Evaluation of Antibody Response Directed against Porcine Reproductive and Respiratory Syndrome Virus Structural Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Reagents
2.2. Serum Samples
2.3. Plasmid Construction and Protein Expression
2.4. Immunofluorescence Microscopy
2.5. SDS-PAGE and Immunoblotting
2.6. Determination of the Serological Status of the Test Serum Samples
2.7. Luciferase-Immunoprecipitation System
2.8. Statistical Analysis
3. Results
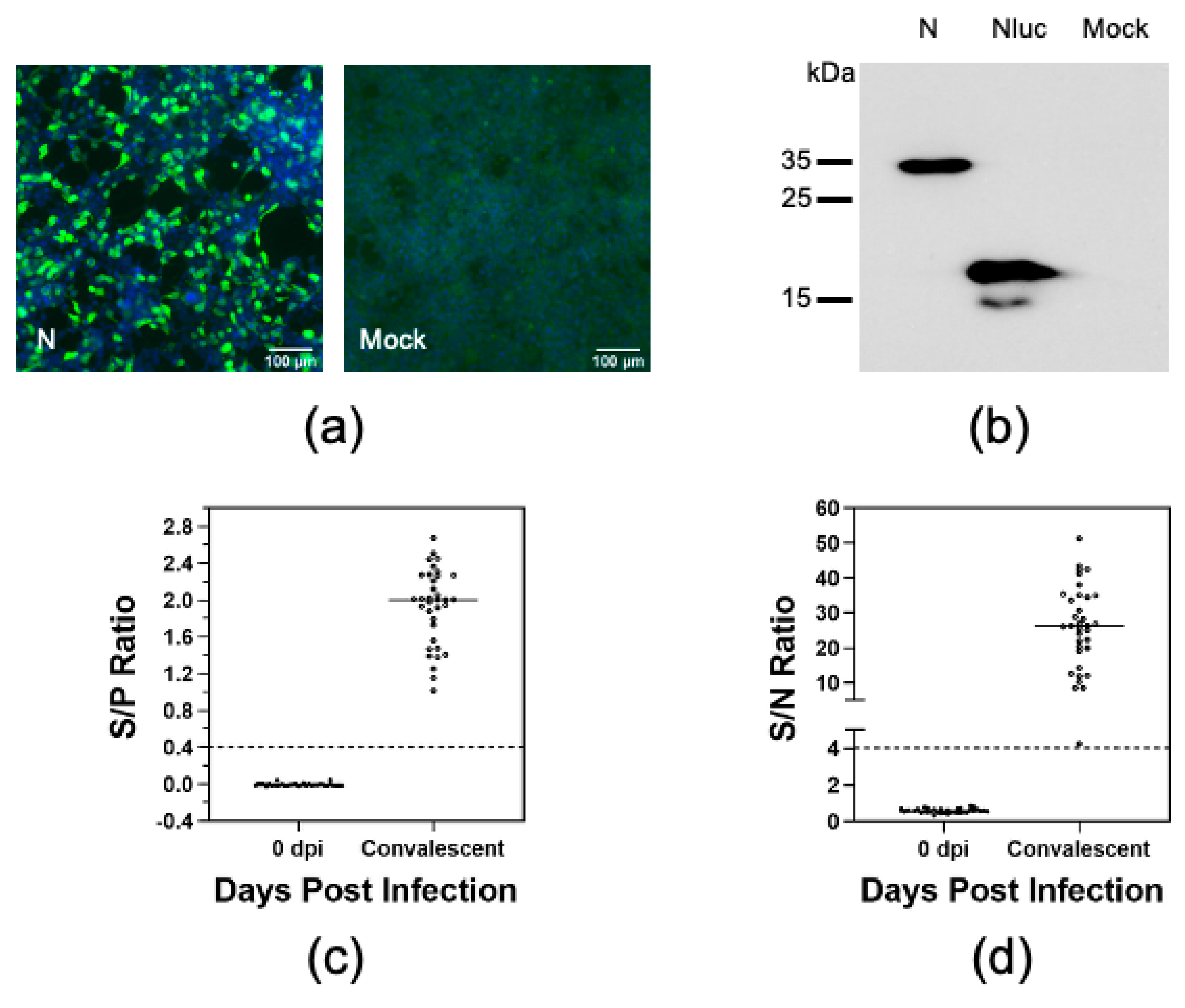
3.1. Establishment of a Luciferase Immunoprecipitation System to Detect Antibodies against PRRSV
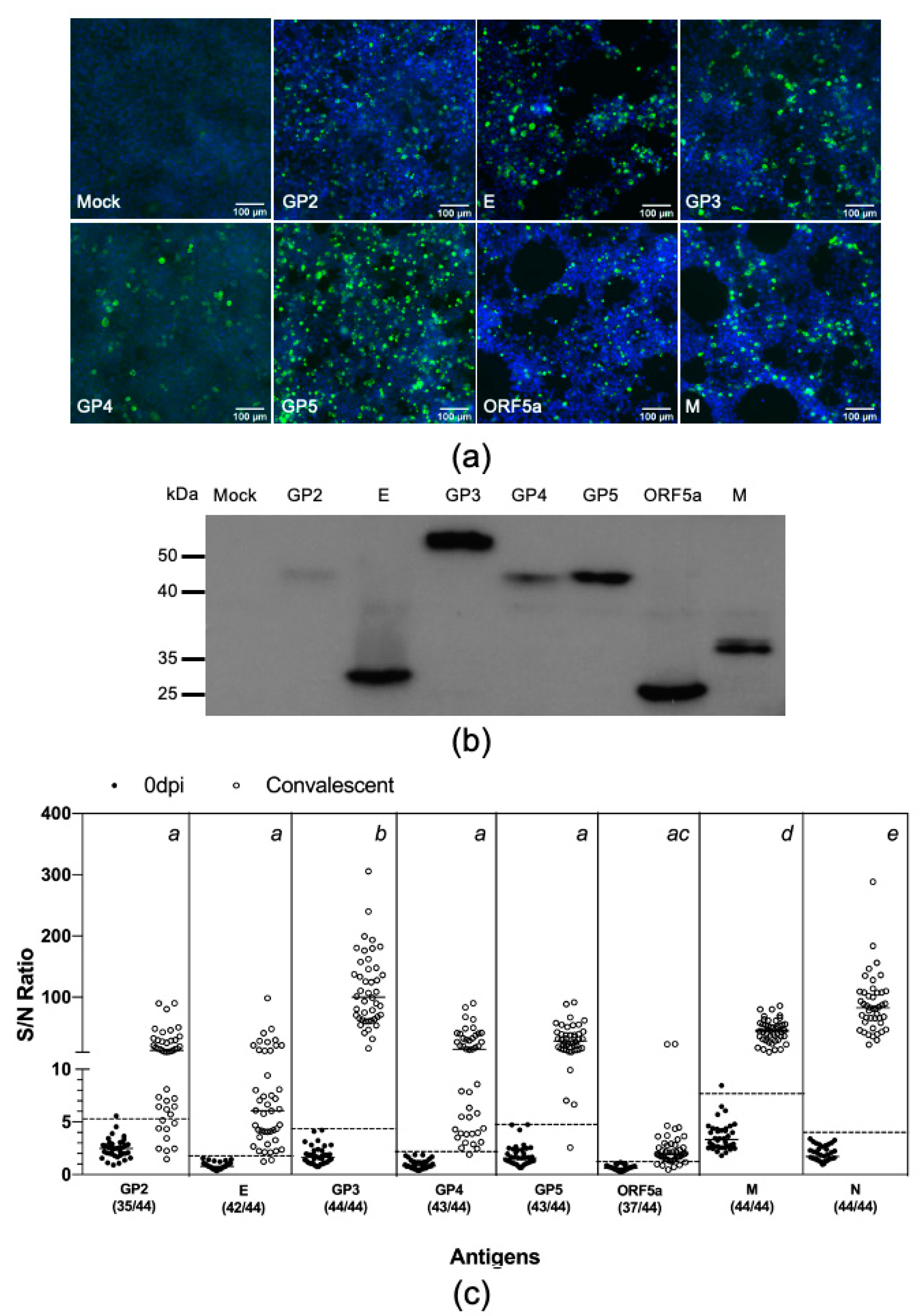
3.2. Swine Antibody Response to PRRSV Structural Proteins
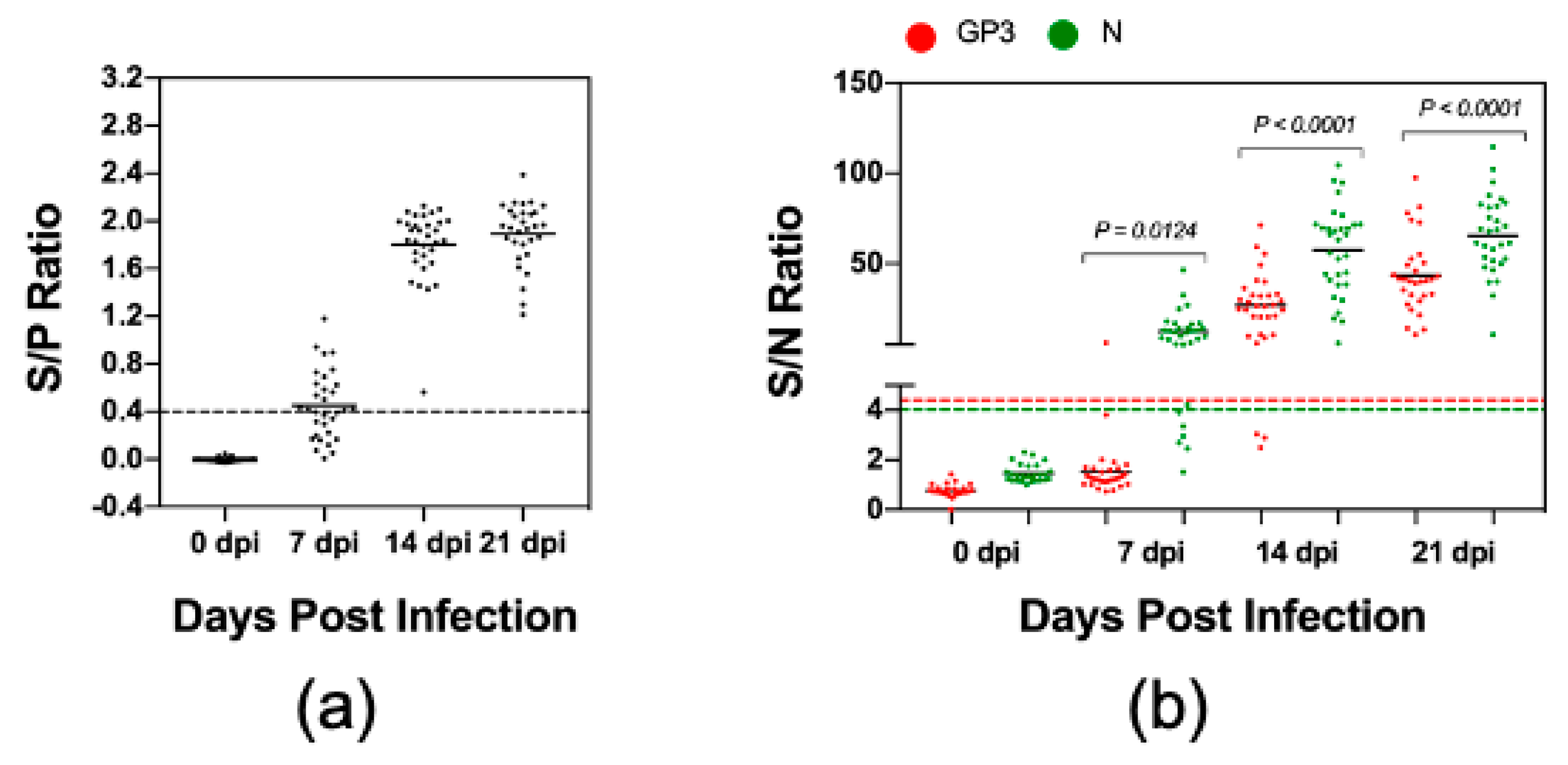
3.3. Comparison of Kinetics of Antibody Response against GP3 and N Protein
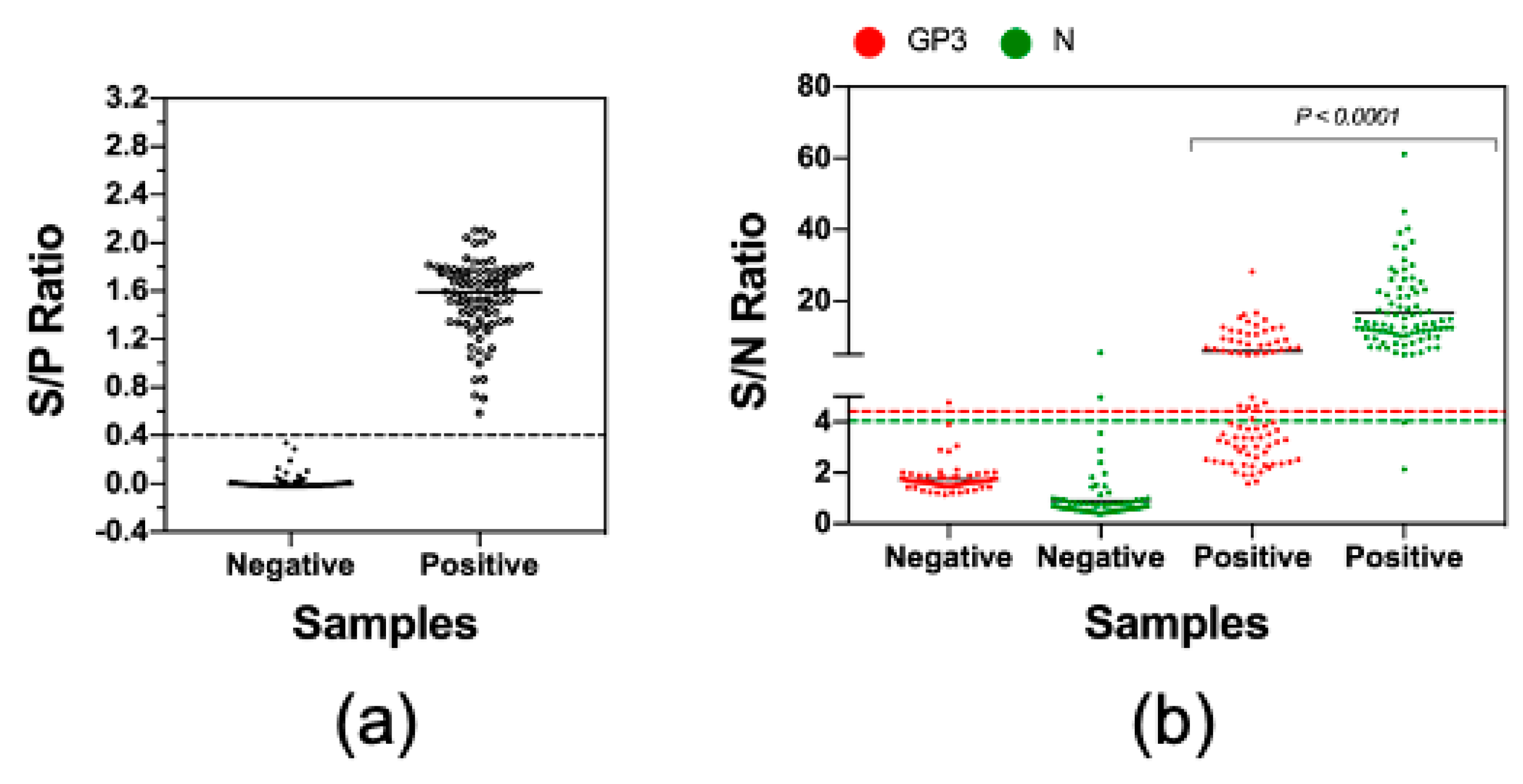
3.4. Measurement of Antibody Reactivities against GP3 and N with Clinical Serum Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holtkamp, D.J.; Kliebenstein, J.B.; Neumann, E.J.; Zimmerman, J.; Rotto, H.F.; Yoder, T.K.; Wang, C.; Yeske, P.E.; Mowrer, C.L.; Haley, C.A. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J. Swine Health Prod. 2013, 21, 72–84. [Google Scholar]
- Snijder, E.J.; Kikkert, M.; Fang, Y. Arterivirus molecular biology and pathogenesis. J. Gen. Virol. 2013, 94, 2141–2163. [Google Scholar] [CrossRef]
- Fang, Y.; Snijder, E.J. The PRRSV replicase: Exploring the multifunctionality of an intriguing set of nonstructural proteins. Virus Res. 2010, 154, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Meulenberg, J.; Petersen-den Besten, A. Identification and Characterization of a Sixth Structural Protein of Lelystad Virus: The Glycoprotein GP2Encoded by ORF2 Is Incorporated in Virus Particles. Virology 1996, 225, 44–51. [Google Scholar] [PubMed]
- Van Nieuwstadt, A.; Meulenberg, J.; van Essen-Zanbergen, A.; Petersen-den Besten, A.; Bende, R.; Moormann, R.; Wensvoort, G. Proteins encoded by open reading frames 3 and 4 of the genome of Lelystad virus (Arteriviridae) are structural proteins of the virion. J. Virol. 1996, 70, 4767–4772. [Google Scholar] [PubMed]
- Gonin, P.; Mardassi, H.; Gagnon, C.; Massie, B.; Dea, S. A nonstructural and antigenic glycoprotein is encoded by ORF3 of the IAF-Klop strain of porcine reproductive and respiratory syndrome virus. Arch. Virol. 1998, 143, 1927–1940. [Google Scholar] [PubMed]
- Meulenberg, J.; Van Nieuwstadt, A.; van Essen-Zandbergen, A.; Langeveld, J. Posttranslational processing and identification of a neutralization domain of the GP4 protein encoded by ORF4 of Lelystad virus. J. Virol. 1997, 71, 6061–6067. [Google Scholar] [PubMed]
- Wissink, E.H.; Kroese, M.V.; van Wijk, H.A.; Rijsewijk, F.A.; Meulenberg, J.J.; Rottier, P.J. Envelope protein requirements for the assembly of infectious virions of porcine reproductive and respiratory syndrome virus. J. Virol. 2005, 79, 12495–12506. [Google Scholar] [CrossRef]
- Das, P.B.; Dinh, P.X.; Ansari, I.H.; de Lima, M.; Osorio, F.A.; Pattnaik, A.K. The minor envelope glycoproteins GP2a and GP4 of porcine reproductive and respiratory syndrome virus interact with the receptor CD163. J. Virol. 2010, 84, 1731–1740. [Google Scholar] [CrossRef]
- Calvert, J.G.; Slade, D.E.; Shields, S.L.; Jolie, R.; Mannan, R.M.; Ankenbauer, R.G.; Welch, S.K. CD163 expression confers susceptibility to porcine reproductive and respiratory syndrome viruses. J. Virol. 2007, 81, 7371–7379. [Google Scholar] [CrossRef]
- Ansari, I.H.; Kwon, B.; Osorio, F.A.; Pattnaik, A.K. Influence of N-linked glycosylation of porcine reproductive and respiratory syndrome virus GP5 on virus infectivity, antigenicity, and ability to induce neutralizing antibodies. J. Virol. 2006, 80, 3994–4004. [Google Scholar] [CrossRef] [PubMed]
- Doan, D.N.P.; Dokland, T. Structure of the Nucleocapsid Protein of Porcine Reproductive and Respiratory Syndrome Virus. Structure 2003, 11, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.H.; Fang, Y.; Farwell, R.; Steffen-Bien, M.; Rowland, R.R.; Christopher-Hennings, J.; Nelson, E.A. A 10-kDa structural protein of porcine reproductive and respiratory syndrome virus encoded by ORF2b. Virology 2001, 287, 183–191. [Google Scholar] [CrossRef]
- Lee, C.; Yoo, D. The small envelope protein of porcine reproductive and respiratory syndrome virus possesses ion channel protein-like properties. Virology 2006, 355, 30–43. [Google Scholar] [CrossRef]
- Johnson, C.R.; Griggs, T.F.; Gnanandarajah, J.; Murtaugh, M.P. Novel structural protein in porcine reproductive and respiratory syndrome virus encoded by an alternative ORF5 present in all arteriviruses. J. Gen. Virol. 2011, 92, 1107–1116. [Google Scholar] [CrossRef]
- Sun, L.; Li, Y.; Liu, R.; Wang, X.; Gao, F.; Lin, T.; Huang, T.; Yao, H.; Tong, G.; Fan, H.; et al. Porcine reproductive and respiratory syndrome virus ORF5a protein is essential for virus viability. Virus Res. 2013, 171, 178–185. [Google Scholar] [CrossRef]
- Yoon, K.J.; Zimmerman, J.J.; Swenson, S.L.; McGinley, M.J.; Eernisse, K.A.; Brevik, A.; Rhinehart, L.L.; Frey, M.L.; Hill, H.T.; Platt, K.B. Characterization of the humoral immune response to porcine reproductive and respiratory syndrome (PRRS) virus infection. J. Vet. Diagn. Investig. 1995, 7, 305–312. [Google Scholar]
- Nelson, E.A.; Christopher-Hennings, J.; Benfield, D.A. Serum immune responses to the proteins of porcine reproductive and respiratory syndrome (PRRS) virus. J. Vet. Diagn. Investig. 1994, 6, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Roof, M.; Vaughn, E.; Christopher-Hennings, J.; Johnson, C.R.; Murtaugh, M.P. Pathogenic and humoral immune responses to porcine reproductive and respiratory syndrome virus (PRRSV) are related to viral load in acute infection. Vet. Immunol. Immunopathol. 2004, 102, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Lopez, O.J.; Osorio, F.A. Role of neutralizing antibodies in PRRSV protective immunity. Vet. Immunol. Immunopathol. 2004, 102, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.J.; Wu, L.L.; Zimmerman, J.J.; Hill, H.T.; Platt, K.B. Antibody-dependent enhancement (ADE) of porcine reproductive and respiratory syndrome virus (PRRSV) infection in pigs. Viral Immunol. 1996, 9, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.; Galeota, J.A.; Jar, A.M.; Platt, K.B.; Osorio, F.A.; Lopez, O.J. Identification of neutralizing and nonneutralizing epitopes in the porcine reproductive and respiratory syndrome virus GP5 ectodomain. J. Virol. 2002, 76, 4241–4250. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.L.; Kwon, B.; Yoon, K.J.; Laegreid, W.W.; Pattnaik, A.K.; Osorio, F.A. Immune evasion of porcine reproductive and respiratory syndrome virus through glycan shielding involves both glycoprotein 5 as well as glycoprotein 3. J. Virol. 2011, 85, 5555–5564. [Google Scholar] [CrossRef]
- Vanhee, M.; Van Breedam, W.; Costers, S.; Geldhof, M.; Noppe, Y.; Nauwynck, H. Characterization of antigenic regions in the porcine reproductive and respiratory syndrome virus by the use of peptide-specific serum antibodies. Vaccine 2011, 29, 4794–4804. [Google Scholar] [CrossRef]
- Loemba, H.D.; Mounir, S.; Mardassi, H.; Archambault, D.; Dea, S. Kinetics of humoral immune response to the major structural proteins of the porcine reproductive and respiratory syndrome virus. Arch. Virol. 1996, 141, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, K.; Podgorska, K.; Tyszka, A.; Stadejek, T. Comparison of six commercial ELISAs for the detection of antibodies against porcine reproductive and respiratory syndrome virus (PRRSV) in field serum samples. Res. Vet. Sci. 2018, 121, 40–45. [Google Scholar] [CrossRef]
- Molina, R.M.; Cha, S.H.; Chittick, W.; Lawson, S.; Murtaugh, M.P.; Nelson, E.A.; Christopher-Hennings, J.; Yoon, K.J.; Evans, R.; Rowland, R.R.; et al. Immune response against porcine reproductive and respiratory syndrome virus during acute and chronic infection. Vet. Immunol. Immunopathol. 2008, 126, 283–292. [Google Scholar] [CrossRef]
- De Lima, M.; Pattnaik, A.K.; Flores, E.F.; Osorio, F.A. Serologic marker candidates identified among B-cell linear epitopes of Nsp2 and structural proteins of a North American strain of porcine reproductive and respiratory syndrome virus. Virology 2006, 353, 410–421. [Google Scholar] [CrossRef]
- Brown, E.; Lawson, S.; Welbon, C.; Gnanandarajah, J.; Li, J.; Murtaugh, M.P.; Nelson, E.A.; Molina, R.M.; Zimmerman, J.J.; Rowland, R.R.; et al. Antibody response of nonstructural proteins: Implications for diagnostic detection and differentiation of Type I and Type II porcine reproductive and respiratory syndrome viruses. Clin. Vaccine Immunol. 2009, 16, 628–635. [Google Scholar] [CrossRef]
- Burbelo, P.D.; Ching, K.H.; Bush, E.R.; Han, B.L.; Iadarola, M.J. Antibody-profiling technologies for studying humoral responses to infectious agents. Expert Rev. Vaccines 2010, 9, 567–578. [Google Scholar] [CrossRef]
- Burbelo, P.D.; Lebovitz, E.E.; Notkins, A.L. Luciferase immunoprecipitation systems for measuring antibodies in autoimmune and infectious diseases. Transl. Res. 2015, 165, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Das, P.B.; Vu, H.L.; Dinh, P.X.; Cooney, J.L.; Kwon, B.; Osorio, F.A.; Pattnaik, A.K. Glycosylation of minor envelope glycoproteins of porcine reproductive and respiratory syndrome virus in infectious virus recovery, receptor interaction, and immune response. Virology 2011, 410, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.L.; Kwon, B.; de Lima, M.; Pattnaik, A.K.; Osorio, F.A. Characterization of a serologic marker candidate for development of a live-attenuated DIVA vaccine against porcine reproductive and respiratory syndrome virus. Vaccine 2013, 31, 4330–4337. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vu, H.L.; Ma, F.; Laegreid, W.W.; Pattnaik, A.K.; Steffen, D.; Doster, A.R.; Osorio, F.A. A Synthetic Porcine Reproductive and Respiratory Syndrome Virus Strain Confers Unprecedented Levels of Heterologous Protection. J. Virol. 2015, 89, 12070–12083. [Google Scholar] [CrossRef] [PubMed]
- Truong, H.M.; Lu, Z.; Kutish, G.F.; Galeota, J.; Osorio, F.A.; Pattnaik, A.K. A highly pathogenic porcine reproductive and respiratory syndrome virus generated from an infectious cDNA clone retains the in vivo virulence and transmissibility properties of the parental virus. Virology 2004, 325, 308–319. [Google Scholar] [CrossRef]
- Longo, P.A.; Kavran, J.M.; Kim, M.S.; Leahy, D.J. Transient mammalian cell transfection with polyethylenimine (PEI). Methods Enzymol. 2013, 529, 227–240. [Google Scholar] [CrossRef]
- Burbelo, P.D.; Ching, K.H.; Klimavicz, C.M.; Iadarola, M.J. Antibody profiling by Luciferase Immunoprecipitation Systems (LIPS). J. Vis. Exp. 2009, e1549. [Google Scholar] [CrossRef]
- England, C.G.; Ehlerding, E.B.; Cai, W. NanoLuc: A Small Luciferase Is Brightening Up the Field of Bioluminescence. Bioconjugate Chem. 2016, 27, 1175–1187. [Google Scholar] [CrossRef]
- Seuberlich, T.; Tratschin, J.D.; Thur, B.; Hofmann, M.A. Nucleocapsid protein-based enzyme-linked immunosorbent assay for detection and differentiation of antibodies against European and North American porcine reproductive and respiratory syndrome virus. Clin. Diagn. Lab. Immunol. 2002, 9, 1183–1191. [Google Scholar] [CrossRef]
- Ferrin, N.H.; Fang, Y.; Johnson, C.R.; Murtaugh, M.P.; Polson, D.D.; Torremorell, M.; Gramer, M.L.; Nelson, E.A. Validation of a blocking enzyme-linked immunosorbent assay for detection of antibodies against porcine reproductive and respiratory syndrome virus. Clin. Diagn. Lab. Immunol. 2004, 11, 503–514. [Google Scholar] [CrossRef]
- Denac, H.; Moser, C.; Tratschin, J.D.; Hofmann, M.A. An indirect ELISA for the detection of antibodies against porcine reproductive and respiratory syndrome virus using recombinant nucleocapsid protein as antigen. J. Virol. Methods 1997, 65, 169–181. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Yang, D.; Zhao, B.; Zhao, Y.; Liu, Y.; Cai, X.; Nan, Y.; Zhou, E.M.; Wu, C. Development of luciferase-linked antibody capture assay based on luciferase immunoprecipitation systems for antibody detection of porcine reproductive and respiratory syndrome virus. BMC Biotechnol. 2018, 18, 73. [Google Scholar] [CrossRef] [PubMed]
- Kappes, M.A.; Miller, C.L.; Faaberg, K.S. Highly divergent strains of porcine reproductive and respiratory syndrome virus incorporate multiple isoforms of nonstructural protein 2 into virions. J. Virol. 2013, 87, 13456–13465. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Gao, P.; Kong, C.; Zhou, L.; Ge, X.; Guo, X.; Han, J.; Yang, H. The nsp2 Hypervariable Region of Porcine Reproductive and Respiratory Syndrome Virus Strain JXwn06 Is Associated with Viral Cellular Tropism to Primary Porcine Alveolar Macrophages. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.R.; Yu, W.; Murtaugh, M.P. Cross-reactive antibody responses to nsp1 and nsp2 of Porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2007, 88, 1184–1195. [Google Scholar] [CrossRef]
- De Lima, M.; Ansari, I.H.; Das, P.B.; Ku, B.J.; Martinez-Lobo, F.J.; Pattnaik, A.K.; Osorio, F.A. GP3 is a structural component of the PRRSV type II (US) virion. Virology 2009, 390, 31–36. [Google Scholar] [CrossRef]
- Mardassi, H.; Gonin, P.; Gagnon, C.A.; Massie, B.; Dea, S. A subset of porcine reproductive and respiratory syndrome virus GP3 glycoprotein is released into the culture medium of cells as a non-virion-associated and membrane-free (soluble) form. J. Virol. 1998, 72, 6298–6306. [Google Scholar] [CrossRef]
- Kapur, V.; Elam, M.R.; Pawlovich, T.M.; Murtaugh, M.P. Genetic variation in porcine reproductive and respiratory syndrome virus isolates in the midwestern United States. J. Gen. Virol. 1996, 77 Pt 6, 1271–1276. [Google Scholar] [CrossRef]

| Primers | Nucleotide Sequence (5′-3′) |
|---|---|
| ORF2F | ATATGCTAGCGCCACCATGAAATGGGGTCCATGCAAAGCC |
| ORF2R | ATATGAATTCCCGTGAGTTCGAAGGAAAAATTGC |
| ORF2bF | ATATGCTAGCGCCACCATGGGGTCCATGCAAAGCCTC |
| ORF2bR | ATATGAATTCCATAGCGTCAAGTTGTAAATC |
| ORF3F | ATATGCTAGCGCCACCATGGCTAATAGCTGTGCACTCC |
| ORF3R | ATATGAATTCTCGCCGCGCGGCACTGAGAGC |
| ORF4F | ATATGCTAGCGCCACCATGGCTGCGCCCCTTCTTTTCC |
| ORF4R | ATATGAATTCAATTGCCAGTAAGATGGC |
| ORF5F | ATATGCTAGCGCCACCATGTTGGGGAGATGCTTGACCG |
| ORF5R | ATATGAATTCAAGACGACCCCATTGTTCCG |
| ORF5aF | ATATGCTAGCGCCACCATGTTCAAGTATGTTGGGGAG |
| ORF5aR | ATATGAATTCCATAGCGTCAAGTTGTAAATC |
| ORF6F | ATATGCTAGCGCCACCATGGGGTCGTCTTTAGACGAC |
| ORF6R | ATATGAATTCTTTGGCATATTTGACAAGG |
| ORF7F | ATATGCTAGCGCCACCATGCCAAATAACAACGGCAAG ATATGAATTCCGCTGATGATGGCGCTGTG |
| ORF7R | ATATGAATTCCCGTGAGTTCGAAGGAAAAATTGC |
| Characteristics | Value |
|---|---|
| Optimized cutoff (S/N) | 4.02 |
| Diagnostic sensitivity (%) | 100.0 |
| 95% confidence interval | 87.68–100.0 |
| Diagnostic specific (%) | 100.0 |
| 95% confidence interval | 87.68–100.0 |
| AUC | 1.0 |
| 95% confidence interval | 1.0–1.0 |
| Kappa coefficient 1 | 1.0 |
| 95% confidence interval | 1.0–1.0 |
| Characteristics | Value for Each Structural Protein | |||||||
|---|---|---|---|---|---|---|---|---|
| GP2 | E | GP3 | GP4 | GP5 | ORF5a | M | N | |
| Optimized cutoff (S/N) | 5.32 | 1.78 | 4.41 | 2.24 | 4.85 | 1.30 | 7.741 | 4.02 |
| Diagnostic sensitivity (%) | 79.55 | 95.45 | 100.0 | 97.73 | 97.73 | 84.09 | 100.0 | 100.0 |
| 95% confidence interval | 64.25–89.67 | 83.30–99.21 | 89.99–100.0 | 86.49–99.88 | 86.49–99.88 | 69.33–92.84 | 89.99–100.0 | 89.99–100.0 |
| Diagnostic specific (%) | 97.37 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 97.37 | 100.0 |
| 95% confidence interval | 84.57–99.86 | 88.57–100.0 | 88.57–100.0 | 88.57–100.0 | 88.57–100.0 | 88.57–100.0 | 84.57–99.86 | 88.57–100.0 |
| AUC | 0.9372 | 0.9952 | 1.0 | 1.0 | 0.9970 | 0.9695 | 1.0 | 1.0 |
| 95% confidence interval | 0.8804–0.9940 | 0.9868–1.0 | 1.0–1.0 | 1.0–1.0 | 0.9903–1.0 | 0.9338–1.0 | 1.0–1.0 | 1.0–1.0 |
| Kappa coefficient | 0.758 | 0.951 | 1.0 | 0.976 | 0.976 | 0.830 | 0.975 | 1.0 1 |
| 95% confidence interval | 0.620–0.896 | 0.884–1.0 | 1.0–1.0 | 0.928–1.0 | 0.928–1.0 | 0.712–0.949 | 0.928–1.0 | 1.0–1.0 |
| Characteristics | Value for GP3 | Value for N |
|---|---|---|
| Optimized cutoff (S/N) | 4.41 | 4.02 |
| Diagnostic sensitivity (%) | 47.62 | 97.62 |
| 95% confidence interval | 36.72–58.74 | 90.86–99.59 |
| Diagnostic specific (%) | 98.81 | 97.62 |
| 95% confidence interval | 92.63–99.93 | 90.86–99.59 |
| AUC | 0.9636 | 0.9987 |
| 95% confidence interval | 0.9365–0.9906 | 0.9966–1.000 |
| Kappa coefficient | 0.464 | 0.952 1 |
| 95% confidence interval | 0.349–0.579 | 0.906–0.998 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luong, H.Q.; Lai, H.T.L.; Vu, H.L.X. Evaluation of Antibody Response Directed against Porcine Reproductive and Respiratory Syndrome Virus Structural Proteins. Vaccines 2020, 8, 533. https://doi.org/10.3390/vaccines8030533
Luong HQ, Lai HTL, Vu HLX. Evaluation of Antibody Response Directed against Porcine Reproductive and Respiratory Syndrome Virus Structural Proteins. Vaccines. 2020; 8(3):533. https://doi.org/10.3390/vaccines8030533
Chicago/Turabian StyleLuong, Hung Q., Huong T. L. Lai, and Hiep L. X. Vu. 2020. "Evaluation of Antibody Response Directed against Porcine Reproductive and Respiratory Syndrome Virus Structural Proteins" Vaccines 8, no. 3: 533. https://doi.org/10.3390/vaccines8030533
APA StyleLuong, H. Q., Lai, H. T. L., & Vu, H. L. X. (2020). Evaluation of Antibody Response Directed against Porcine Reproductive and Respiratory Syndrome Virus Structural Proteins. Vaccines, 8(3), 533. https://doi.org/10.3390/vaccines8030533







