 2018, 200, 2670–2676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, S.; Jin, H.; Korn, T.; Liu, S.M.; Oukka, M.; Lim, B.; Kuchroo, V.K. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J. Immunol. 2008, 181, 2277–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammerschmidt, S.I.; Friedrichsen, M.; Boelter, J.; Lyszkiewicz, M.; Kremmer, E.; Pabst, O.; Forster, R. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J. Clin. Investig. 2011, 121, 3051–3061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.; Sande, J.L.; Pufnock, J.S.; Blattman, J.N.; Greenberg, P.D. Retinoic acid as a vaccine adjuvant enhances CD8+ T cell response and mucosal protection from viral challenge. J. Virol. 2011, 85, 8316–8327. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Tu, C.; Qin, T.; Zhu, L.; Yin, Y.; Yang, Q. Retinoic acid facilitates inactivated transmissible gastroenteritis virus induction of CD8(+) T-cell migration to the porcine gut. Sci. Rep. 2016, 6, 24152. [Google Scholar] [CrossRef] [Green Version]
- Christensen, D.; Bollehuus Hansen, L.; Leboux, R.; Jiskoot, W.; Christensen, J.P.; Andersen, P.; Dietrich, J. A Liposome-Based Adjuvant Containing Two Delivery Systems with the Ability to Induce Mucosal Immunoglobulin A Following a Parenteral Immunization. ACS Nano 2019, 13, 1116–1126. [Google Scholar] [CrossRef] [Green Version]
- Trovato, M.; De Berardinis, P. Novel antigen delivery systems. World J. Virol. 2015, 4, 156–168. [Google Scholar] [CrossRef]
- Guo, S.; Yan, W.; McDonough, S.P.; Lin, N.; Wu, K.J.; He, H.; Xiang, H.; Yang, M.; Moreira, M.A.; Chang, Y.F. The recombinant Lactococcus lactis oral vaccine induces protection against C. difficile spore challenge in a mouse model. Vaccine 2015, 33, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, S.; Karasaki, M.; Tafuku, S.; Aoki, W.; Sewaki, T.; Ueda, M. Oral Immunization Against Candidiasis Using Lactobacillus casei Displaying Enolase 1 from Candida albicans. Sci. Pharm. 2014, 82, 697–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.; Abdul-Wahid, A.; Faubert, G.M. Comparison of the local immune response against Giardia lamblia cyst wall protein 2 induced by recombinant Lactococcus lactis and Streptococcus gordonii. Microbes Infect. 2009, 11, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Chang, J. Viral vectors for vaccine applications. Clin. Exp. Vaccine Res. 2013, 2, 97–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauer, K.B.; Borrow, R.; Blanchard, T.J. Multivalent and Multipathogen Viral Vector Vaccines. Clin. Vaccine Immunol. 2017, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, M.R.; Kaminski, J.J.; Kurt-Jones, E.A.; Fitzgerald, K.A. Pattern recognition receptors and the innate immune response to viral infection. Viruses 2011, 3, 920–940. [Google Scholar] [CrossRef] [Green Version]
- Sayedahmed, E.E.; Hassan, A.O.; Kumari, R.; Cao, W.; Gangappa, S.; York, I.; Sambhara, S.; Mittal, S.K. A Bovine Adenoviral Vector-Based H5N1 Influenza -Vaccine Provides Enhanced Immunogenicity and Protection at a Significantly Low Dose. Mol. Ther. Methods Clin. Dev. 2018, 10, 210–222. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Wang, Q.; Shan, C.; Yang, C.; Feng, Y.; Wu, J.; Liu, X.; Zhou, Y.; Jiang, R.; Hu, P.; et al. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat. Commun. 2020, 11, 4207. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Sayedahmed, E.E.; Elkashif, A.; Alhashimi, M.; Sambhara, S.; Mittal, S.K. Adenoviral Vector-Based Vaccine Platforms for Developing the Next Generation of Influenza Vaccines. Vaccines 2020, 8, 574. [Google Scholar] [CrossRef]
- Ricobaraza, A.; Gonzalez-Aparicio, M.; Mora-Jimenez, L.; Lumbreras, S.; Hernandez-Alcoceba, R. High-Capacity Adenoviral Vectors: Expanding the Scope of Gene Therapy. Int. J. Mol. Sci. 2020, 21, 3643. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zheng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Mese, K.; Bunz, O.; Ehrhardt, A. State-of-the-art human adenovirus vectorology for therapeutic approaches. FEBS Lett. 2019, 593, 3609–3622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Top, F.H., Jr. Control of adenovirus acute respiratory disease in U.S. Army trainees. Yale J. Biol. Med. 1975, 48, 185–195. [Google Scholar] [PubMed]
- Chen, H.; Xiang, Z.Q.; Li, Y.; Kurupati, R.K.; Jia, B.; Bian, A.; Zhou, D.M.; Hutnick, N.; Yuan, S.; Gray, C.; et al. Adenovirus-based vaccines: Comparison of vectors from three species of adenoviridae. J. Virol. 2010, 84, 10522–10532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatsis, N.; Ertl, H.C. Adenoviruses as vaccine vectors. Mol. Ther. J. Am. Soc. Gene Ther. 2004, 10, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H. Novel adenovirus vector-based vaccines for HIV-1. Curr. Opin. HIV Aids 2010, 5, 386–390. [Google Scholar] [CrossRef]
- Moore, A.C.; Dora, E.G.; Peinovich, N.; Tucker, K.P.; Lin, K.; Cortese, M.; Tucker, S.N. Pre-clinical studies of a recombinant adenoviral mucosal vaccine to prevent SARS-CoV-2 infection. bioRxiv 2020. [Google Scholar] [CrossRef]
- Monteil, M.; Le Pottier, M.F.; Ristov, A.A.; Cariolet, R.; L’Hospitalier, R.; Klonjkowski, B.; Eloit, M. Single inoculation of replication-defective adenovirus-vectored vaccines at birth in piglets with maternal antibodies induces high level of antibodies and protection against pseudorabies. Vaccine 2000, 18, 1738–1742. [Google Scholar] [CrossRef]
- Fuenmayor, J.; Godia, F.; Cervera, L. Production of virus-like particles for vaccines. New Biotechnol. 2017, 39, 174–180. [Google Scholar] [CrossRef]
- Zeltins, A. Construction and characterization of virus-like particles: A review. Mol. Biotechnol. 2013, 53, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Pumpens, P.U.R.; Sasnauskas, K.; Kazaks, A.; Ose, V.; Grens, E. Construction of Novel Vaccines on the Basis of the Virus-Like Particles: Hepatitis B Virus Proteins as Vaccine Carriers, 1st ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Frazer, I.H. Prevention of cervical cancer through papillomavirus vaccination. Nat. Rev. Immunol. 2004, 4, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Mirza, Z.; Soto, E.R.; Dikengil, F.; Levitz, S.M.; Ostroff, G.R. Beta-Glucan Particles as Vaccine Adjuvant Carriers. Methods Mol. Biol. 2017, 1625, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhao, D.; Li, D.; Wang, X.; Jin, Z.; Zhao, K. Polymer-Based Nanomaterials and Applications for Vaccines and Drugs. Polymers 2018, 10, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Xu, C.; Yin, H.; Hill, J.; Pi, F.; Guo, P. Tuning the size, shape and structure of RNA nanoparticles for favorable cancer targeting and immunostimulation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1582. [Google Scholar] [CrossRef] [PubMed]
- Duran-Lobato, M.; Munoz-Rubio, I.; Holgado, M.A.; Alvarez-Fuentes, J.; Fernandez-Arevalo, M.; Martin-Banderas, L. Enhanced cellular uptake and biodistribution of a synthetic cannabinoid loaded in surface-modified poly(lactic-co-glycolic acid) nanoparticles. J. Biomed. Nanotechnol. 2014, 10, 1068–1079. [Google Scholar] [CrossRef]
- Plapied, L.; Duhem, N.; des Rieux, A.; Preat, V. Fate of polymeric nanocarriers for oral drug delivery. Curr Opin Colloid 2011, 16, 228–237. [Google Scholar] [CrossRef]
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit: A technology evaluation. Expert Opin. Drug Deliv. 2013, 10, 131–149. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Bernasconi, V.; Norling, K.; Bally, M.; Hook, F.; Lycke, N.Y. Mucosal Vaccine Development Based on Liposome Technology. J. Immunol. Res. 2016, 2016, 5482087. [Google Scholar] [CrossRef]
- Schwendener, R.A. Liposomes as vaccine delivery systems: A review of the recent advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Mishra, V.; Kesharwani, P. Bilosomes in the context of oral immunization: Development, challenges and opportunities. Drug Discov. Today 2016, 21, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, N.; Golocorbin-Kon, S.; Ethanic, M.; Stanimirov, B.; Al-Salami, H.; Stankov, K.; Mikov, M. Bile Acids and Their Derivatives as Potential Modifiers of Drug Release and Pharmacokinetic Profiles. Front. Pharmacol. 2018, 9, 1283. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.T.; Brown, L.E.; Deliyannis, G.; Pearse, M.J. ISCOM-based vaccines: The second decade. Immunol. Cell Biol. 2005, 83, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.S.; Gonzalez, A.M.; Yuan, L.; Jeong, K.I.; Iosef, C.; Van Nguyen, T.; Lovgren-Bengtsson, K.; Morein, B.; Saif, L.J. An oral versus intranasal prime/boost regimen using attenuated human rotavirus or VP2 and VP6 virus-like particles with immunostimulating complexes influences protection and antibody-secreting cell responses to rotavirus in a neonatal gnotobiotic pig model. Clin. Vaccine Immunol. 2010, 17, 420–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duran-Lobato, M.; Carrillo-Conde, B.; Khairandish, Y.; Peppas, N.A. Surface-modified P(HEMA-co-MAA) nanogel carriers for oral vaccine delivery: Design, characterization, and in vitro targeting evaluation. Biomacromolecules 2014, 15, 2725–2734. [Google Scholar] [CrossRef] [Green Version]
- Mabbott, N.A.; Donaldson, D.S.; Ohno, H.; Williams, I.R.; Mahajan, A. Microfold (M) cells: Important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013, 6, 666–677. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Gao, Z.; Zhang, Z.; Pan, L.; Zhang, Y. Roles of M cells in infection and mucosal vaccines. Hum. Vaccines Immunother. 2014, 10, 3544–3551. [Google Scholar] [CrossRef] [Green Version]
- Giannasca, P.J.; Giannasca, K.T.; Leichtner, A.M.; Neutra, M.R. Human intestinal M cells display the sialyl Lewis A antigen. Infect. Immun. 1999, 67, 946–953. [Google Scholar] [CrossRef] [Green Version]
- Hase, K.; Kawano, K.; Nochi, T.; Pontes, G.S.; Fukuda, S.; Ebisawa, M.; Kadokura, K.; Tobe, T.; Fujimura, Y.; Kawano, S.; et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature 2009, 462, 226–230. [Google Scholar] [CrossRef]
- Kim, S.H.; Jung, D.I.; Yang, I.Y.; Kim, J.; Lee, K.Y.; Nochi, T.; Kiyono, H.; Jang, Y.S. M cells expressing the complement C5a receptor are efficient targets for mucosal vaccine delivery. Eur. J. Immunol. 2011, 41, 3219–3229. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Hase, K. Glycoprotein 2 (GP2): Grabbing the FimH bacteria into M cells for mucosal immunity. Gut Microbes 2010, 1, 407–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lencer, W.I.; Moe, S.; Rufo, P.A.; Madara, J.L. Transcytosis of cholera toxin subunits across model human intestinal epithelia. Proc. Natl. Acad. Sci. USA 1995, 92, 10094–10098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florence, A.T. The oral absorption of micro- and nanoparticulates: Neither exceptional nor unusual. Pharm. Res. 1997, 14, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.D.; Leaphart, C.; Levy, R.; Prince, J.; Billiar, T.R.; Watkins, S.; Li, J.; Cetin, S.; Ford, H.; Schreiber, A.; et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J. Immunol. 2006, 176, 3070–3079. [Google Scholar] [CrossRef]
- Ye, L.; Zeng, R.; Bai, Y.; Roopenian, D.C.; Zhu, X. Efficient mucosal vaccination mediated by the neonatal Fc receptor. Nat. Biotechnol. 2011, 29, 158–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, E.S.; Ober, R.J. Targeting FcRn to Generate Antibody-Based Therapeutics. Trends Pharmacol. Sci. 2018, 39, 892–904. [Google Scholar] [CrossRef]
- Rath, T.; Baker, K.; Pyzik, M.; Blumberg, R.S. Regulation of immune responses by the neonatal fc receptor and its therapeutic implications. Front. Immunol. 2014, 5, 664. [Google Scholar] [CrossRef] [PubMed]
- Mina-Osorio, P. The moonlighting enzyme CD13: Old and new functions to target. Trends Mol. Med. 2008, 14, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Shapiro, L.H. In vitro Ag Cross-presentation and in vivo Ag Cross-presentation by Dendritic Cells in the Mouse. Bio-Protocol. 2012, 2, e305. [Google Scholar] [CrossRef] [Green Version]
- Villasenor-Cardoso, M.I.; Frausto-Del-Rio, D.A.; Ortega, E. Aminopeptidase N (CD13) is involved in phagocytic processes in human dendritic cells and macrophages. Biomed Res. Int. 2013, 2013, 562984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auray, G.; Keller, I.; Python, S.; Gerber, M.; Bruggmann, R.; Ruggli, N.; Summerfield, A. Characterization and Transcriptomic Analysis of Porcine Blood Conventional and Plasmacytoid Dendritic Cells Reveals Striking Species-Specific Differences. J. Immunol. 2016, 197, 4791–4806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, R.; Kiyota, A.; Suzaki, E.; Kataoka, K.; Ohe, Y.; Miyamoto, K.; Senda, T.; Fujimoto, T. Human coronavirus 229E binds to CD13 in rafts and enters the cell through caveolae. J. Virol. 2004, 78, 8701–8708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolb, A.F.; Hegyi, A.; Maile, J.; Heister, A.; Hagemann, M.; Siddell, S.G. Molecular analysis of the coronavirus-receptor function of aminopeptidase N. Adv. Exp. Med. Biol. 1998, 440, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Hulswit, R.J.G.; Kenney, S.P.; Widjaja, I.; Jung, K.; Alhamo, M.A.; van Dieren, B.; van Kuppeveld, F.J.M.; Saif, L.J.; Bosch, B.J. Broad receptor engagement of an emerging global coronavirus may potentiate its diverse cross-species transmissibility. Proc. Natl. Acad. Sci. USA 2018, 115, E5135–E5143. [Google Scholar] [CrossRef] [Green Version]
- Soderberg, C.; Giugni, T.D.; Zaia, J.A.; Larsson, S.; Wahlberg, J.M.; Moller, E. CD13 (human aminopeptidase N) mediates human cytomegalovirus infection. J. Virol. 1993, 67, 6576–6585. [Google Scholar] [CrossRef] [Green Version]
- Ho, H.T.; Tsai, I.F.; Wu, C.L.; Lu, Y.T. Aminopeptidase N facilitates entry and intracellular survival of Mycobacterium tuberculosis in monocytes. Respirology 2014, 19, 109–115. [Google Scholar] [CrossRef]
- Verdonck, F.; Cox, E.; Vancaeneghem, S.; Goddeeris, B.M. The interaction of F4 fimbriae with porcine enterocytes as analysed by surface plasmon resonance. FEMS Immunol. Med Microbiol. 2004, 41, 243–248. [Google Scholar] [CrossRef]
- Xia, P.; Wang, Y.; Zhu, C.; Zou, Y.; Yang, Y.; Liu, W.; Hardwidge, P.R.; Zhu, G. Porcine aminopeptidase N binds to F4+ enterotoxigenic Escherichia coli fimbriae. Vet. Res. 2016, 47, 24. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S. Pattern recognition receptors: Doubling up for the innate immune response. Cell 2002, 111, 927–930. [Google Scholar] [CrossRef] [Green Version]
- Jahan, S.T.; Sadat, S.M.; Haddadi, A. Design and immunological evaluation of anti-CD205-tailored PLGA-based nanoparticulate cancer vaccine. Int. J. Nanomed. 2018, 13, 367–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volckmar, J.; Gereke, M.; Ebensen, T.; Riese, P.; Philipsen, L.; Lienenklaus, S.; Wohlleber, D.; Klopfleisch, R.; Stegemann-Koniszewski, S.; Muller, A.J.; et al. Targeted antigen delivery to dendritic cells elicits robust antiviral T cell-mediated immunity in the liver. Sci. Rep. 2017, 7, 43985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boscardin, S.B.; Hafalla, J.C.; Masilamani, R.F.; Kamphorst, A.O.; Zebroski, H.A.; Rai, U.; Morrot, A.; Zavala, F.; Steinman, R.M.; Nussenzweig, R.S.; et al. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J. Exp. Med. 2006, 203, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.W.; Thompson, C.; Reid, D.M.; Wong, S.Y.; Tough, D.F. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. J. Immunol. 2006, 177, 2276–2284. [Google Scholar] [CrossRef] [Green Version]
- He, L.Z.; Crocker, A.; Lee, J.; Mendoza-Ramirez, J.; Wang, X.T.; Vitale, L.A.; O’Neill, T.; Petromilli, C.; Zhang, H.F.; Lopez, J.; et al. Antigenic targeting of the human mannose receptor induces tumor immunity. J. Immunol. 2007, 178, 6259–6267. [Google Scholar] [CrossRef] [Green Version]
- Sancho, D.; Mourao-Sa, D.; Joffre, O.P.; Schulz, O.; Rogers, N.C.; Pennington, D.J.; Carlyle, J.R.; Reis e Sousa, C. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J. Clin. Investig. 2008, 118, 2098–2110. [Google Scholar] [CrossRef]
- Caminschi, I.; Proietto, A.I.; Ahmet, F.; Kitsoulis, S.; Shin Teh, J.; Lo, J.C.; Rizzitelli, A.; Wu, L.; Vremec, D.; van Dommelen, S.L.; et al. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood 2008, 112, 3264–3273. [Google Scholar] [CrossRef]
- Baumann, J.; Park, C.G.; Mantis, N.J. Recognition of secretory IgA by DC-SIGN: Implications for immune surveillance in the intestine. Immunol. Lett. 2010, 131, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Kretz-Rommel, A.; Qin, F.; Dakappagari, N.; Torensma, R.; Faas, S.; Wu, D.; Bowdish, K.S. In vivo targeting of antigens to human dendritic cells through DC-SIGN elicits stimulatory immune responses and inhibits tumor growth in grafted mouse models. J. Immunother. 2007, 30, 715–726. [Google Scholar] [CrossRef]
- Yang, L.; Yang, H.; Rideout, K.; Cho, T.; Joo, K.I.; Ziegler, L.; Elliot, A.; Walls, A.; Yu, D.; Baltimore, D.; et al. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat. Biotechnol. 2008, 26, 326–334. [Google Scholar] [CrossRef] [Green Version]
- Gosselin, E.J.; Wardwell, K.; Gosselin, D.R.; Alter, N.; Fisher, J.L.; Guyre, P.M. Enhanced antigen presentation using human Fc gamma receptor (monocyte/macrophage)-specific immunogens. J. Immunol. 1992, 149, 3477–3481. [Google Scholar] [PubMed]
- Gil, M.; Bieniasz, M.; Wierzbicki, A.; Bambach, B.J.; Rokita, H.; Kozbor, D. Targeting a mimotope vaccine to activating Fcgamma receptors empowers dendritic cells to prime specific CD8+ T cell responses in tumor-bearing mice. J. Immunol. 2009, 183, 6808–6818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawool, D.B.; Bitsaktsis, C.; Li, Y.; Gosselin, D.R.; Lin, Y.; Kurkure, N.V.; Metzger, D.W.; Gosselin, E.J. Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J. Immunol. 2008, 180, 5548–5557. [Google Scholar] [CrossRef] [PubMed]
- Wenink, M.H.; Santegoets, K.C.; Roelofs, M.F.; Huijbens, R.; Koenen, H.J.; van Beek, R.; Joosten, I.; Meyer-Wentrup, F.; Mathsson, L.; Ronnelid, J.; et al. The inhibitory Fc gamma IIb receptor dampens TLR4-mediated immune responses and is selectively up-regulated on dendritic cells from rheumatoid arthritis patients with quiescent disease. J. Immunol. 2009, 183, 4509–4520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, G.H.; Iglesias, B.V.; Gosselin, E.J. Fc receptor-targeting of immunogen as a strategy for enhanced antigen loading, vaccination, and protection using intranasally administered antigen-pulsed dendritic cells. Vaccine 2014, 32, 5212–5220. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Meng, G.; Dickinson, B.L.; Li, X.; Mizoguchi, E.; Miao, L.; Wang, Y.; Robert, C.; Wu, B.; Smith, P.D.; et al. MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J. Immunol. 2001, 166, 3266–3276. [Google Scholar] [CrossRef] [Green Version]
- Latvala, S.; Jacobsen, B.; Otteneder, M.B.; Herrmann, A.; Kronenberg, S. Distribution of FcRn Across Species and Tissues. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2017, 65, 321–333. [Google Scholar] [CrossRef] [Green Version]
- Stirling, C.M.; Charleston, B.; Takamatsu, H.; Claypool, S.; Lencer, W.; Blumberg, R.S.; Wileman, T.E. Characterization of the porcine neonatal Fc receptor--potential use for trans-epithelial protein delivery. Immunology 2005, 114, 542–553. [Google Scholar] [CrossRef]
- de Jong, S.E.; Olin, A.; Pulendran, B. The Impact of the Microbiome on Immunity to Vaccination in Humans. Cell Host Microbe 2020, 28, 169–179. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Takanashi, S.; Miyazaki, A.; Rajashekara, G.; Saif, L.J. How the gut microbiome regulates host immune responses to viral vaccines. Curr. Opin. Virol. 2019, 37, 16–25. [Google Scholar] [CrossRef]
- Rampelli, S.; Candela, M.; Turroni, S.; Biagi, E.; Collino, S.; Franceschi, C.; O’Toole, P.W.; Brigidi, P. Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging 2013, 5, 902–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huda, M.N.; Lewis, Z.; Kalanetra, K.M.; Rashid, M.; Ahmad, S.M.; Raqib, R.; Qadri, F.; Underwood, M.A.; Mills, D.A.; Stephensen, C.B. Stool microbiota and vaccine responses of infants. Pediatrics 2014, 134, e362–e372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, V.C.; Haak, B.W.; Handley, S.A.; Jiang, B.; Velasquez, D.E.; Hykes, B.L., Jr.; Droit, L.; Berbers, G.A.M.; Kemper, E.M.; van Leeuwen, E.M.M.; et al. Effect of Antibiotic-Mediated Microbiome Modulation on Rotavirus Vaccine Immunogenicity: A Human, Randomized-Control Proof-of-Concept Trial. Cell Host Microbe 2018, 24, 197–207.e194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.Z.; Ravindran, R.; Chassaing, B.; Carvalho, F.A.; Maddur, M.S.; Bower, M.; Hakimpour, P.; Gill, K.P.; Nakaya, H.I.; Yarovinsky, F.; et al. TLR5-Mediated Sensing of Gut Microbiota Is Necessary for Antibody Responses to Seasonal Influenza Vaccination. Immunity 2014, 41, 478–492. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, P.; Curtis, N. The influence of probiotics on vaccine response—A systematic review. Vaccine 2018, 36, 207–213. [Google Scholar] [CrossRef]
- Philipps, J.; Schonfeld, C.; Schleehauf, D.M.; Beuerle, W.; Bienzle, U.; Kremsner, P.G. [Side effects of travel vaccinations. Data collection via telephone survey in Berlin]. Wien. Klin. Wochenschr. 1996, 108, 615–620. [Google Scholar]
- Klein, S.L.; Passaretti, C.; Anker, M.; Olukoya, P.; Pekosz, A. The impact of sex, gender and pregnancy on 2009 H1N1 disease. Biol. Sex Differ. 2010, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Umlauf, B.J.; Haralambieva, I.H.; Ovsyannikova, I.G.; Kennedy, R.B.; Pankratz, V.S.; Jacobson, R.M.; Poland, G.A. Associations between demographic variables and multiple measles-specific innate and cell-mediated immune responses after measles vaccination. Viral Immunol. 2012, 25, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Furman, D.; Hejblum, B.P.; Simon, N.; Jojic, V.; Dekker, C.L.; Thiebaut, R.; Tibshirani, R.J.; Davis, M.M. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 869–874. [Google Scholar] [CrossRef] [Green Version]
- Flanagan, K.L.; Plebanski, M. Sex-differential heterologous (non-specific) effects of vaccines: An emerging public health issue that needs to be understood and exploited. Expert Rev. Vaccines 2017, 16, 5–13. [Google Scholar] [CrossRef]
- Fischinger, S.; Boudreau, C.M.; Butler, A.L.; Streeck, H.; Alter, G. Sex differences in vaccine-induced humoral immunity. Semin. Immunopathol. 2019, 41, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haralambieva, I.H.; Ovsyannikova, I.G.; Kennedy, R.B.; Larrabee, B.R.; Shane Pankratz, V.; Poland, G.A. Race and sex-based differences in cytokine immune responses to smallpox vaccine in healthy individuals. Hum. Immunol. 2013, 74, 1263–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haralambieva, I.H.; Salk, H.M.; Lambert, N.D.; Ovsyannikova, I.G.; Kennedy, R.B.; Warner, N.D.; Pankratz, V.S.; Poland, G.A. Associations between race, sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine 2014, 32, 1946–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, R.B.; Ovsyannikova, I.G.; Haralambieva, I.H.; Lambert, N.D.; Pankratz, V.S.; Poland, G.A. Genome-wide SNP associations with rubella-specific cytokine responses in measles-mumps-rubella vaccine recipients. Immunogenetics 2014, 66, 493–499. [Google Scholar] [CrossRef] [Green Version]
- Christy, C.; Pichichero, M.E.; Reed, G.F.; Decker, M.D.; Anderson, E.L.; Rennels, M.B.; Englund, J.A.; Edwards, K.M.; Steinhoff, M.C. Effect of gender, race, and parental education on immunogenicity and reported reactogenicity of acellular and whole-cell pertussis vaccines. Pediatrics 1995, 96, 584–587. [Google Scholar]
- McQuillan, G.M.; Kruszon-Moran, D.; Hyde, T.B.; Forghani, B.; Bellini, W.; Dayan, G.H. Seroprevalence of measles antibody in the US population, 1999-2004. J. Infect. Dis. 2007, 196, 1459–1464. [Google Scholar] [CrossRef] [Green Version]
- Poland, G.A.; Jacobson, R.M.; Colbourne, S.A.; Thampy, A.M.; Lipsky, J.J.; Wollan, P.C.; Roberts, P.; Jacobsen, S.J. Measles antibody seroprevalence rates among immunized Inuit, Innu and Caucasian subjects. Vaccine 1999, 17, 1525–1531. [Google Scholar] [CrossRef]
- Greenberg, D.P.; Vadheim, C.M.; Partridge, S.; Chang, S.J.; Chiu, C.Y.; Ward, J.I. Immunogenicity of Haemophilus influenzae type b tetanus toxoid conjugate vaccine in young infants. The Kaiser-UCLA Vaccine Study Group. J. Infect. Dis. 1994, 170, 76–81. [Google Scholar] [CrossRef]
- Guthridge, S.; McIntyre, P.; Isaacs, D.; Hanlon, M.; Patel, M. Differing serologic responses to an haemophilus influenzae type b polysaccharide-neisseria meningitidis outer membrane protein conjugate (PRP-OMPC) vaccine in australian aboriginal and caucasian infants-implications for disease epidemiology. Vaccine 2000, 18, 2584–2591. [Google Scholar] [CrossRef]
- Kurupati, R.; Kossenkov, A.; Haut, L.; Kannan, S.; Xiang, Z.; Li, Y.; Doyle, S.; Liu, Q.; Schmader, K.; Showe, L.; et al. Race-related differences in antibody responses to the inactivated influenza vaccine are linked to distinct pre-vaccination gene expression profiles in blood. Oncotarget 2016, 7, 62898–62911. [Google Scholar] [CrossRef]
- Naylor, C.; Lu, M.; Haque, R.; Mondal, D.; Buonomo, E.; Nayak, U.; Mychaleckyj, J.C.; Kirkpatrick, B.; Colgate, R.; Carmolli, M.; et al. Environmental Enteropathy, Oral Vaccine Failure and Growth Faltering in Infants in Bangladesh. EBioMedicine 2015, 2, 1759–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelly, A.; Gupta, P.; Ahuja, R.; Srichandan, S.; Meena, J.; Majumdar, T. Impact of Microbiota: A Paradigm for Evolving Herd Immunity against Viral Diseases. Viruses 2020, 12, 1150. [Google Scholar] [CrossRef] [PubMed]
- Devriendt, B.; Gallois, M.; Verdonck, F.; Wache, Y.; Bimczok, D.; Oswald, I.P.; Goddeeris, B.M.; Cox, E. The food contaminant fumonisin B(1) reduces the maturation of porcine CD11R1(+) intestinal antigen presenting cells and antigen-specific immune responses, leading to a prolonged intestinal ETEC infection. Vet. Res. 2009, 40, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of mycotoxin on immune response and consequences for pig health. Anim. Nutr. 2016, 2, 63–68. [Google Scholar] [CrossRef] [PubMed]
| Oral adjuvants | |
| Toxin derivates | dmLT [20,21,22,23], mmCT [24] |
| PRR ligands | β-glucans [25,26], MPL [27], Flagellin [28,29], CpG [30,31] |
| NKT-ligands | α-galactosyl ceramide [32,33] |
| Delivery systems | |
| Living delivery systems | Recombinant bacteria [34,35,36,37,38,39,40,41,42,43,44] Viral vectors [45,46,47,48,49,50,51,52,53,54,55,56] |
| Non-living delivery systems | Virus-like particles [57] |
| Micro- and nanoparticles [58,59,60] | |
| Lipid-based delivery systems [61,62,63,64,65,66,67,68,69] | |
| Nanogels [70] | |
| Targeted delivery | |
| M-cells | Dectin-1 [71], GP2 [72], C5aR [73] |
| Enterocytes | FcRn [74] Aminopeptidase N [60,75,76,77,78,79] |
| Pathogen Recognition Receptor (PRR) | Cellular Localization | Ligand |
|---|---|---|
| Toll-like receptors (TLR) [102,103,104,105,106] | ||
| TLR1 | Plasma membrane | Peptidoglycans/lipoproteins |
| TLR2 | Plasma membrane | Peptidoglycans/lipoproteins |
| TLR3 | Endosome | dsRNA |
| TLR4 | Plasma membrane | LPS |
| TLR5 | Plasma membrane | Flagellin |
| TLR6 | Plasma membrane | Lipoproteins |
| TLR7 | Endosome | ssRNA |
| TLR8 | Endosome | ssRNA |
| TLR9 | Endosome | Unmethylated CpG |
| TLR10 | Endosome | Unknown |
| NOD-like receptors (NLR)* [107,108] | ||
| NOD1/2 | Cytoplasm | Peptidoglycans |
| NLRP3 | Cytoplasm | PAMP, DAMP ** |
| NLRC4 | Cytoplasm | Cytosolic flagellin |
| C-type lectin receptors (CLR) [109] | ||
| Dectin-1 | Plasma membrane | β-glucans |
| Clec9A | Plasma membrane | F-actin |
| DC-SIGN | Plasma membrane | Mannose |
| Mannose receptor | Plasma membrane | Glycans |
| RIG-I-like receptors (RLR) [110] | ||
| RIG-I | Cytoplasm | dsRNA |
| MDA-5 | Cytoplasm | dsRNA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van der Weken, H.; Cox, E.; Devriendt, B. Advances in Oral Subunit Vaccine Design. Vaccines 2021, 9, 1. https://doi.org/10.3390/vaccines9010001
Van der Weken H, Cox E, Devriendt B. Advances in Oral Subunit Vaccine Design. Vaccines. 2021; 9(1):1. https://doi.org/10.3390/vaccines9010001
Chicago/Turabian StyleVan der Weken, Hans, Eric Cox, and Bert Devriendt. 2021. "Advances in Oral Subunit Vaccine Design" Vaccines 9, no. 1: 1. https://doi.org/10.3390/vaccines9010001
APA StyleVan der Weken, H., Cox, E., & Devriendt, B. (2021). Advances in Oral Subunit Vaccine Design. Vaccines, 9(1), 1. https://doi.org/10.3390/vaccines9010001