Cross-Reactive Effects of Vaccines: Heterologous Immunity between Tetanus and Chlamydia
Abstract
1. Introduction
2. Materials and Methods
2.1. Protein Sequence Alignments
2.2. Antigens
2.3. Tetanus-Specific Monoclonal Antibodies
2.4. Inhibition of mAbs Binding to Tetanus by Chlamydia
2.5. In Vitro Neutralization Assay—A Percentage of Neutralization
2.6. Ethics Statement
2.7. Experimental Animals
2.8. C. caviae Conjunctival Infections
2.9. Pathology Scoring
2.10. Statistical Analysis
3. Results
3.1. Identification of Potentially Cross-Reactive Sequences between Tetanus and Chlamydial Proteins
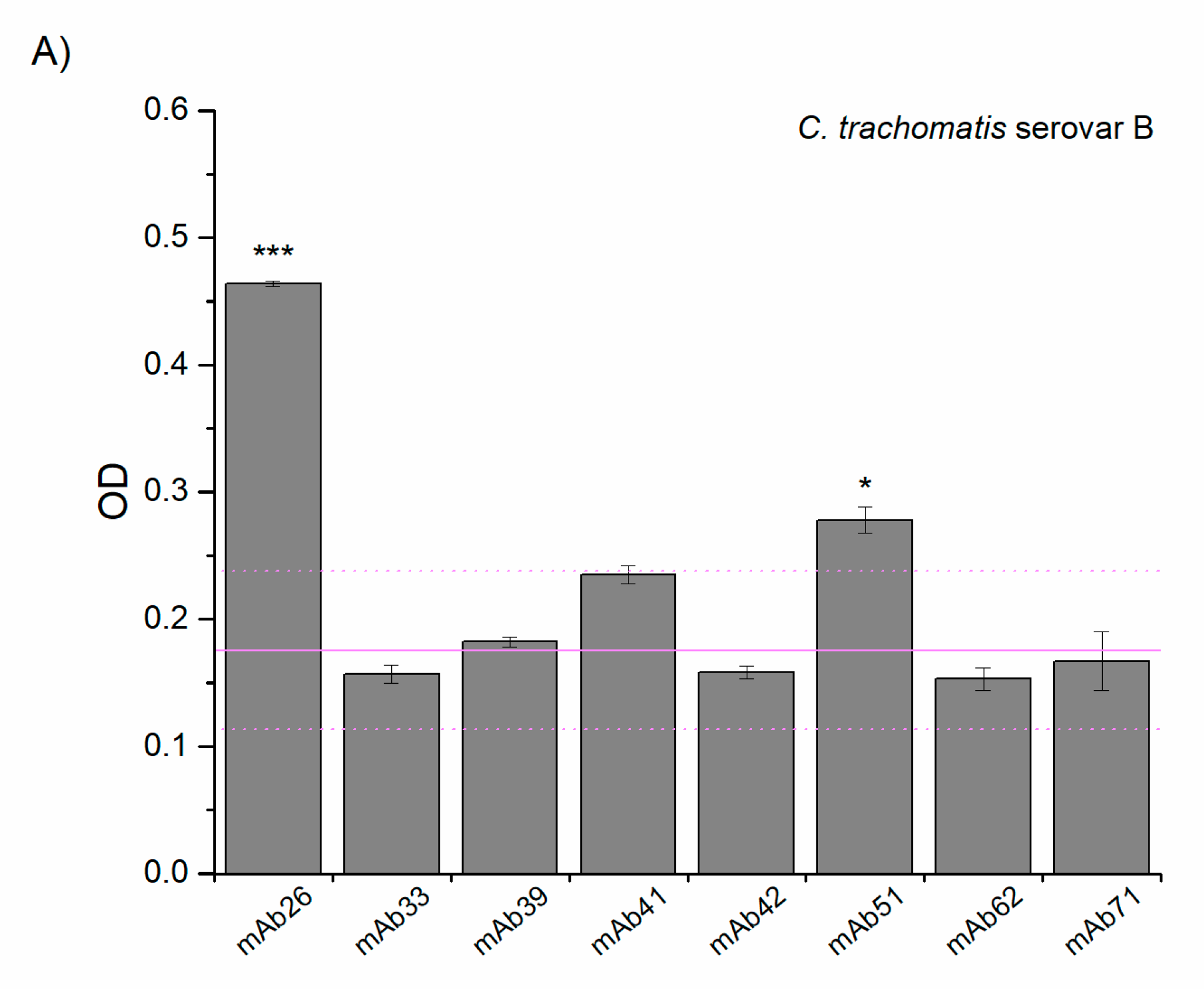
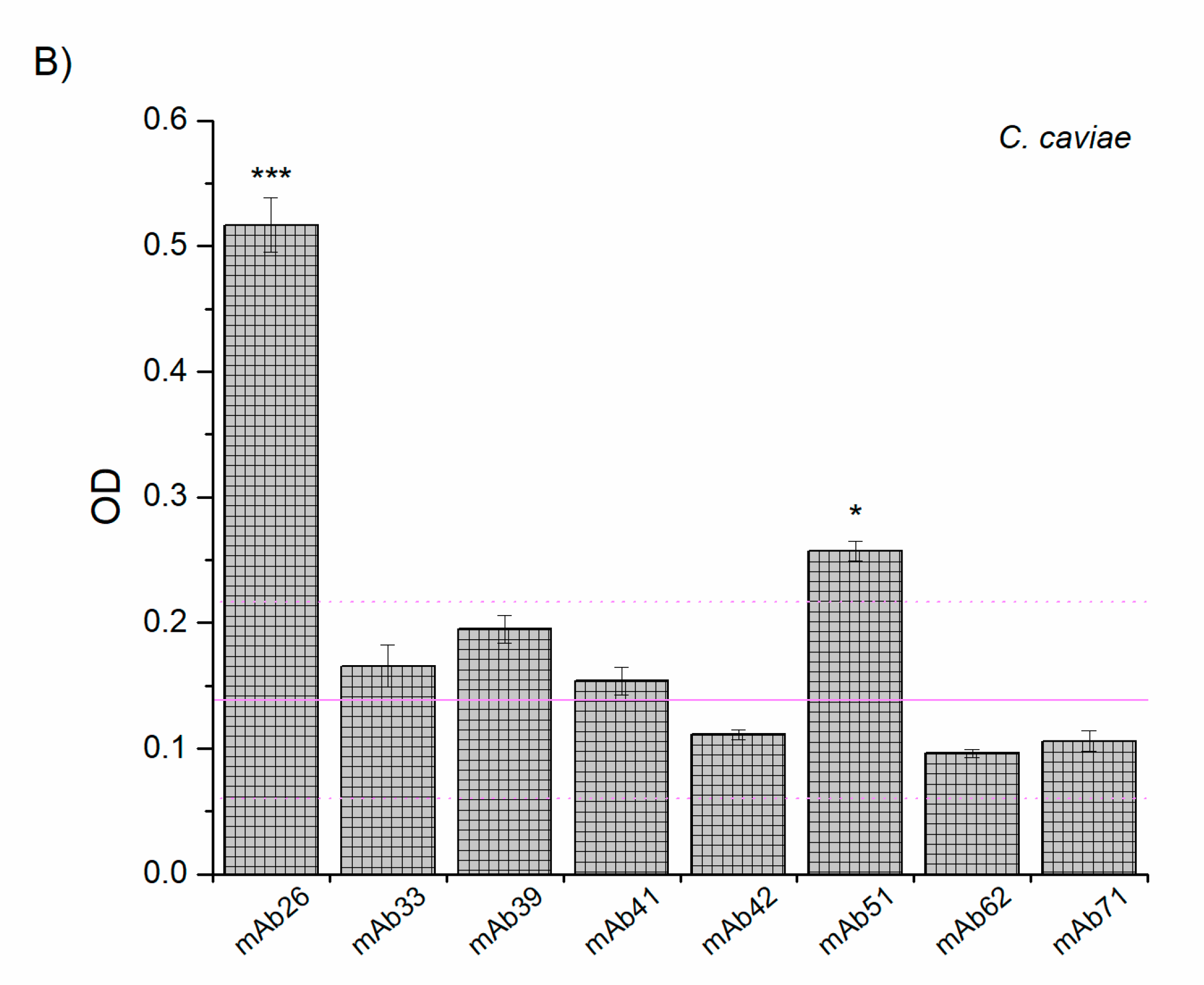
3.2. Cross-Reactivity of Anti-TTd mAbs and Chlamydial Proteins
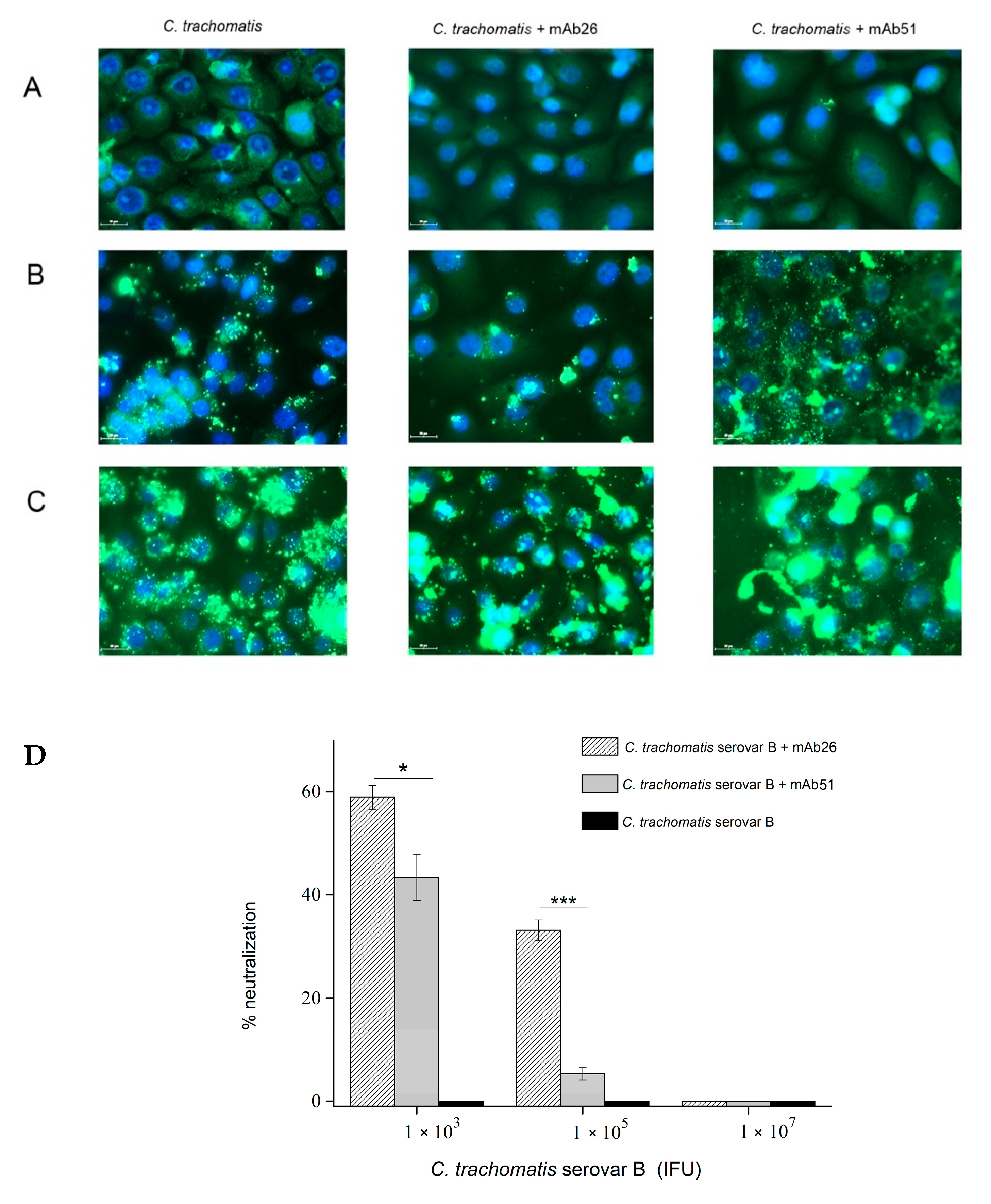
3.3. The Ability of mAb26 and mAb51 to Inhibit Chlamydial Infection In Vitro
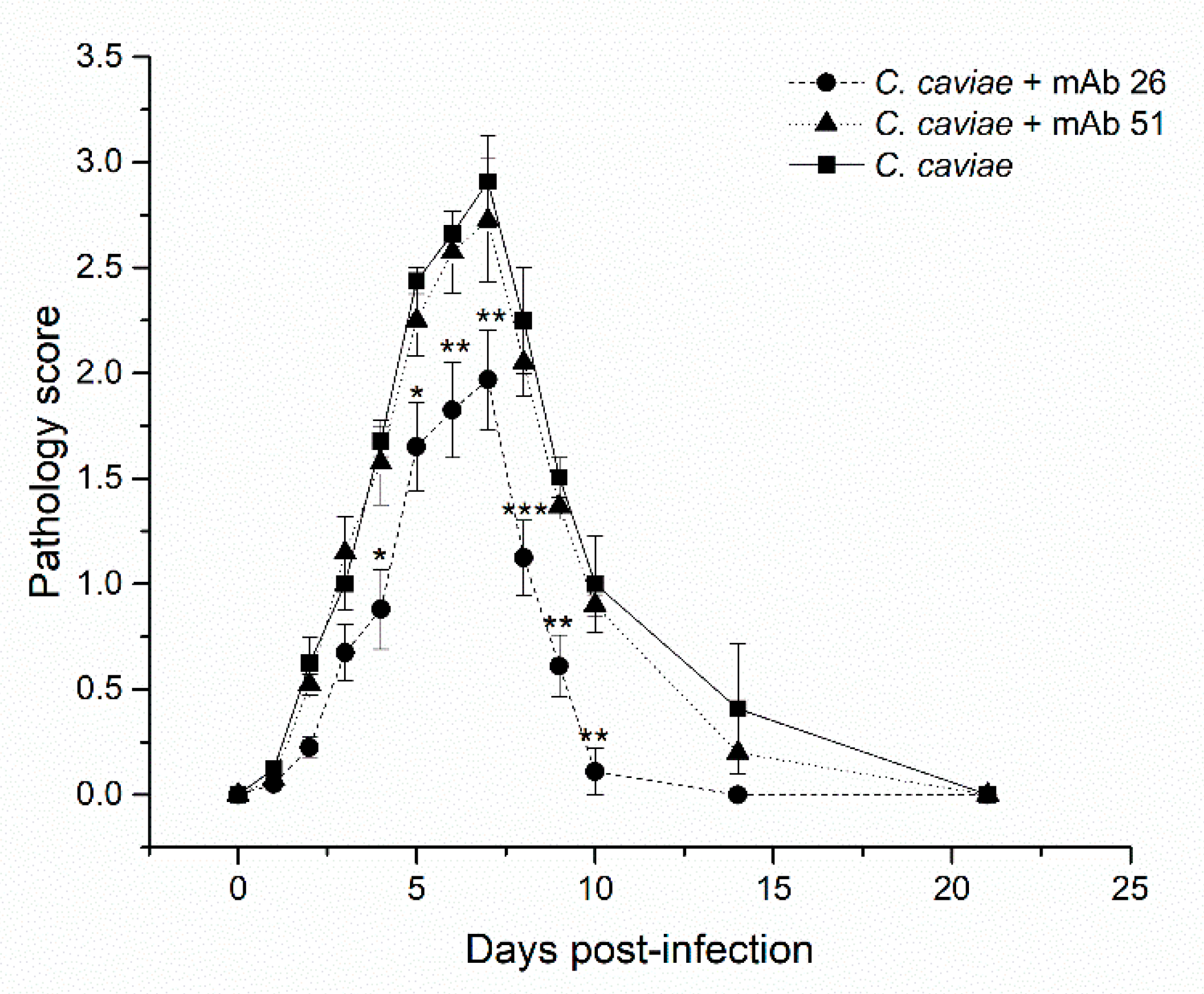
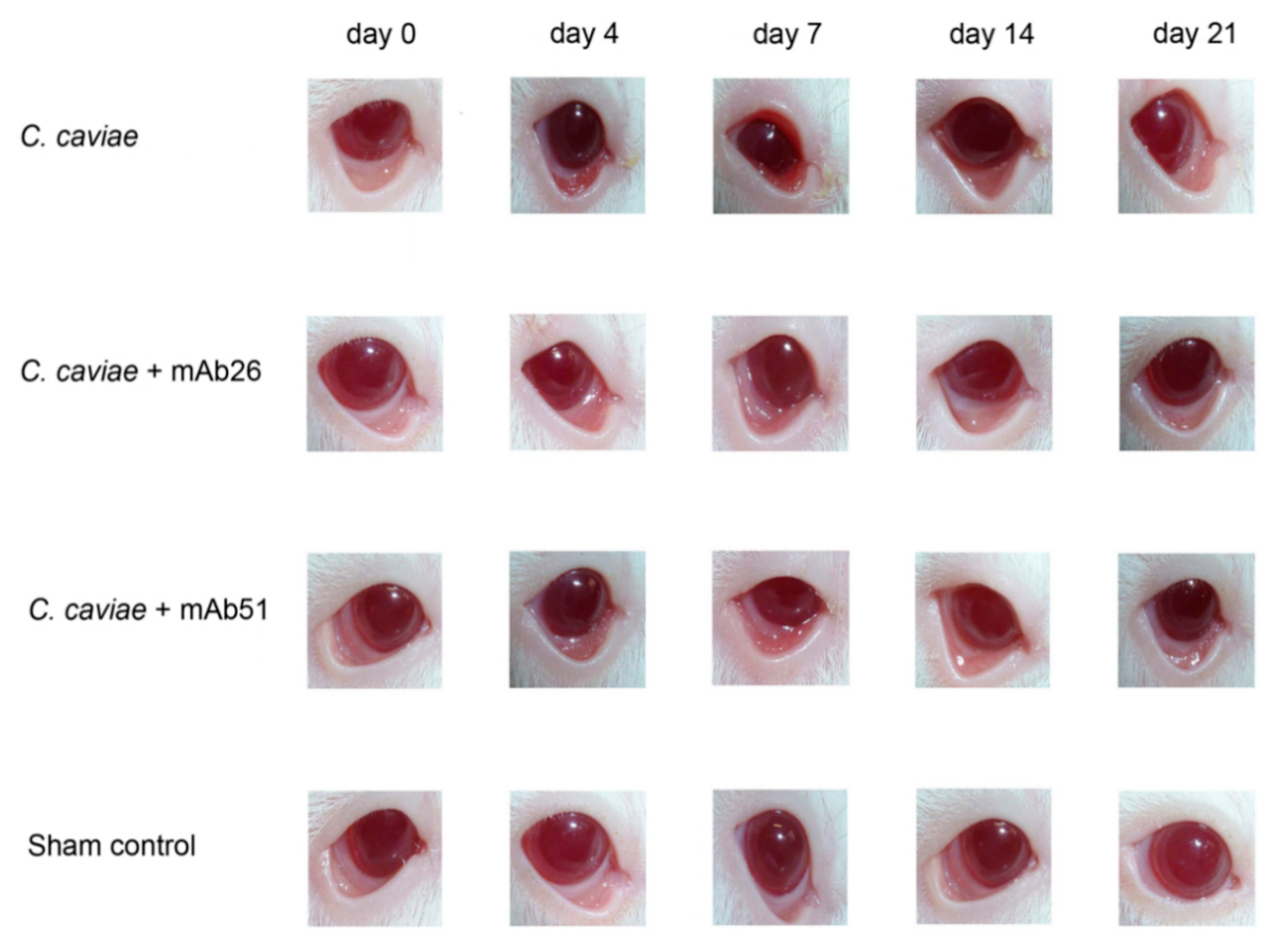
3.4. The Ability of mAb26 and mAb51 to Prevent Ocular Chlamydial Infection In Vivo: A Model of Guinea Pig Inclusive Conjunctivitis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plotkin, S.A.; Mortimer, E.A. Vaccines; Saunders: Philadelphia, PA, USA, 1988. [Google Scholar]
- Fauci, A.S.; Marston, H.D. Public health. Toward an hiv vaccine: A scientific journey. Science 2015, 349, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Richner, J.M.; Diamond, M.S. Zika virus vaccines: Immune response, current status, and future challenges. Curr. Opin. Immunol. 2018, 53, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.; Gholipour, N.; Zolfaghari, M.R.; Moradi Binabaj, M.; Yegane Moghadam, A.; Motalleb, G. Hepatitis c virus and vaccine development. Int. J. Mol. Cell. Med. 2014, 3, 207–215. [Google Scholar]
- De la Maza, L.M.; Zhong, G.; Brunham, R.C. Update on chlamydia trachomatis vaccinology. Clin. Vaccine Immunol. 2017, 24, e00543-16. [Google Scholar] [CrossRef]
- Strnad, M.; Grubhoffer, L.; Rego, R.O.M. Novel targets and strategies to combat borreliosis. Appl. Microbiol. Biotechnol. 2020, 104, 1915–1925. [Google Scholar] [CrossRef]
- Amanat, F.; Krammer, F. Sars-cov-2 vaccines: Status report. Immunity 2020, 52, 583–589. [Google Scholar] [CrossRef]
- Kandasamy, R.; Voysey, M.; McQuaid, F.; de Nie, K.; Ryan, R.; Orr, O.; Uhlig, U.; Sande, C.; O’Connor, D.; Pollard, A.J. Non-specific immunological effects of selected routine childhood immunisations: Systematic review. BMJ 2016, 355, i5225. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Ahmed, S.S.; Curtis, N.; Kollmann, T.R.; Levy, O.; Netea, M.G.; Pollard, A.J.; van Crevel, R.; Wilson, C.B. Harnessing the beneficial heterologous effects of vaccination. Nat. Rev. Immunol. 2016, 16, 392–400. [Google Scholar] [CrossRef]
- Benn, C.S.; Netea, M.G.; Selin, L.K.; Aaby, P. A small jab—A big effect: Nonspecific immunomodulation by vaccines. Trends Immunol. 2013, 34, 431–439. [Google Scholar] [CrossRef]
- Rehermann, B.; Shin, E.C. Private aspects of heterologous immunity. J. Exp. Med. 2005, 201, 667–670. [Google Scholar] [CrossRef]
- Gil, A.; Kenney, L.L.; Mishra, R.; Watkin, L.B.; Aslan, N.; Selin, L.K. Vaccination and heterologous immunity: Educating the immune system. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Saadatian-Elahi, M.; Aaby, P.; Shann, F.; Netea, M.G.; Levy, O.; Louis, J.; Picot, V.; Greenberg, M.; Warren, W. Heterologous vaccine effects. Vaccine 2016, 34, 3923–3930. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, B. Heterologous immunity: Role in natural and vaccine-induced resistance to infections. Front. Immunol. 2019, 10, 2631. [Google Scholar] [CrossRef] [PubMed]
- Messina, N.L.; Zimmermann, P.; Curtis, N. The impact of vaccines on heterologous adaptive immunity. Clin. Microbiol. Infect. 2019, 25, 1484–1493. [Google Scholar] [CrossRef]
- Moulder, J.W. Interaction of chlamydiae and host cells in vitro. Microbiol. Rev. 1991, 55, 143–190. [Google Scholar] [CrossRef]
- Grieshaber, S.; Grieshaber, N.; Yang, H.; Baxter, B.; Hackstadt, T.; Omsland, A. Impact of active metabolism on chlamydia trachomatis elementary body transcript profile and infectivity. J. Bacteriol. 2018, 200. [Google Scholar] [CrossRef]
- Kari, L.; Whitmire, W.M.; Crane, D.D.; Reveneau, N.; Carlson, J.H.; Goheen, M.M.; Peterson, E.M.; Pal, S.; de la Maza, L.M.; Caldwell, H.D. Chlamydia trachomatis native major outer membrane protein induces partial protection in nonhuman primates: Implication for a trachoma transmission-blocking vaccine. J. Immunol. 2009, 182, 8063–8070. [Google Scholar] [CrossRef]
- Olsen, A.W.; Follmann, F.; Erneholm, K.; Rosenkrands, I.; Andersen, P. Vaccine promoted neutralizing antibodies directed to the vd4 of momp protect against chlamydia trachomatis infection and upper genital tract pathology. J. Infect. Dis. 2015, 15, 978–989. [Google Scholar] [CrossRef]
- Rank, R.G.; Batteiger, B.E. Protective role of serum antibody in immunity to chlamydial genital infection. Infect. Immun. 1989, 57, 299–301. [Google Scholar] [CrossRef]
- Longbottom, D. Chlamydial vaccine development. J. Med. Microbiol. 2003, 52, 537–540. [Google Scholar] [CrossRef]
- Rank, R.G.; White, H.J.; Barron, A.L. Humoral immunity in the resolution of genital infection in female guinea pigs infected with the agent of guinea pig inclusion conjunctivitis. Infect. Immun. 1979, 26, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Rank, R.G.; Barron, A.L. Humoral immune response in acquired immunity to chlamydial genital infection of female guinea pigs. Infect. Immun. 1983, 39, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Batteiger, B.E.; Rank, R.G. Analysis of the humoral immune response to chlamydial genital infection in guinea pigs. Infect. Immun. 1987, 55, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.H.; Soderberg, L.S.; Rank, R.G. Resolution of chlamydial genital infection in b-cell-deficient mice and immunity to reinfection. Infect. Immun. 1988, 56, 1320–1325. [Google Scholar] [CrossRef]
- Malaviarachchi, P.A.; Mercado, M.A.B.; McSorley, S.J.; Li, L.X. Antibody, but not b-cell-dependent antigen presentation, plays an essential role in preventing chlamydia systemic dissemination in mice. Eur. J. Immunol. 2020, 50, 676–684. [Google Scholar] [CrossRef]
- Li, L.X.; McSorley, S.J. A re-evaluation of the role of b cells in protective immunity to chlamydia infection. Immunol. Lett. 2015, 164, 88–93. [Google Scholar] [CrossRef]
- Rockey, D.D.; Wang, J.; Lei, L.; Zhong, G. Chlamydia vaccine candidates and tools for chlamydial antigen discovery. Expert Rev. Vaccines 2009, 8, 1365–1377. [Google Scholar] [CrossRef]
- Brunham, R.C.; Rey-Ladino, J. Immunology of chlamydia infection: Implications for a chlamydia trachomatis vaccine. Nat. Rev. Immunol. 2005, 5, 149–161. [Google Scholar] [CrossRef]
- Fotinou, C.; Emsley, P.; Black, I.; Ando, H.; Ishida, H.; Kiso, M.; Sinha, K.A.; Fairweather, N.F.; Isaacs, N.W. The crystal structure of tetanus toxin hc fragment complexed with a synthetic gt1b analogue suggests cross-linking between ganglioside receptors and the toxin. J. Biol. Chem. 2001, 276, 32274–32281. [Google Scholar] [CrossRef]
- Gill, D.M. Bacterial toxins: A table of lethal amounts. Microbiol. Rev. 1982, 46, 86–94. [Google Scholar] [CrossRef]
- Manghi, M.A.; Pasetti, M.F.; Brero, M.L.; Deluchi, S.; di Paola, G.; Mathet, V.; Eriksson, P.V. Development of an elisa for measuring the activity of tetanus toxoid in vaccines and comparison with the toxin neutralization test in mice. J. Immunol. Methods 1994, 168, 17–24. [Google Scholar] [CrossRef]
- Bizzini, B.; Turpin, A.; Raynaud, M. Production et purification de la toxine tétanique. Ann. Inst. Pasteur 1969, 116, 686–712. [Google Scholar]
- Johnsen, S.; Andersen, P.L.; Stanek, G.; Christiansen, G.; Birkelund, S.; Berthelsen, L.M.; Østergaard, L. Chlamydia antibody response in healthy volunteers immunized with nonchlamydial antigens: A randomized, double-blind, placebo-controlled study. Clin. Infect. Dis. 2003, 36, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Lukic, I.; Filipovic, A.; Inic-Kanada, A.; Marinkovic, E.; Miljkovic, R.; Stojanovic, M. Cooperative binding of anti-tetanus toxin monoclonal antibodies: Implications for designing an efficient biclonal preparation to prevent tetanus toxin intoxication. Vaccine 2018, 36, 3764–3771. [Google Scholar] [CrossRef]
- Lukić, I.; Marinković, E.; Filipović, A.; Krnjaja, O.; Kosanović, D.; Inić-Kanada, A.; Stojanović, M. Key protection factors against tetanus: Anti-tetanus toxin antibody affinity and its ability to prevent tetanus toxin—Ganglioside interaction. Toxicon 2015, 103, 135–144. [Google Scholar] [CrossRef]
- Inic-Kanada, A.; Stojanovic, M.; Zivkovic, I.; Kosec, D.; Micic, M.; Petrusic, V.; Zivancevic-Simonovic, S.; Dimitrijevic, L. Murine monoclonal antibody 26 raised against tetanus toxoid cross-reacts with beta2-glycoprotein i: Its characteristics and role in molecular mimicry. Am. J. Reprod. Immunol. 2009, 61, 39–51. [Google Scholar] [CrossRef]
- Seatović, S.; Inić-Kanada, A.; Stojanović, M.; Zivković, I.; Jankov, R.M.; Dimitrijević, L. Development of sandwich enzyme-linked immunosorbent assay for determination of tetanus toxoid concentration. J. Immunoass. Immunochem. 2004, 25, 31–44. [Google Scholar] [CrossRef]
- Rank, R.G.; Batteiger, B.E.; Soderberg, L.S. Susceptibility to reinfection after a primary chlamydial genital infection. Infect. Immun. 1988, 56, 2243–2249. [Google Scholar] [CrossRef]
- Belij-Rammerstorfer, S.; Inic-Kanada, A.; Stojanovic, M.; Marinkovic, E.; Lukic, I.; Stein, E.; Montanaro, J.; Bintner, N.; Schurer, N.; Ghasemian, E.; et al. Infectious dose and repeated infections are key factors influencing immune response characteristics in guinea pig ocular chlamydial infection. Microbes Infect. 2016, 18, 254–262. [Google Scholar] [CrossRef]
- Filipovic, A.; Ghasemian, E.; Inic-Kanada, A.; Lukic, I.; Stein, E.; Marinkovic, E.; Djokic, R.; Kosanovic, D.; Schuerer, N.; Chalabi, H.; et al. The effect of infectious dose on humoral and cellular immune responses in chlamydophila caviae primary ocular infection. PLoS ONE 2017, 12, e0180551. [Google Scholar] [CrossRef]
- Barisani-Asenbauer, T.; Inic-Kanada, A.; Belij, S.; Marinkovic, E.; Stojicevic, I.; Montanaro, J.; Stein, E.; Bintner, N.; Stojanovic, M. The ocular conjunctiva as a mucosal immunization route: A profile of the immune response to the model antigen tetanus toxoid. PLoS ONE 2013, 8, e60682. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inic-Kanada, A.; Stojanovic, M.; Schlacher, S.; Stein, E.; Belij-Rammerstorfer, S.; Marinkovic, E.; Lukic, I.; Montanaro, J.; Schuerer, N.; Bintner, N.; et al. Delivery of a chlamydial adhesin n-pmpc subunit vaccine to the ocular mucosa using particulate carriers. PLoS ONE 2015, 10, e0144380. [Google Scholar] [CrossRef] [PubMed]
- Mullan, L. Pairwise sequence alignment—It’s all about us! Brief Bioinform. 2006, 7, 113–115. [Google Scholar] [CrossRef][Green Version]
- Brunham, R.C.; Peeling, R.W. Chlamydia trachomatis antigens: Role in immunity and pathogenesis. Infect. Agents Dis. 1994, 3, 218–233. [Google Scholar] [PubMed]
- Sanchez-Campillo, M.; Bini, L.; Comanducci, M.; Raggiaschi, R.; Marzocchi, B.; Pallini, V.; Ratti, G. Identification of immunoreactive proteins of chlamydia trachomatis by western blot analysis of a two-dimensional electrophoresis map with patient sera. Electrophoresis 1999, 20, 2269–2279. [Google Scholar] [CrossRef]
- Gomes, J.P.; Hsia, R.C.; Mead, S.; Borrego, M.J.; Dean, D. Immunoreactivity and differential developmental expression of known and putative chlamydia trachomatis membrane proteins for biologically variant serovars representing distinct disease groups. Microbes Infect. 2005, 7, 410–420. [Google Scholar] [CrossRef]
- Crane, D.D.; Carlson, J.H.; Fischer, E.R.; Bavoil, P.; Hsia, R.C.; Tan, C.; Kuo, C.C.; Caldwell, H.D. Chlamydia trachomatis polymorphic membrane protein d is a species-common pan-neutralizing antigen. Proc. Natl. Acad. Sci. USA 2006, 103, 1894–1899. [Google Scholar] [CrossRef]
- Frikha-Gargouri, O.; Gdoura, R.; Znazen, A.; Gargouri, B.; Gargouri, J.; Rebai, A.; Hammami, A. Evaluation of an in silico predicted specific and immunogenic antigen from the omcb protein for the serodiagnosis of chlamydia trachomatis infections. BMC Microbiol. 2008, 8, 217. [Google Scholar] [CrossRef]
- Fadel, S.; Eley, A. Chlamydia trachomatis omcb protein is a surface-exposed glycosaminoglycan-dependent adhesin. J. Med. Microbiol. 2007, 56, 15–22. [Google Scholar] [CrossRef]
- Peng, H.P.; Lee, K.H.; Jian, J.W.; Yang, A.S. Origins of specificity and affinity in antibody-protein interactions. Proc. Natl. Acad. Sci. USA 2014, 111, E2656–E2665. [Google Scholar] [CrossRef]
- Kringelum, J.V.; Nielsen, M.; Padkjær, S.B.; Lund, O. Structural analysis of b-cell epitopes in antibody:Protein complexes. Mol. Immunol. 2013, 53, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Lo Conte, L.; Chothia, C.; Janin, J. The atomic structure of protein-protein recognition sites. J. Mol. Biol. 1999, 285, 2177–2198. [Google Scholar] [CrossRef] [PubMed]
- Bogan, A.A.; Thorn, K.S. Anatomy of hot spots in protein interfaces. J. Mol. Biol. 1998, 280, 1–9. [Google Scholar] [CrossRef]
- Clackson, T.; Wells, J.A. A hot spot of binding energy in a hormone-receptor interface. Science 1995, 267, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Zacharias, M. Prediction of protein-protein interaction sites using electrostatic desolvation profiles. Biophys. J. 2010, 98, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Inic-Kanada, A.; Stojanovic, M.; Marinkovic, E.; Becker, E.; Stein, E.; Lukic, I.; Djokic, R.; Schuerer, N.; Hegemann, J.H.; Barisani-Asenbauer, T. A probiotic adjuvant lactobacillus rhamnosus enhances specific immune responses after ocular mucosal immunization with chlamydial polymorphic membrane protein c. PLoS ONE 2016, 11, e0157875. [Google Scholar] [CrossRef]
- Pal, S.; Favaroni, A.; Tifrea, D.F.; Hanisch, P.T.; Luczak, S.E.T.; Hegemann, J.H.; de la Maza, L.M. Comparison of the nine polymorphic membrane proteins of chlamydia trachomatis for their ability to induce protective immune responses in mice against a c. Muridarum challenge. Vaccine 2017, 35, 2543–2549. [Google Scholar] [CrossRef]
- Becker, E.; Hegemann, J.H. All subtypes of the pmp adhesin family are implicated in chlamydial virulence and show species-specific function. Microbiol. Open 2014, 3, 544–556. [Google Scholar] [CrossRef]
- Molleken, K.; Schmidt, E.; Hegemann, J.H. Members of the pmp protein family of chlamydia pneumoniae mediate adhesion to human cells via short repetitive peptide motifs. Mol. Microbiol. 2010, 78, 1004–1017. [Google Scholar] [CrossRef]
- Feher, V.A.; Randall, A.; Baldi, P.; Bush, R.M.; de la Maza, L.M.; Amaro, R.E. A 3-dimensional trimeric β-barrel model for chlamydia momp contains conserved and novel elements of gram-negative bacterial porins. PLoS ONE 2013, 8, e68934. [Google Scholar] [CrossRef]
- Su, H.; Watkins, N.G.; Zhang, Y.X.; Caldwell, H.D. Chlamydia trachomatis-host cell interactions: Role of the chlamydial major outer membrane protein as an adhesin. Infect. Immun. 1990, 58, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Tifrea, D.F.; Ralli-Jain, P.; Pal, S.; de la Maza, L.M. Vaccination with the recombinant major outer membrane protein elicits antibodies to the constant domains and induces cross-serovar protection against intranasal challenge with chlamydia trachomatis. Infect. Immun. 2013, 81, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Pal, S.; Weiland, J.; Peterson, E.M.; de la Maza, L.M. Protection against an intranasal challenge by vaccines formulated with native and recombinant preparations of the chlamydia trachomatis major outer membrane protein. Vaccine 2009, 27, 5020–5025. [Google Scholar] [CrossRef] [PubMed]
- Yeruva, L.; Bowlin, A.K.; Spencer, N.; Maurelli, A.T.; Rank, R.G. Chlamydial variants differ in ability to ascend the genital tract in the guinea pig model of chlamydial genital infection. Infect. Immun. 2015, 83, 3176–3183. [Google Scholar] [CrossRef][Green Version]
- Woodland, R.M.; Johnson, A.P.; Tuffrey, M. Animal models of chlamydial infection. Br. Med. Bull. 1983, 39, 175–180. [Google Scholar] [CrossRef]
- Wang, Y.; Nagarajan, U.; Hennings, L.; Bowlin, A.K.; Rank, R.G. Local host response to chlamydial urethral infection in male guinea pigs. Infect. Immun. 2010, 78, 1670–1681. [Google Scholar] [CrossRef][Green Version]
- Rank, R.G.; Whittum-Hudson, J.A. Animal models for ocular infections. Methods Enzymol. 1994, 235, 69–83. [Google Scholar]
- Monnickendam, M.A.; Darougar, S.; Tilbury, A.M. Ocular and dermal delayed hypersensitivity reactions in guinea-pigs following infection with guinea-pig inclusion conjunctivitis agent (chlamydia psittaci). Clin. Exp. Immunol. 1981, 44, 57–62. [Google Scholar]
- Inic-Kanada, A.; Stein, E.; Stojanovic, M.; Schuerer, N.; Ghasemian, E.; Filipovic, A.; Marinkovic, E.; Kosanovic, D.; Barisani-Asenbauer, T. Effects of iota-carrageenan on ocular chlamydia trachomatis infection in vitro and in vivo. J. Appl. Phycol. 2018, 30, 2601–2610. [Google Scholar] [CrossRef]
- Inic-Kanada, A.; Stojanovic, M.; Miljkovic, R.; Stein, E.; Filipovic, A.; Frohns, A.; Zöller, N.; Kuratli, J.; Barisani-Asenbauer, T.; Borel, N. Water-filtered infrared a and visible light (wira/vis) treatment reduces chlamydia caviae-induced ocular inflammation and infectious load in a guinea pig model of inclusion conjunctivitis. J. Photochem. Photobiol. Biol. 2020, 209, 111953. [Google Scholar] [CrossRef]
- Wali, S.; Gupta, R.; Veselenak, R.L.; Li, Y.; Yu, J.J.; Murthy, A.K.; Cap, A.P.; Guentzel, M.N.; Chambers, J.P.; Zhong, G.; et al. Use of a guinea pig-specific transcriptome array for evaluation of protective immunity against genital chlamydial infection following intranasal vaccination in guinea pigs. PLoS ONE 2014, 9, e114261. [Google Scholar] [CrossRef] [PubMed]

| Chlamydial Antigens | Serovar | UniProt ID | MW (kDa) | Max Score | E Value | Nr. AA | TeNT Region with Max. Overlap |
|---|---|---|---|---|---|---|---|
| PmpC | Ct | Q6HA51 | 188.1 | 25.0 | 0.071 | 84 | HC |
| PmpD | CtD * | O84818 | 160.7 | 20.0 | 1.9 | 40 | LC |
| PmpH | Ct | Q2TCH7 | 105.6 | 18.1 | 4.3 | 37 | HC |
| OmcB | CtC * | P26758 | 58.6 | 19.6 | 0.86 | 61 | HC |
| Hsp60 | CtA * | Q3KMQ9 | 58.1 | 17.3 | 3.6 | 18 | LC |
| MOMP-A | CtA * | P23732 | 42.9 | 16.9 | 3.3 | 17 | HC |
| MOMP-B | CtB * | P23421 | 42.5 | 24,6 | 0.016 | 60 | HC |
| MOMP-C | CtC * | P08780 | 42.9 | 17.7 | 2.0 | 30 | HC |
| MOMP-D | CtD * | Q46409 | 42.5 | 19.2 | 0.79 | 66 | HC |
| MOMP-CC | CC | Q824U2 | 41.9 | 16.9 | 3.6 | 9 | HC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanovic, M.; Lukic, I.; Marinkovic, E.; Kovacevic, A.; Miljkovic, R.; Tobias, J.; Schabussova, I.; Zlatović, M.; Barisani-Asenbauer, T.; Wiedermann, U.; et al. Cross-Reactive Effects of Vaccines: Heterologous Immunity between Tetanus and Chlamydia. Vaccines 2020, 8, 719. https://doi.org/10.3390/vaccines8040719
Stojanovic M, Lukic I, Marinkovic E, Kovacevic A, Miljkovic R, Tobias J, Schabussova I, Zlatović M, Barisani-Asenbauer T, Wiedermann U, et al. Cross-Reactive Effects of Vaccines: Heterologous Immunity between Tetanus and Chlamydia. Vaccines. 2020; 8(4):719. https://doi.org/10.3390/vaccines8040719
Chicago/Turabian StyleStojanovic, Marijana, Ivana Lukic, Emilija Marinkovic, Ana Kovacevic, Radmila Miljkovic, Joshua Tobias, Irma Schabussova, Mario Zlatović, Talin Barisani-Asenbauer, Ursula Wiedermann, and et al. 2020. "Cross-Reactive Effects of Vaccines: Heterologous Immunity between Tetanus and Chlamydia" Vaccines 8, no. 4: 719. https://doi.org/10.3390/vaccines8040719
APA StyleStojanovic, M., Lukic, I., Marinkovic, E., Kovacevic, A., Miljkovic, R., Tobias, J., Schabussova, I., Zlatović, M., Barisani-Asenbauer, T., Wiedermann, U., & Inic-Kanada, A. (2020). Cross-Reactive Effects of Vaccines: Heterologous Immunity between Tetanus and Chlamydia. Vaccines, 8(4), 719. https://doi.org/10.3390/vaccines8040719







