Protection against the New Equine Influenza Virus Florida Clade I Outbreak Strain Provided by a Whole Inactivated Virus Vaccine
Abstract
1. Introduction
2. Materials and Methods
2.1. Horses
2.2. Vaccine
2.3. EIV Viruses
2.4. Vaccination and Challenge Infection Protocol
2.5. Sample Collection
2.6. HI Assay
2.7. Observations of Clinical Signs
2.8. Shedding of Live EIV
2.9. Data Analysis and Statistics
3. Results
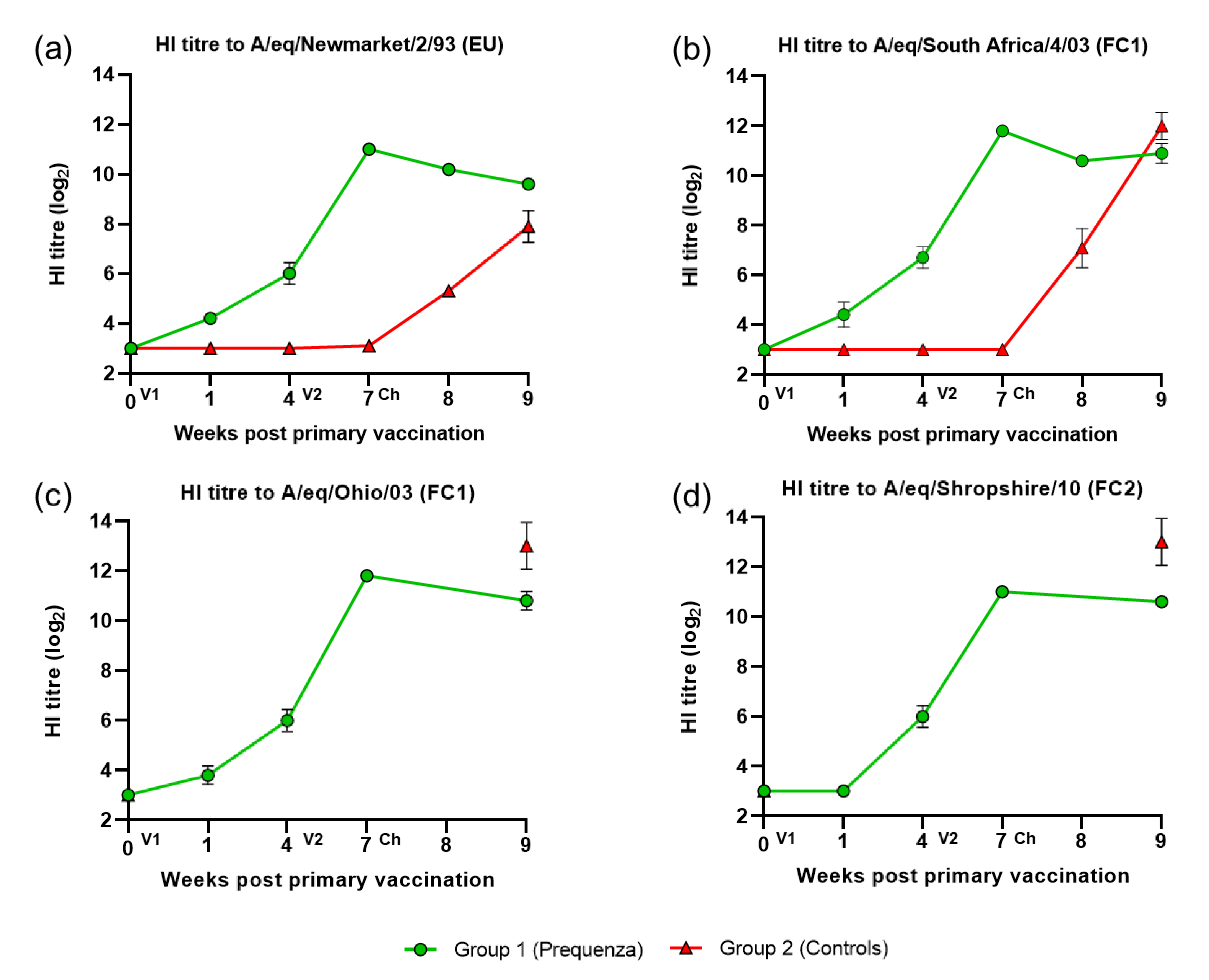
3.1. HI Antibody Response
3.2. Clinical Signs of Disease after Challenge with H3N8 A/equine/Venlo/19 (FC1 Sub-lineage)
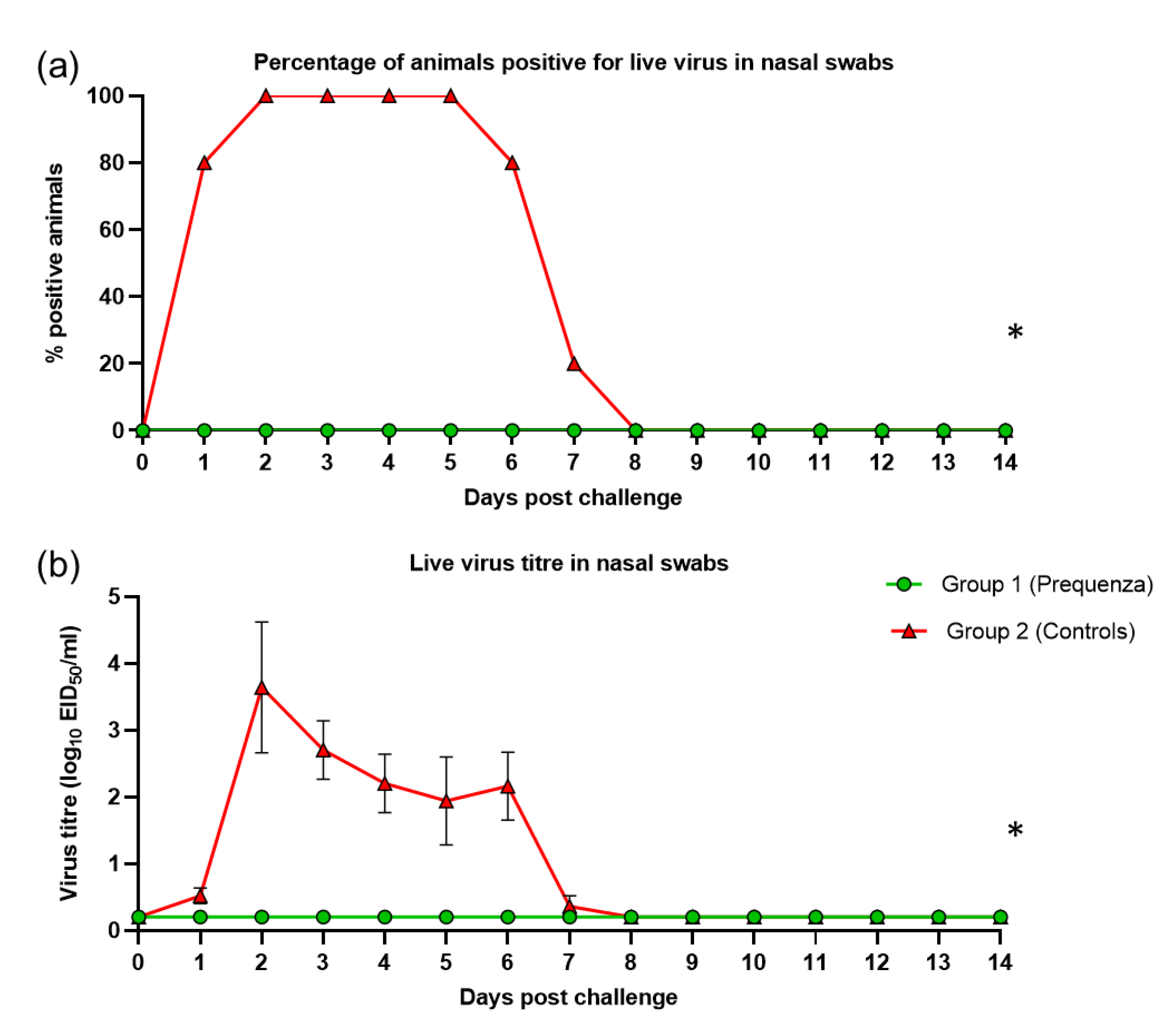
3.3. Virus Shedding after Challenge with H3N8 A/equine/Venlo/19 (FC1 Sub-Lineage)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paillot, R. A systematic review of recent advances in equine influenza vaccination. Vaccines 2014, 2, 797–831. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S.; Daly, J.M.; Russell, C.A.; Horton, D.L.; Skepner, E.; Bryant, N.A.; Burke, D.F.; Rash, A.S.; Wood, J.L.; Chambers, T.M.; et al. Antigenic and genetic evolution of equine influenza A (H3N8) virus from 1968 to 2007. J. Virol. 2011, 85, 12742–12749. [Google Scholar] [CrossRef]
- Landolt, G.; Townsend, H.G.; Lunn, D.P. Equine influenza infection. In Equine Infectious Diseases, 2nd ed.; Sellon, D.C., Long, M.T., Eds.; Saunders Elsevier: Amsterdam, The Netherland, 2013; pp. 141–151. [Google Scholar]
- Fougerolle, S.; Fortier, C.; Legrand, L.; Jourdan, M.; Marcillaud-Pitel, C.; Pronost, S.; Paillot, R. Success and Limitation of Equine Influenza Vaccination: The First Incursion in a Decade of a Florida Clade 1 Equine Influenza Virus that Shakes Protection Despite High Vaccine Coverage. Vaccines 2019, 7, 174. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.R.; Perez, R.; Rodriguez, S.; Bassetti, L.; Negro, R.; Vidal, R. Epidemiological and virological findings during an outbreak of equine influenza in Uruguay in 2018. Rev. Sci. Tech. 2019, 38, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Olguin-Perglione, C.; Vissani, M.A.; Alamos, F.; Tordoya, M.S.; Barrandeguy, M. Multifocal outbreak of equine influenza in vaccinated horses in Argentina in 2018: Epidemiological aspects and molecular characterisation of the involved virus strains. Equine Vet. J. 2020, 52, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Pitel, P.H.; Pronost, S.; Legrand, L.; Fougerolle, S.; Jourdan, M.; Marcillaud-Pitel, C. Florida clade 1 equine influenza virus in France. Vet. Rec. 2019, 184, 101. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Grimmett, H.; Elton, D.; Daly, J.M. Protection, systemic IFNgamma, and antibody responses induced by an ISCOM-based vaccine against a recent equine influenza virus in its natural host. Vet. Res. 2008, 39, 21. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Equine influenza vaccine (inactivated) monograph 0249, 2-3-2-2-2. Haemagglutinin-inhibition test. In European Pharmacopoeia, 9th ed.; Directorate for the Quality of Medicines & HealthCare of the Council of Europe (EDQM): Strasbourg, France, 2017; p. 1052. [Google Scholar]
- The World Organisation for Animal Health (OIE). Equine Influenza: Chapter 3.5.7. Available online: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.05.07_EQ_INF.pdf (accessed on 1 August 2019).
- Anonymous. Equine influenza vaccine (inactivated) monograph 0249. In European Pharmacopoeia, 9th ed.; Directorate for the Quality of Medicines & HealthCare of the Council of Europe (EDQM): Strasbourg, France, 2017; pp. 1051–1053. [Google Scholar]
- Reemers, S.; Sonnemans, D.; Horspool, L.; van Bommel, S.; Cao, Q.; van de Zande, S. Determining Equine Influenza Virus Vaccine Efficacy-The Specific Contribution of Strain Versus Other Vaccine Attributes. Vaccines 2020, 8, 501. [Google Scholar] [CrossRef] [PubMed]
- Geeraedts, F.; Bungener, L.; Pool, J.; ter Veer, W.; Wilschut, J.; Huckriede, A. Whole inactivated virus influenza vaccine is superior to subunit vaccine in inducing immune responses and secretion of proinflammatory cytokines by DCs. Influenza Other Respir. Viruses 2008, 2, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Stoel, M.; Pool, J.; de Vries-Idema, J.; Zaaraoui-Boutahar, F.; Bijl, M.; Andeweg, A.C.; Wilschut, J.; Huckriede, A. Innate responses induced by whole inactivated virus or subunit influenza vaccines in cultured dendritic cells correlate with immune responses in vivo. PLoS ONE 2015, 10, e0125228. [Google Scholar] [CrossRef] [PubMed]
- Budimir, N.; Huckriede, A.; Meijerhof, T.; Boon, L.; Gostick, E.; Price, D.A.; Wilschut, J.; de Haan, A. Induction of heterosubtypic cross-protection against influenza by a whole inactivated virus vaccine: The role of viral membrane fusion activity. PLoS ONE 2012, 7, 30898. [Google Scholar] [CrossRef] [PubMed]
- Bennink, J.R.; Yewdell, J.W.; Gerhard, W. A viral polymerase involved in recognition of influenza virus-infected cells by a cytotoxic T-cell clone. Nature 1982, 296, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Townsend, A.R.; Skehel, J.J. The influenza A virus nucleoprotein gene controls the induction of both subtype specific and cross-reactive cytotoxic T cells. J. Exp. Med. 1984, 160, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Doherty, P.C.; Kelso, A. Toward a broadly protective influenza vaccine. J. Clin. Investig. 2008, 118, 3273–3275. [Google Scholar] [CrossRef] [PubMed]


| Clinical Observation | Score | |
|---|---|---|
| General Health | No abnormality found | 0 |
| Diarrhea | 1 | |
| Malaise/depression/normal eating | 1 | |
| Malaise/depression/reduced appetite | 2 | |
| Dehydration | 2 | |
| Anorexia | 4 | |
| Colic | 5 | |
| Down/unable to stand | 30 | |
| Dead | 100 | |
| Respiratory | Hyperpnoea | 2 |
| Dyspnea | 4 | |
| Cough/2–5× in 10 min | 1 | |
| Cough/6–20× in 10 min | 2 | |
| Cough/>20× in 10 min | 3 | |
| Ocular | Lachrymation | 1 |
| Mild mucopurulent discharge | 2 | |
| Marked mucopurulent discharge | 4 | |
| Mild conjunctivitis | 2 | |
| Marked conjunctivitis | 4 | |
| Nasal | Nasal serous discharge | 1 |
| Mild nasal mucopurulent | 2 | |
| Marked nasal mucopurulent | 4 | |
| Sneeze/2–5× in 10 min | 1 | |
| Sneeze/6–20× in 10 min | 2 | |
| Sneeze/>20× in 10 min | 3 | |
| Temperature (°C) | <38.5 | 0 |
| 38.5–39.0 | 1 | |
| 39.1–39.5 | 2 | |
| 39.6–40.0 | 3 | |
| >40.0 | 4 | |
| Group Comparison 1 = Control, 2 = Equilis Prequenza | |
|---|---|
| Parameter | 2 vs. 1 |
| Rectal temperature over time a | 0.0156 |
| Peak change rectal temperature post-challenge a | 0.0478 |
| Clinical score a | <0.0001 |
| Total clinical score a | <0.0001 |
| Viral shedding nasal swabs +/− b | <0.0001 |
| Viral shedding nasal swab titre b | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reemers, S.; van Bommel, S.; Cao, Q.; Sutton, D.; van de Zande, S. Protection against the New Equine Influenza Virus Florida Clade I Outbreak Strain Provided by a Whole Inactivated Virus Vaccine. Vaccines 2020, 8, 784. https://doi.org/10.3390/vaccines8040784
Reemers S, van Bommel S, Cao Q, Sutton D, van de Zande S. Protection against the New Equine Influenza Virus Florida Clade I Outbreak Strain Provided by a Whole Inactivated Virus Vaccine. Vaccines. 2020; 8(4):784. https://doi.org/10.3390/vaccines8040784
Chicago/Turabian StyleReemers, Sylvia, Sander van Bommel, Qi Cao, David Sutton, and Saskia van de Zande. 2020. "Protection against the New Equine Influenza Virus Florida Clade I Outbreak Strain Provided by a Whole Inactivated Virus Vaccine" Vaccines 8, no. 4: 784. https://doi.org/10.3390/vaccines8040784
APA StyleReemers, S., van Bommel, S., Cao, Q., Sutton, D., & van de Zande, S. (2020). Protection against the New Equine Influenza Virus Florida Clade I Outbreak Strain Provided by a Whole Inactivated Virus Vaccine. Vaccines, 8(4), 784. https://doi.org/10.3390/vaccines8040784





