Vaccination into the Dermal Compartment: Techniques, Challenges, and Prospects
Abstract
1. Introduction
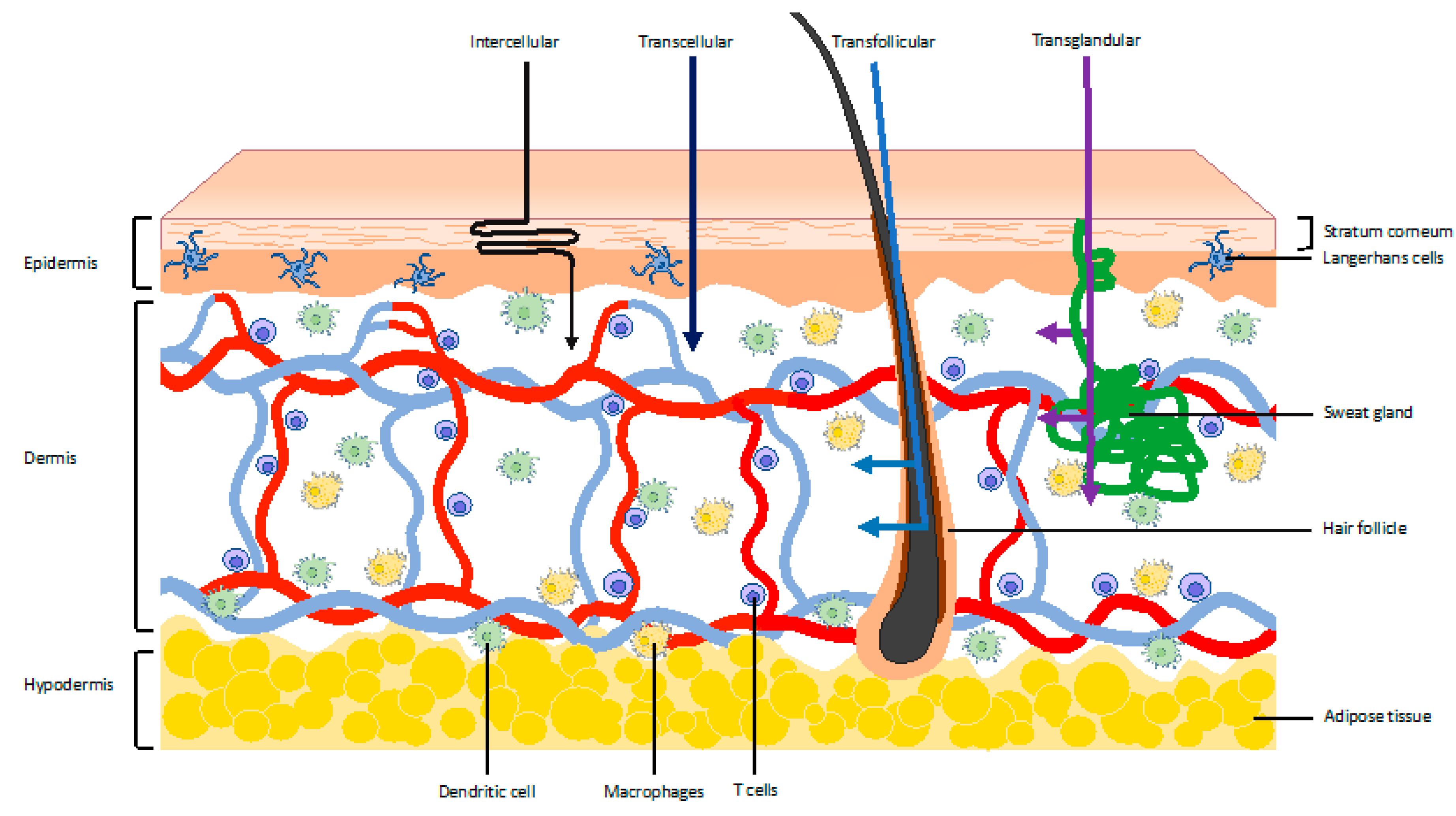
2. The Skin as a Vaccination Target
3. Rationale for Vaccination into the Dermal Compartment
4. Vaccination into the Dermal Compartment by Needle and Syringe
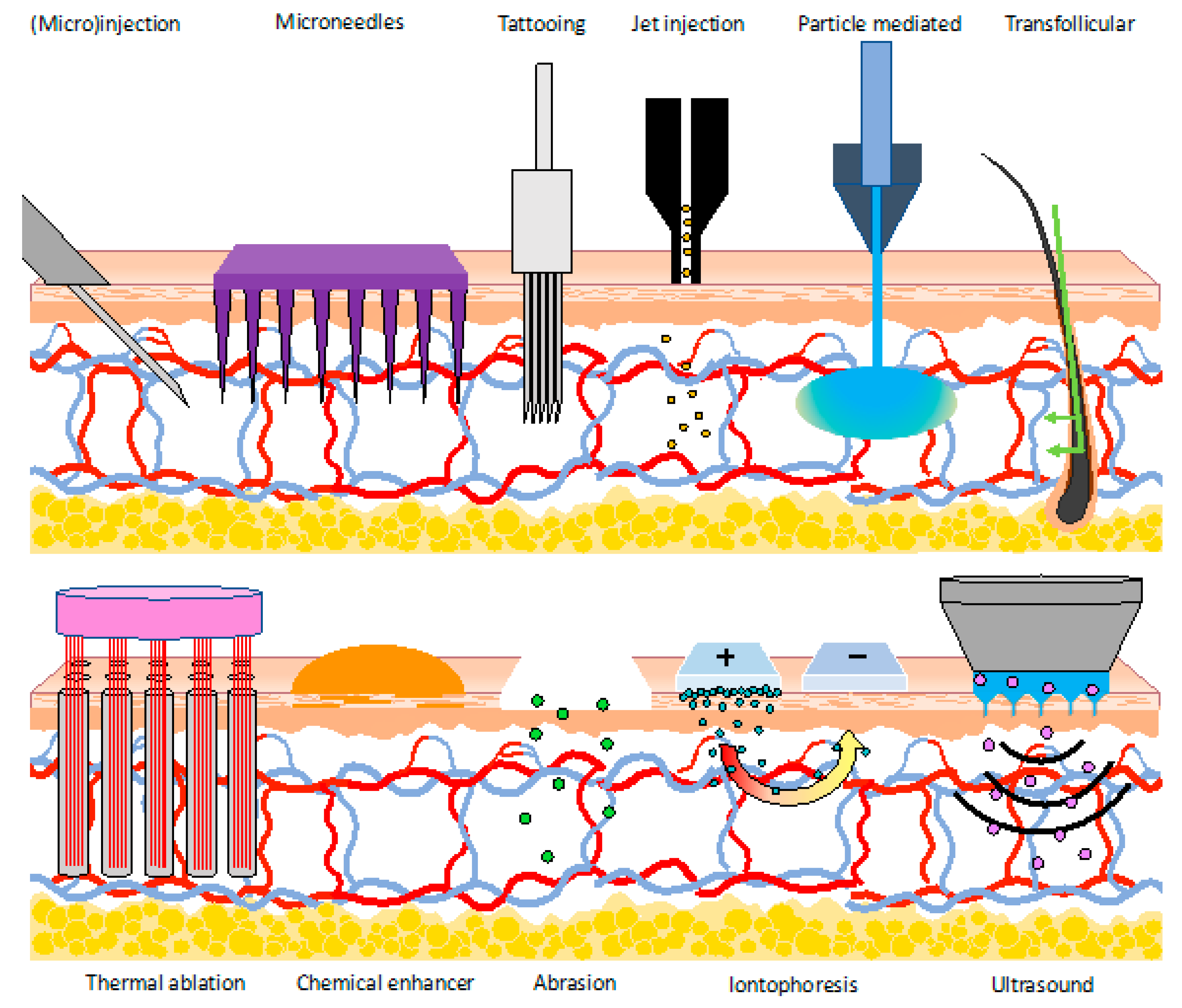
5. Vaccination into the Dermal Compartment without Needle and Syringe
5.1. Injection Methods
5.1.1. Microneedles
Solid Microneedles
Coated Microneedles
Hollow Microneedles
Dissolving Microneedles
Hydrogel Microneedles
5.1.2. DNA Tattooing
5.2. Jet and Ballistic Delivery
5.2.1. Gene Gun
5.2.2. Jet Injection
5.3. Permeabilization of the Skin
5.3.1. Thermal Ablation
5.3.2. Chemical Enhancers
5.3.3. Abrasion
5.3.4. Transfollicular Vaccination
5.3.5. Electroporation
5.3.6. Iontophoresis
5.3.7. Ultrasound
6. Conclusions
7. Methods
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Ten Threats to Global Health in 2019. Available online: https://anmj.org.au/whos-top-10-threats-to-global-health-in-2019/ (accessed on 9 April 2019).
- Lambert, P.H.; Laurent, P.E. Intradermal vaccine delivery: Will new delivery systems transform vaccine administration? Vaccine 2008, 26, 3197–3208. [Google Scholar] [CrossRef] [PubMed]
- Egawa, M.; Hirao, T.; Takahashi, M. In vivo estimation of stratum corneum thickness from water concentration profiles obtained with raman spectroscopy. Acta Derm. Venereol. 2007, 87, 4–8. [Google Scholar] [CrossRef]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Amagai, M. Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 2015, 27, 269–280. [Google Scholar] [CrossRef]
- Finnin, B.C.; Morgan, T.M. Transdermal penetration enhancers: Applications, limitations, and potential. J. Pharm. Sci. 1999, 88, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Trommer, H.; Neubert, R.H.H. Overcoming the stratum corneum: The modulation of skin penetration: A review. Skin Pharmacol. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef]
- Mulholland, W.J.; Arbuthnott, E.A.H.; Bellhouse, B.J.; Cornhill, J.F.; Austyn, J.M.; Kendall, M.A.F.; Cui, Z.; Tirlapur, U.K. Multiphoton high-resolution 3D imaging of Langerhans cells and keratinocytes in the mouse skin model adopted for epidermal powdered immunization. J. Invest. Dermatol. 2006, 126, 1541–1548. [Google Scholar] [CrossRef]
- Kubo, A.; Nagao, K.; Yokouchi, M.; Sasaki, H.; Amagai, M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 2009, 206, 2937–2946. [Google Scholar] [CrossRef]
- Ouchi, T.; Kubo, A.; Yokouchi, M.; Adachi, T.; Kobayashi, T.; Kitashima, D.Y.; Fujii, H.; Clausen, B.E.; Koyasu, S.; Amagai, M.; et al. Langerhans cell antigen capture through tight junctions confers preemptive immunity in experimental staphylococcal scalded skin syndrome. J. Exp. Med. 2011, 208, 2607–2613. [Google Scholar] [CrossRef]
- Tay, S.S.; Roediger, B.; Tong, P.L.; Tikoo, S.; Weninger, W. The Skin-Resident Immune Network. Curr. Dermatol. Rep. 2014, 3, 13–22. [Google Scholar] [CrossRef]
- Wang, X.N.; McGovern, N.; Gunawan, M.; Richardson, C.; Windebank, M.; Siah, T.W.; Lim, H.Y.; Fink, K.; Li, J.L.Y.; Ng, L.G.; et al. A three-dimensional atlas of human dermal leukocytes, lymphatics, and blood vessels. J. Invest. Dermatol. 2014, 134, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Matejuk, A. Skin Immunity. Arch. Immunol. Ther. Exp. 2018, 66, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef]
- Saitoh, A.; Aizawa, Y.; Sato, I.; Hirano, H.; Sakai, T.; Mori, M. Skin thickness in young infants and adolescents: Applications for intradermal vaccination. Vaccine 2015, 33, 3384–3391. [Google Scholar] [CrossRef] [PubMed]
- Van Mulder, T.J.S.; de Koeijer, M.; Theeten, H.; Willems, D.; Van Damme, P.; Demolder, M.; De Meyer, G.; Beyers, K.C.L.; Vankerckhoven, V. High frequency ultrasound to assess skin thickness in healthy adults. Vaccine 2017, 35, 1810–1815. [Google Scholar] [CrossRef]
- Laurent, A.; Mistretta, F.; Bottigioli, D.; Dahel, K.; Goujon, C.; Nicolas, J.F.; Hennino, A.; Laurent, P.E. Echographic measurement of skin thickness in adults by high frequency ultrasound to assess the appropriate microneedle length for intradermal delivery of vaccines. Vaccine 2007, 25, 6423–6430. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The human microbiome: Our second genome. Annu. Rev. Genom. Hum. Genet. 2012, 13, 151–170. [Google Scholar] [CrossRef]
- Belkaid, Y.; Tamoutounour, S. The influence of skin microorganisms on cutaneous immunity. Nat. Rev. Immunol. 2016, 16, 353–366. [Google Scholar] [CrossRef]
- de Jong, S.E.; Olin, A.; Pulendran, B. The Impact of the Microbiome on Immunity to Vaccination in Humans. Cell Host Microbe 2020, 28, 169–179. [Google Scholar] [CrossRef]
- Arnou, R.; Eavis, P.; De Juanes Pardo, J.R.; Ambrozaitis, A.; Kazek, M.P.; Weber, F. Immunogenicity, large scale safety and lot consistency of an intradermal influenza vaccine in adults aged 18–60 years: Randomized, controlled, phase III trial. Hum. Vaccin. 2010, 6, 346–354. [Google Scholar] [CrossRef]
- Lang, J.; Hoa, D.Q.; Gioi, N.V.; Tho, L.T.; Vien, N.C.; Rouyrre, N.; Forrat, R. Immunogenicity and safety of low-dose intradermal rabies vaccination given during an Expanded Programme on Immunization session in Viet Nam: Results of a comparative randomized trial. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 208–213. [Google Scholar] [CrossRef]
- Okayasu, H.; Sein, C.; Chang Blanc, D.; Gonzalez, A.R.; Zehrung, D.; Jarrahian, C.; Macklin, G.; Sutter, R.W. Intradermal Administration of Fractional Doses of Inactivated Poliovirus Vaccine: A Dose-Sparing Option for Polio Immunization. J. Infect. Dis. 2017, 216, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Sutter, R.W.; Cochi, S.L. Inactivated Poliovirus Vaccine Supply Shortage: Is There Light at the End of the Tunnel? J. Infect. Dis. 2019, 220, 1545–1546. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Molodecky, N.A.; Pallansch, M.A.; Sutter, R.W. Immunogenicity to poliovirus type 2 following two doses of fractional intradermal inactivated poliovirus vaccine: A novel dose sparing immunization schedule. Vaccine 2017, 35, 2993–2998. [Google Scholar] [CrossRef]
- Snider, C.J.; Zaman, K.; Estivariz, C.F.; Yunus, M.; Weldon, W.C.; Wannemuehler, K.A.; Oberste, M.S.; Pallansch, M.A.; Wassilak, S.G.; Bari, T.I.A.; et al. Immunogenicity of full and fractional dose of inactivated poliovirus vaccine for use in routine immunisation and outbreak response: An open-label, randomised controlled trial. Lancet 2019, 393, 2624–2634. [Google Scholar] [CrossRef]
- Kenney, R.T.; Frech, S.A.; Muenz, L.R.; Villar, C.P.; Glenn, G.M. Dose sparing with intradermal injection of influenza vaccine. N. Engl. J. Med. 2004, 351, 2295–2301. [Google Scholar] [CrossRef]
- Boonnak, K.; Dhitavat, J.; Thantamnu, N.; Kosoltanapiwat, N.; Auayporn, M.; Jiang, L.; Puthavathana, P.; Pitisuttithum, P. Immune responses to intradermal and intramuscular inactivated influenza vaccine among older age group. Vaccine 2017, 35, 7339–7346. [Google Scholar] [CrossRef]
- World Health Organization; Program for Appropriate Technology in Health (PATH). Intradermal Delivery of Vaccines—A Review of the Literature and the Potential for Development for Use in Low and Middle Income Countries; Program for Appropriate Technology in Health (PATH): Seattle, WA, USA, 2009. [Google Scholar]
- Hickling, J.K.; Jones, K.R.; Friede, M.; Zehrung, D.; Chen, D.; Kristensenc, D. Intradermal delivery of vaccines: Potential benefits and current challenges. Bull. World Health Organ. 2011, 89, 221–226. [Google Scholar] [CrossRef]
- Grunwald, T.; Ulbert, S. Improvement of DNA vaccination by adjuvants and sophisticated delivery devices: Vaccine-platforms for the battle against infectious diseases. Clin. Exp. Vaccine Res. 2015, 4, 1–10. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Orsi, A.; Ansaldi, F.; Gasparini, R.; Icardi, G. Fluzone® intra-dermal (Intanza®/Istivac® Intra-dermal): An updated overview. Hum. Vaccines Immunother. 2016, 12, 2616–2627. [Google Scholar] [CrossRef]
- Choi, Y.H.; Perez-Cuevas, M.B.; Kodani, M.; Zhang, X.; Prausnitz, M.R.; Kamili, S.; O’connor, S.M. Feasibility of Hepatitis B Vaccination by Microneedle Patch: Cellular and Humoral Immunity Studies in Rhesus Macaques. J. Infect. Dis. 2019, 220, 1926–1934. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Erdos, G.; Huang, S.; Kenniston, T.W.; Balmert, S.C.; Carey, C.D.; Raj, V.S.; Epperly, M.W.; Klimstra, W.B.; Haagmans, B.L.; et al. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine 2020. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Ewing, D.; Blevins, M.; Sun, P.; Sundaram, A.K.; Raviprakash, K.S.; Porter, K.R.; Sanders, J.W. Enhanced immunogenicity and protective efficacy of a tetravalent dengue DNA vaccine using electroporation and intradermal delivery. Vaccine 2019, 37, 4444–4453. [Google Scholar] [CrossRef] [PubMed]
- Roos, A.K.; Moreno, S.; Leder, C.; Pavlenko, M.; King, A.; Pisa, P. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol. Ther. 2006, 13, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Lombard, M.; Pastoret, P.P.; Moulin, A.M. A brief history of vaccines and vaccination. OIE Rev. Sci. Tech. 2007, 26, 29–48. [Google Scholar] [CrossRef]
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef]
- Wright, L.T. Intradermal vaccination against smallpox. J. Am. Med. Assoc. 1918, 71, 654–657. [Google Scholar] [CrossRef]
- Tuft, L. Active immunization against typhoid fever, with particular reference to an intradermal method. J. Lab. Clin. Med. 1931, 16, 552–556. [Google Scholar] [CrossRef]
- Tuft, L.; Yagle, E.M.; Rogers, S. Comparative Study of the Antibody Response After Various Methods of Administration of Mixed Typhoid Vaccine: With Particular Reference to the Intradermal and Oral Methods. J. Infect. Dis. 1932. [Google Scholar] [CrossRef]
- Rivers, T.M.; Ward, S.M. Jennerian prophylaxis by means of in: Tradermal injections of culture vaccine virus. J. Exp. Med. 1935, 62, 549–560. [Google Scholar] [CrossRef]
- Wallgren, A. Intradermal vaccinations with B C G virus: Preliminary note. J. Am. Med. Assoc. 1928, 91, 1876–1881. [Google Scholar] [CrossRef]
- Toomey, J.A. Intradermal vaccination. Am. J. Dis. Child. 1928. [Google Scholar] [CrossRef]
- Francis, T.; Magill, T.P. The antibody response of human subjects vaccinated with the virus of human influenza. J. Exp. Med. 1937, 65, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.A. Pronged Vaccinating and Testing Needle. U.S. Patent US3194237A, 13 July 1965. [Google Scholar]
- Belongia, E.A.; Naleway, A.L. Smallpox vaccine: The good, the bad, and the ugly. Clin. Med. Res. 2003, 1, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.J.; Gupta, J.; Patel, S.R.; Park, S.; Jarrahian, C.; Zehrung, D.; Prausnitz, M.R. Reliability and accuracy of intradermal injection by Mantoux technique, hypodermic needle adapter, and hollow microneedle in pigs. Drug Deliv. Transl. Res. 2014, 4, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Tsals, I. Methods and Devices for Intradermal Injection. U.S. Patent US8556861B2, 15 October 2013. [Google Scholar]
- Path Technology Solutions for Global Health (PATH). Intradermal Adapter; PATH: Seattle, WA, USA, 2018. [Google Scholar]
- Icardi, G.; Orsi, A.; Ceravolo, A.; Ansaldi, F. Current evidence on intradermal influenza vaccines administered by Soluvia™ licensed micro injection system. Hum. Vaccines Immunother. 2012, 8, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Ezell, J. BD Soluvia™ Microinjection System Used For First Approved Intradermal Influenza Vaccine in the European Union; Becton, Dickinson and Company (BD): Franklin Lakes, NJ, USA, 2009. [Google Scholar]
- Ansaldi, F.; De Florentiis, D.; Durando, P.; Icardi, G. Fluzone® Intradermal vaccine: A promising new chance to increase the acceptability of influenza vaccination in adults. Expert Rev. Vaccines 2012, 11, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Levin, Y.; Kochba, E.; Hung, I.; Kenney, R. Intradermal vaccination using the novel microneedle device MicronJet600: Past, present, and future. Hum. Vaccines Immunother. 2015, 11, 991–997. [Google Scholar] [CrossRef]
- Hung, I.F.N.; Levin, Y.; To, K.K.W.; Chan, K.H.; Zhang, A.J.; Li, P.; Li, C.; Xu, T.; Wong, T.Y.; Yuen, K.Y. Dose sparing intradermal trivalent influenza (2010/2011) vaccination overcomes reduced immunogenicity of the 2009 H1N1 strain. Vaccine 2012, 30, 6427–6435. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, H.J.; Kim, D.R.; Lee, H.; Jin, J.E.; Kim, Y.R.; Lee, M.S.; Cho, S.N.; Kang, Y.A. Safety and efficacy of tuberculin skin testing with microneedle MicronJet600E in healthy adults. Int. J. Tuberc. Lung Dis. 2016, 20, 500–504. [Google Scholar] [CrossRef]
- Levin, Y.; Kochba, E.; Shukarev, G.; Rusch, S.; Herrera-Taracena, G.; van Damme, P. A phase 1, open-label, randomized study to compare the immunogenicity and safety of different administration routes and doses of virosomal influenza vaccine in elderly. Vaccine 2016, 34, 5262–5272. [Google Scholar] [CrossRef] [PubMed]
- Dul, M.; Stefanidou, M.; Porta, P.; Serve, J.; O’Mahony, C.; Malissen, B.; Henri, S.; Levin, Y.; Kochba, E.; Wong, F.S.; et al. Hydrodynamic gene delivery in human skin using a hollow microneedle device. J. Control. Release 2017, 265, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Zaman, K.; Estívariz, C.F.; Yunus, M.; Gary, H.E.; Weldon, W.C.; Bari, T.I.; Steven Oberste, M.; Wassilak, S.G.; Luby, S.P.; et al. Early priming with inactivated poliovirus vaccine (IPV) and intradermal fractional dose IPV administered by a microneedle device: A randomized controlled trial. Vaccine 2015, 33, 6816–6822. [Google Scholar] [CrossRef] [PubMed]
- Van Mulder, T.J.S.; Withanage, K.; Beyers, K.C.L.; Vankerckhoven, V.V.J.; Theeten, H.; Van Damme, P. Immunogenicity and safety of intradermal delivery of hepatitis B booster vaccine using the novel drug delivery device VAX-ID™. Vaccine 2019, 37, 581–586. [Google Scholar] [CrossRef]
- Arakane, R.; Nakatani, H.; Fujisaki, E.; Takahama, A.; Ishida, K.; Yoshiike, M.; Nakayama, T.; Takeshita, F. Immunogenicity and safety of the new intradermal influenza vaccine in adults and elderly: A randomized phase 1/2 clinical trial. Vaccine 2015, 33, 6340–6350. [Google Scholar] [CrossRef][Green Version]
- Arakane, R.; Annaka, R.; Takahama, A.; Ishida, K.; Yoshiike, M.; Nakayama, T.; Takeshita, F. Superior immunogenicity profile of the new intradermal influenza vaccine compared to the standard subcutaneous vaccine in subjects 65 years and older: A randomized controlled phase III study. Vaccine 2015, 33, 6650–6658. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Atmar, R.L.; Patel, S.M.; Keitel, W.A. Intanza®: A new intradermal vaccine for seasonal influenza. Expert Rev. Vaccines 2010, 9, 1399–1409. [Google Scholar] [CrossRef]
- Lambert, L.C.; Fauci, A.S. Influenza vaccines for the future. N. Engl. J. Med. 2010, 363, 2036–2044. [Google Scholar] [CrossRef]
- Kennedy, A.; Basket, M.; Sheedy, K. Vaccine Attitudes, Concerns, and Information Sources Reported by Parents of Young Children: Results From the 2009 HealthStyles Survey. Pediatrics 2011, 17, 92–99. [Google Scholar] [CrossRef]
- Jacobson, M.A.; Sinclair, E.; Bredt, B.; Agrillo, L.; Black, D.; Epling, C.L.; Carvidi, A.; Ho, T.; Bains, R.; Girling, V.; et al. Safety and immunogenicity of Towne cytomegalovirus vaccine with or without adjuvant recombinant interleukin-12. Vaccine 2006, 24, 5311–5319. [Google Scholar] [CrossRef]
- McLenon, J.; Rogers, M.A.M. The fear of needles: A systematic review and meta-analysis. J. Adv. Nurs. 2019, 75, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, T.; Motta, M.; Sylvester, S.; Lunz Trujillo, K.; Blackburn, C.C. Parent psychology and the decision to delay childhood vaccination. Soc. Sci. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Frijstein, G.; Hortensius, J.; Zaaijer, H.L. Needlestick injuries and infectious patients in a major academic medical centre from 2003 to 2010. Neth. J. Med. 2011, 69, 465–468. [Google Scholar]
- Wittmann, A.; Hofmann, F.; Kralj, N. Needle stick injuries—Risk from blood contact in dialysis. J. Ren. Care 2007, 33, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Prüss-Üstün, A.; Rapiti, E.; Hutin, Y. Estimation of the global burden of disease attributable to contaminated sharps injuries among health-care workers. Am. J. Ind. Med. 2005, 48, 482–490. [Google Scholar] [CrossRef]
- Mannocci, A.; De Carli, G.; Di Bari, V.; Saulle, R.; Unim, B.; Nicolotti, N.; Carbonari, L.; Puro, V.; La Torre, G. How Much do Needlestick Injuries Cost? A Systematic Review of the Economic Evaluations of Needlestick and Sharps Injuries among Healthcare Personnel. Infect. Control Hosp. Epidemiol. 2016, 37, 635–646. [Google Scholar] [CrossRef]
- Singh, A.; Sethi, S.; Verma, A.; Gangwar, K.; Rathore, D.D.K.S. Needle Stick Injuries among Healthcare Waste Handlers in a Tertiary Care Hospital of Delhi. Epidemiol. Int. 2017, 20, 3. [Google Scholar] [CrossRef]
- Drape, R.J.; Macklin, M.D.; Barr, L.J.; Jones, S.; Haynes, J.R.; Dean, H.J. Epidermal DNA vaccine for influenza is immunogenic in humans. Vaccine 2006, 24, 4475–4481. [Google Scholar] [CrossRef]
- Rottinghaus, S.T.; Poland, G.A.; Jacobson, R.M.; Barr, L.J.; Roy, M.J. Hepatitis B DNA vaccine induces protective antibody responses in human non-responders to conventional vaccination. Vaccine 2003, 21, 4604–4608. [Google Scholar] [CrossRef]
- McBurney, S.P.; Sunshine, J.E.; Gabriel, S.; Huynh, J.P.; Sutton, W.F.; Fuller, D.H.; Haigwood, N.L.; Messer, W.B. Evaluation of protection induced by a dengue virus serotype 2 envelope domain III protein scaffold/DNA vaccine in non-human primates. Vaccine 2016, 34, 3500–3507. [Google Scholar] [CrossRef]
- Aps, L.R.M.M.; Tavares, M.B.; Rozenfeld, J.H.K.; Lamy, M.T.; Ferreira, L.C.S.; Diniz, M.O. Bacterial spores as particulate carriers for gene gun delivery of plasmid DNA. J. Biotechnol. 2016, 228, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Fernando, G.J.P.; Hickling, J.; Jayashi Flores, C.M.; Griffin, P.; Anderson, C.D.; Skinner, S.R.; Davies, C.; Witham, K.; Pryor, M.; Bodle, J.; et al. Safety, tolerability, acceptability and immunogenicity of an influenza vaccine delivered to human skin by a novel high-density microprojection array patch (Nanopatch™). Vaccine 2018, 36, 3779–3788. [Google Scholar] [CrossRef] [PubMed]
- Boopathy, A.V.; Mandal, A.; Kulp, D.W.; Menis, S.; Bennett, N.R.; Watkins, H.C.; Wang, W.; Martin, J.T.; Thai, N.T.; He, Y.; et al. Enhancing humoral immunity via sustained-release implantable microneedle patch vaccination. Proc. Natl. Acad. Sci. USA 2019, 116, 16473–16478. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar]
- Eriksson, E.; Yao, F.; Svensjö, T.; Winkler, T.; Slama, J.; MacKlin, M.D.; Andree, C.; McGregor, M.; Hinshaw, V.; Swain, W.F. In vivo gene transfer to skin and wound by microseeding. J. Surg. Res. 1998, 78, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Moulin, V.; Morgan, M.E.; Eleveld-Trancikova, D.; Haanen, J.B.A.G.; Wielders, E.; Looman, M.W.G.; Janssen, R.A.J.; Figdor, C.G.; Jansen, B.J.H.; Adema, G.J. Targeting dendritic cells with antigen via dendritic cell-associated promoters. Cancer Gene Ther. 2012, 19, 303–311. [Google Scholar] [CrossRef]
- Kim, Y.C.; Jarrahian, C.; Zehrung, D.; Mitragotri, S.; Prausnitz, M.R. Delivery systems for intradermal vaccination. Curr. Top. Microbiol. Immunol. 2012. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar]
- van den Berg, J.H. Formulation and Delivery of Dermal DNA Vaccines. Ph.D. Thesis, University of Utrecht, Utrecht, The Netherlands, 2009. [Google Scholar]
- Shaw, J.; Urquhart, J. Programmed, systemic drug delivery by the transdermal route. Trends Pharmacol. Sci. 1979. [Google Scholar] [CrossRef]
- Prausnitz, M.R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 2004. [Google Scholar] [CrossRef]
- Prausnitz, M.R. Engineering Microneedle Patches for Vaccination and Drug Delivery to Skin. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 177–200. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Raj Singh, T.R.; Woolfson, A.D. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010, 17, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Tuan-Mahmood, T.M.; McCrudden, M.T.C.; Torrisi, B.M.; McAlister, E.; Garland, M.J.; Singh, T.R.R.; Donnelly, R.F. Microneedles for intradermal and transdermal delivery. Eur. J. Pharm. Sci. 2013, 50, 623–637. [Google Scholar] [CrossRef]
- Ripolin, A.; Quinn, J.; Larrañeta, E.; Vicente-Perez, E.M.; Barry, J.; Donnelly, R.F. Successful application of large microneedle patches by human volunteers. Int. J. Pharm. 2017, 521, 92–101. [Google Scholar] [CrossRef]
- Norman, J.J.; Arya, J.M.; McClain, M.A.; Frew, P.M.; Meltzer, M.I.; Prausnitz, M.R. Microneedle patches: Usability and acceptability for self-vaccination against influenza. Vaccine 2014, 32, 1856–1862. [Google Scholar] [CrossRef]
- Rouphael, N.G.; Paine, M.; Mosley, R.; Henry, S.; McAllister, D.V.; Kalluri, H.; Pewin, W.; Frew, P.M.; Yu, T.; Thornburg, N.J.; et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): A randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 2017, 390, 649–658. [Google Scholar] [CrossRef]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Fu, Y.; Song, Y. Recent advances of microneedles for biomedical applications: Drug delivery and beyond. Acta Pharm. Sin. B 2019, 9, 469–483. [Google Scholar] [CrossRef]
- Ding, Z.; Verbaan, F.J.; Bivas-Benita, M.; Bungener, L.; Huckriede, A.; van den Berg, D.J.; Kersten, G.; Bouwstra, J.A. Microneedle arrays for the transcutaneous immunization of diphtheria and influenza in BALB/c mice. J. Control. Release 2009, 136, 71–78. [Google Scholar] [CrossRef]
- Schepens, B.; Vos, P.J.; Saelens, X.; van der Maaden, K. Vaccination with influenza hemagglutinin-loaded ceramic nanoporous microneedle arrays induces protective immune responses. Eur. J. Pharm. Biopharm. 2019, 136, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Matriano, J.A.; Cormier, M.; Johnson, J.; Young, W.A.; Buttery, M.; Nyam, K.; Daddona, P.E. Macroflux® microprojection array patch technology: A new and efficient approach for intracutaneous immunization. Pharm. Res. 2002, 19, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Fernando, G.J.P.; Chen, X.; Prow, T.W.; Crichton, M.L.; Fairmaid, E.J.; Roberts, M.S.; Frazer, I.H.; Brown, L.E.; Kendall, M.A.F. Potent immunity to low doses of influenza vaccine by probabilistic guided micro-targeted skin delivery in a mouse model. PLoS ONE 2010, 5, e10266. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Söderholm, J.; Prausnitz, M.R.; Sällberg, M. Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine. Gene Ther. 2010, 17, 811–814. [Google Scholar] [CrossRef]
- Mikszta, J.A.; Alarcon, J.B.; Brittingham, J.M.; Sutter, D.E.; Pettis, R.J.; Harvey, N.G. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat. Med. 2002, 8, 415–419. [Google Scholar] [CrossRef]
- Kadavakollu, M.; Karri, V.V.S.R.; Gowthamarajan, K.; Radhakrishnan, A.; Palanisamy, D.; Balasubramanian, S.; Bhojraj, S. The novel coronavirus and its possible treatment by vaccines, therapeutics and drug delivery systems: Current status and future perspectives. Int. J. Res. Pharm. Sci. 2020, 11, 54–61. [Google Scholar] [CrossRef]
- World Health Organization. DRAFT Landscape of COVID-19 Candidate Vaccines—23 April 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Niu, L.; Chu, L.Y.; Burton, S.A.; Hansen, K.J.; Panyam, J. Intradermal delivery of vaccine nanoparticles using hollow microneedle array generates enhanced and balanced immune response. J. Control. Release 2019, 294, 268–278. [Google Scholar] [CrossRef]
- Poirier, D.; Renaud, F.; Dewar, V.; Strodiot, L.; Wauters, F.; Janimak, J.; Shimada, T.; Nomura, T.; Kabata, K.; Kuruma, K.; et al. Hepatitis B surface antigen incorporated in dissolvable microneedle array patch is antigenic and thermostable. Biomaterials 2017, 145, 256–265. [Google Scholar] [CrossRef]
- Donadei, A.; Kraan, H.; Ophorst, O.; Flynn, O.; O’Mahony, C.; Soema, P.C.; Moore, A.C. Skin delivery of trivalent Sabin inactivated poliovirus vaccine using dissolvable microneedle patches induces neutralizing antibodies. J. Control. Release 2019, 311, 96–103. [Google Scholar] [CrossRef]
- Pastor, Y.; Larrañeta, E.; Erhard, Á.; Quincooces, G.; Peñuelas, I.; Irache, J.M.; Donnelly, R.; Gamazo, C. Dissolving microneedles for intradermal vaccination against shigellosis. Vaccines 2019, 7, 159. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lai, K.Y.; Chiu, Y.H.; Wu, Y.W.; Shiau, A.L.; Chen, M.C. Implantable microneedles with an immune-boosting function for effective intradermal influenza vaccination. Acta Biomater. 2019, 97, 230–238. [Google Scholar] [CrossRef]
- Cole, G.; Ali, A.A.; McErlean, E.; Mulholland, E.J.; Short, A.; McCrudden, C.M.; McCaffrey, J.; Robson, T.; Kett, V.L.; Coulter, J.A.; et al. DNA vaccination via RALA nanoparticles in a microneedle delivery system induces a potent immune response against the endogenous prostate cancer stem cell antigen. Acta Biomater. 2019, 96, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Bernelin-Cottet, C.; Urien, C.; McCaffrey, J.; Collins, D.; Donadei, A.; McDaid, D.; Jakob, V.; Barnier-Quer, C.; Collin, N.; Bouguyon, E.; et al. Electroporation of a nanoparticle-associated DNA vaccine induces higher inflammation and immunity compared to its delivery with microneedle patches in pigs. J. Control. Release 2019, 308, 14–28. [Google Scholar] [CrossRef]
- Cumberbatch, M.; Dearman, R.J.; Kimber, I. Langerhans cells require signals from both tumour necrosis factor- α and interleukin-1β for migration. Immunology 1997, 92, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, D.; Earnest-Silveira, L.; Grubor-Bauk, B.; Wijesundara, D.K.; Boo, I.; Ramsland, P.A.; Vincan, E.; Drummer, H.E.; Gowans, E.J.; Torresi, J. Pre-clinical evaluation of a quadrivalent HCV VLP vaccine in pigs following microneedle delivery. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Sun, W.; Fang, J.; Lee, K.J.; Li, S.; Gu, Z.; Dokmeci, M.R.; Khademhosseini, A. Biodegradable Gelatin Methacryloyl Microneedles for Transdermal Drug Delivery. Adv. Healthc. Mater. 2019, 8, 1801054. [Google Scholar] [CrossRef]
- Leone, M.; Priester, M.I.; Romeijn, S.; Nejadnik, M.R.; Mönkäre, J.; O’Mahony, C.; Jiskoot, W.; Kersten, G.; Bouwstra, J.A. Hyaluronan-based dissolving microneedles with high antigen content for intradermal vaccination: Formulation, physicochemical characterization and immunogenicity assessment. Eur. J. Pharm. Biopharm. 2019, 134, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Erdos, G.; Balmert, S.C.; Carey, C.D.; Falo, G.D.; Patel, N.A.; Zhang, J.; Gambotto, A.; Korkmaz, E.; Falo, L.D. Improved cutaneous genetic immunization by microneedle array delivery of an adjuvanted adenovirus vaccine. J. Investig. Dermatol. 2020. [Google Scholar] [CrossRef]
- Balmert, S.C.; Carey, C.D.; Falo, G.D.; Sethi, S.K.; Erdos, G.; Korkmaz, E.; Falo, L.D. Dissolving undercut microneedle arrays for multicomponent cutaneous vaccination. J. Control. Release 2020, 317, 336–346. [Google Scholar] [CrossRef]
- Leone, M.; Romeijn, S.; Du, G.; Le Dévédec, S.E.; Vrieling, H.; O’Mahony, C.; Bouwstra, J.A.; Kersten, G. Diphtheria toxoid dissolving microneedle vaccination: Adjuvant screening and effect of repeated-fractional dose administration. Int. J. Pharm. 2020. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Garland, M.J.; Migalska, K.; Majithiya, R.; McCrudden, C.M.; Kole, P.L.; Mahmood, T.M.T.; McCarthy, H.O.; Woolfson, A.D. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Adv. Funct. Mater. 2012, 22, 4879–4890. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, A.J.; Rodgers, A.M.; McCrudden, M.T.C.; McCarthy, H.O.; Donnelly, R.F. Novel Hydrogel-Forming Microneedle Array for Intradermal Vaccination in Mice Using Ovalbumin as a Model Protein Antigen. Mol. Pharm. 2018, 16, 118–127. [Google Scholar] [CrossRef]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Hill-Tout, J.E.; Covill, D.; Lau, W.M.; Ng, K.W. Thermoreversible hydrogel-coated microneedles for dermal drug delivery. In Proceedings of the 10th APS International PharmSci Conference, London, UK, 11–12 September 2019. [Google Scholar]
- Lee, K.J.; Jeong, S.S.; Roh, D.H.; Kim, D.Y.; Choi, H.K.; Lee, E.H. A practical guide to the development of microneedle systems—In clinical trials or on the market. Int. J. Pharm. 2020, 573, 118778. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, J.H.; Nuijen, B.; Beijnen, J.H.; Vincent, A.; van Tinteren, H.; Kluge, J.; Woerdeman, L.A.E.; Hennink, W.E.; Storm, G.; Schumacher, T.; et al. Optimization of intradermal vaccination by DNA tattooing in human skin. Hum. Gene Ther. 2009, 20, 181–189. [Google Scholar] [CrossRef]
- Bins, A.D.; Jorritsma, A.; Wolkers, M.C.; Hung, C.F.; Wu, T.C.; Schumacher, T.N.M.; Haanen, J.B.A.G. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nat. Med. 2005, 11, 899–904. [Google Scholar] [CrossRef]
- Oosterhuis, K.; Van Den Berg, J.H.; Schumacher, T.N.; Haanen, J.B.A.G. DNA vaccines and intradermal vaccination by DNA tattooing. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 351, pp. 221–250. ISBN 978-3-642-23689-1. [Google Scholar]
- Samuels, S.; Marijne Heeren, A.; Zijlmans, H.J.M.A.A.; Welters, M.J.P.; van den Berg, J.H.; Philips, D.; Kvistborg, P.; Ehsan, I.; Scholl, S.M.E.; Nuijen, B.; et al. HPV16 E7 DNA tattooing: Safety, immunogenicity, and clinical response in patients with HPV-positive vulvar intraepithelial neoplasia. Cancer Immunol. Immunother. 2017, 66, 1163–1173. [Google Scholar] [CrossRef]
- van de Wall, S.; Walczak, M.; van Rooij, N.; Hoogeboom, B.N.; Meijerhof, T.; Nijman, H.W.; Daemen, T. Tattoo delivery of a semliki forest virus-based vaccine encoding human papillomavirus E6 and E7. Vaccines 2015, 3, 221–238. [Google Scholar] [CrossRef]
- Chiu, Y.; Jiang, X.; Kumar, R.; Hioe, C.; Zolla-Pazner, S.; Kong, X. Skin tattooing as an effective tool for delivering DNA and protein vaccine immunogens. Retrovirology 2012, 9, 338. [Google Scholar] [CrossRef]
- Platteel, A.C.M.; Nieuwenhuizen, N.E.; Domaszewska, T.; Schürer, S.; Zedler, U.; Brinkmann, V.; Sijts, A.J.A.M.; Kaufmann, S.H.E. Efficacy testing of H56 cDNA Tattoo immunization against tuberculosis in a mouse model. Front. Immunol. 2017, 8, 1744. [Google Scholar] [CrossRef]
- Platteel, A.C.M.; Marit de Groot, A.; Keller, C.; Andersen, P.; Ovaa, H.; Kloetzel, P.M.; Mishto, M.; Sijts, A.J.A.M. Strategies to enhance immunogenicity of cDNA vaccine encoded antigens by modulation of antigen processing. Vaccine 2016, 34, 5132–5140. [Google Scholar] [CrossRef]
- Lankelma, J.M.; Wagemakers, A.; Birnie, E.; Haak, B.W.; Trentelman, J.J.A.; Weehuizen, T.A.F.; Ersöz, J.; Roelofs, J.J.T.H.; Hovius, J.W.; Wiersinga, W.J.; et al. Rapid DNA vaccination against Burkholderia pseudomallei flagellin by tattoo or intranasal application. Virulence 2017, 8, 1683–1694. [Google Scholar] [CrossRef]
- Wagemakers, A.; Mason, L.M.K.; Oei, A.; De Wever, B.; Van Der Poll, T.; Bins, A.D.; Hovius, J.W.R. Rapid outer-surface protein C DNA tattoo vaccination protects against Borrelia afzelii infection. Gene Ther. 2014, 21, 1051–1057. [Google Scholar] [CrossRef]
- Yan, G.; Arelly, N.; Farhan, N.; Lobo, S.; Li, H. Enhancing DNA delivery into the skin with a motorized microneedle device. Eur. J. Pharm. Sci. 2014, 52, 215–222. [Google Scholar] [CrossRef]
- Pokorna, D.; Rubio, I.; Müller, M. DNA-vaccination via tattooing induces stronger humoral and cellular immune responses than intramuscular delivery supported by molecular adjuvants. Genet. Vaccines Ther. 2008, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Quaak, S.G.L.; van den Berg, J.H.; Oosterhuis, K.; Beijnen, J.H.; Haanen, J.B.A.G.; Nuijen, B. DNA tattoo vaccination: Effect on plasmid purity and transfection efficiency of different topoisoforms. J. Control. Release 2009, 139, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Verstrepen, B.E.; Bins, A.D.; Rollier, C.S.; Mooij, P.; Koopman, G.; Sheppard, N.C.; Sattentau, Q.; Wagner, R.; Wolf, H.; Schumacher, T.N.M.; et al. Improved HIV-1 specific T-cell responses by short-interval DNA tattooing as compared to intramuscular immunization in non-human primates. Vaccine 2008, 26, 3346–3351. [Google Scholar] [CrossRef] [PubMed]
- Babiarova, K.; Kutinova, L.; Zurkova, K.; Krystofova, J.; Brabcova, E.; Hainz, P.; Musil, J.; Nemeckova, S. Immunization with WT1-derived peptides by Tattooing Inhibits the Growth of TRAMP-C2 Prostate Tumor in Mice. J. Immunother. 2012, 35, 478–487. [Google Scholar] [CrossRef]
- Yoshida, A.; Nagata, T.; Uchijima, M.; Higashi, T.; Koide, Y. Advantage of gene gun-mediated over intramuscular inoculation of plasmid DNA vaccine in reproducible induction of specific immune responses. Vaccine 2000, 18, 1725–1729. [Google Scholar] [CrossRef]
- Torres, C.A.T.; Iwasaki, A.; Barber, B.H.; Robinson, H.L. Differential Dependence on Target Site Tissue for Gene Gun and Intramuscular DNA Immunizations. J. Immunol. 1997, 158, 4529–4532. [Google Scholar]
- Fynan, E.F.; Webster, R.G.; Fuller, D.H.; Haynes, J.R.; Santoro, J.C.; Robinson, H.L. DNA vaccines: Protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc. Natl. Acad. Sci. USA 1993, 90, 11478–11482. [Google Scholar] [CrossRef]
- Lodmell, D.L.; Ray, N.B.; Ewalt, L.C. Gene gun particle-mediated vaccination with plasmid DNA confers protective immunity against rabies virus infection. Vaccine 1998, 16, 115–118. [Google Scholar] [CrossRef]
- Roy, M.J.; Wu, M.S.; Barr, L.J.; Fuller, J.T.; Tussey, L.G.; Speller, S.; Culp, J.; Burkholder, J.K.; Swain, W.F.; Dixon, R.M.; et al. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine 2000, 19, 764–778. [Google Scholar] [CrossRef]
- Trimble, C.; Lin, C.T.; Hung, C.F.; Pai, S.; Juang, J.; He, L.; Gillison, M.; Pardoll, D.; Wu, L.; Wu, T.C. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine 2003, 21, 4036–4042. [Google Scholar] [CrossRef]
- Nguyen-Hoai, T.; Kobelt, D.; Hohn, O.; Vu, M.D.; Schlag, P.M.; Dörken, B.; Norley, S.; Lipp, M.; Walther, W.; Pezzutto, A.; et al. HER2/neu DNA vaccination by intradermal gene delivery in a mouse tumor model: Gene gun is superior to jet injector in inducing ctl responses and protective immunity. Oncoimmunology 2012, 1, 1537–1545. [Google Scholar] [CrossRef]
- Davtyan, H.; Ghochikyan, A.; Movsesyan, N.; Ellefsen, B.; Petrushina, I.; Cribbs, D.H.; Hannaman, D.; Evans, C.F.; Agadjanyan, M.G. Delivery of a DNA vaccine for Alzheimer’s disease by electroporation versus gene gun generates potent and similar immune responses. Neurodegener. Dis. 2012, 10, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, C.; Zhang, L.; Li, J.; Huang, Z.; Lu, S. The relative immunogenicity of DNA vaccines delivered by the intramuscular needle injection, electroporation and gene gun methods. Vaccine 2008, 26, 2100–2110. [Google Scholar] [CrossRef]
- Rosenthal, K.S.; Zimmerman, D.H. Vaccines: All things considered. Clin. Vaccine Immunol. 2006, 13, 821–829. [Google Scholar] [CrossRef]
- Best, S.R.; Peng, S.; Juang, C.M.; Hung, C.F.; Hannaman, D.; Saunders, J.R.; Wu, T.C.; Pai, S.I. Administration of HPV DNA vaccine via electroporation elicits the strongest CD8+ T cell immune responses compared to intramuscular injection and intradermal gene gun delivery. Vaccine 2009, 27, 5450–5459. [Google Scholar] [CrossRef]
- Jones, S.; Evans, K.; McElwaine-Johnn, H.; Sharpe, M.; Oxford, J.; Lambkin-Williams, R.; Mant, T.; Nolan, A.; Zambon, M.; Ellis, J.; et al. DNA vaccination protects against an influenza challenge in a double-blind randomised placebo-controlled phase 1b clinical trial. Vaccine 2009, 27, 2506–2512. [Google Scholar] [CrossRef]
- Nichol, K.L.; Treanor, J.J. Vaccines for seasonal and pandemic influenza. J. Infect. Dis. 2006, 194, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Loudon, P.T.; Yager, E.J.; Lynch, D.T.; Narendran, A.; Stagnar, C.; Franchini, A.M.; Fuller, J.T.; White, P.A.; Nyuandi, J.; Wiley, C.A.; et al. GM-CSF increases mucosal and systemic immunogenicity of an H1N1 influenza DNA vaccine administered into the epidermis of non-human primates. PLoS ONE 2010, 5, e11021. [Google Scholar] [CrossRef]
- Nguyen-Hoai, T.; Hohn, O.; Vu, M.D.; Baldenhofer, G.; Sayed Ahmed, M.S.; Dörken, B.; Norley, S.; Lipp, M.; Pezzutto, A.; Westermann, J. CCL19 as an adjuvant for intradermal gene gun immunization in a Her2/neu mouse tumor model: Improved vaccine efficacy and a role for B cells as APC. Cancer Gene Ther. 2012, 19, 880–887. [Google Scholar] [CrossRef]
- Šmahel, M.; Poláková, I.; Sobotková, E.; Vajdová, E. Systemic administration of CpG oligodeoxynucleotide and levamisole as adjuvants for gene-gun-delivered antitumor DNA vaccines. Clin. Dev. Immunol. 2011. [Google Scholar] [CrossRef]
- Koday, M.T.; Leonard, J.A.; Munson, P.; Forero, A.; Koday, M.; Bratt, D.L.; Fuller, J.T.; Murnane, R.; Qin, S.; Reinhart, T.A.; et al. Multigenic DNA vaccine induces protective cross-reactive T cell responses against heterologous influenza virus in nonhuman primates. PLoS ONE 2017, 12, e0189780. [Google Scholar] [CrossRef]
- Gnjatic, S.; Altorki, N.K.; Ngtang, D.; Tu, S.M.; Kundra, V.; Ritter, G.; Old, L.J.; Logothetis, C.J.; Sharma, P. NY-ESO-1 DNA vaccine induces T-Cell responses that are suppressed by regulatory T Cells. Clin. Cancer Res. 2009, 15, 2130–2139. [Google Scholar] [CrossRef]
- Boudreau, E.F.; Josleyn, M.; Ullman, D.; Fisher, D.; Dalrymple, L.; Sellers-Myers, K.; Loudon, P.; Rusnak, J.; Rivard, R.; Schmaljohn, C.; et al. A Phase 1 clinical trial of Hantaan virus and Puumala virus M-segment DNA vaccines for hemorrhagic fever with renal syndrome. Vaccine 2012, 30, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Weniger, B.G.; Papania, M.J. Alternative vaccine delivery methods. In Vaccines, 6th ed.; Saunders: Philadelphia, PA, USA, 2012; pp. 1200–1231. ISBN 9781455700905. [Google Scholar]
- Sarno, M.J.; Blase, E.; Galindo, N.; Ramirez, R.; Schirmer, C.L.; Trujillo-Juarez, D.F. Clinical immunogenicity of measles, mumps and rubella vaccine delivered by the Injex jet injector: Comparison with standard syringe injection. Pediatr. Infect. Dis. J. 2000, 19, 839–842. [Google Scholar] [CrossRef]
- Graham, B.S.; Enama, M.E.; Nason, M.C.; Gordon, I.J.; Peel, S.A.; Ledgerwood, J.E.; Plummer, S.A.; Mascola, J.R.; Bailer, R.T.; Roederer, M.; et al. DNA Vaccine Delivered by a Needle-Free Injection Device Improves Potency of Priming for Antibody and CD8+ T-Cell Responses after rAd5 Boost in a Randomized Clinical Trial. PLoS ONE 2013, 8, e59340. [Google Scholar] [CrossRef]
- McAllister, L.; Anderson, J.; Werth, K.; Cho, I.; Copeland, K.; Le Cam Bouveret, N.; Plant, D.; Mendelman, P.M.; Cobb, D.K. Needle-free jet injection for administration of influenza vaccine: A randomised non-inferiority trial. Lancet 2014, 384, 674–681. [Google Scholar] [CrossRef]
- Hogan, N.C.; Anahtar, M.N.; Taberner, A.J.; Hunter, I.W. Delivery of immunoreactive antigen using a controllable needle-free jet injector. J. Control. Release 2017, 258, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Viegas, E.O.; Tembe, N.; Nilsson, C.; Meggi, B.; Maueia, C.; Augusto, O.; Stout, R.; Scarlatti, G.; Ferrari, G.; Earl, P.L.; et al. Intradermal HIV-1 DNA Immunization Using Needle-Free Zetajet Injection Followed by HIV-Modified Vaccinia Virus Ankara Vaccination Is Safe and Immunogenic in Mozambican Young Adults: A Phase i Randomized Controlled Trial. AIDS Res. Hum. Retrovir. 2018, 34, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, H.M.; Day, S.; McFarlane, L.R.; Fleck, S.; Miller, A.; Cole, T.; Sousa-Santos, N.; Cope, A.; Cizmeci, D.; Tolazzi, M.; et al. Combined Skin and Muscle DNA Priming Provides Enhanced Humoral Responses to a Human Immunodeficency Virus Type 1 Clade C Envelope Vaccine. Hum. Gene Ther. 2018, 29, 1011–1028. [Google Scholar] [CrossRef]
- Greenberg, R.S.; Maxwell, L.G.; Zahurak, M.; Yaster, M. Preanesthetic medication of children with midazolam using the biojector jet injector. Anesthesiology 1995, 83, 264–269. [Google Scholar] [CrossRef]
- Baer, C.L.; Bennett, W.M.; Folwick, D.A.; Erickson, R.S. Effectiveness of a jet injection system in administering morphine and heparin to healthy adults. Am. J. Crit. Care 1996, 5, 42. [Google Scholar] [CrossRef]
- Zsigmond, E.K.; Darby, P.; Koenig, H.M.; Goll, E.V. A new route, jet injection of lidocaine for skin wheal for painless intravenous catheterization. Int. J. Clin. Pharmacol. Ther. 1999, 37, 90. [Google Scholar]
- Ake, J.A.; Schuetz, A.; Pegu, P.; Wieczorek, L.; Eller, M.A.; Kibuuka, H.; Sawe, F.; Maboko, L.; Polonis, V.; Karasavva, N.; et al. Safety and immunogenicity of PENNVAX-G DNA prime administered by biojector 2000 or CELLECTRA electroporation device with modified vaccinia Ankara-CMDR boost. J. Infect. Dis. 2017, 216, 1080–1090. [Google Scholar] [CrossRef]
- Harris, M.; Joy, R.; Larsen, G.; Valyi, M.; Walker, E.; Frick, L.W.; Palmatier, R.M.; Wring, S.A.; Montaner, J.S.G. Enfuvirtide plasma levels and injection site reactions using a needle-free gas-powered injection system (Biojector). AIDS 2006. [Google Scholar] [CrossRef]
- Resik, S.; Tejeda, A.; Sutter, R.W.; Diaz, M.; Sarmiento, L.; Alemañi, N.; Garcia, G.; Fonseca, M.; Hung, L.H.; Kahn, A.L.; et al. Priming after a fractional dose of inactivated poliovirus vaccine. N. Engl. J. Med. 2013, 368, 416–424. [Google Scholar] [CrossRef]
- Mohammed, A.J.; AlAwaidy, S.; Bawikar, S.; Kurup, P.J.; Elamir, E.; Shaban, M.M.A.; Sharif, S.M.; Van Der Avoort, H.G.A.M.; Pallansch, M.A.; Malankar, P.; et al. Fractional doses of inactivated poliovirus vaccine in Oman. N. Engl. J. Med. 2010, 362, 2351–2359. [Google Scholar] [CrossRef]
- Resik, S.; Tejeda, A.; Lago, P.M.; Diaz, M.; Carmenates, A.; Sarmiento, L.; Alemañi, N.; Galindo, B.; Burton, A.; Friede, M.; et al. Randomized Controlled Clinical Trial of Fractional Doses of Inactivated Poliovirus Vaccine Administered Intradermally by Needle-Free Device in Cuba. J. Infect. Dis. 2010, 201, 1344–1352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garg, T. An evolutionary approachs in development of needle free injection technologies. Int. J. Pharm. Pharm. Sci. 2012, 4, 590–596. [Google Scholar]
- Palermo, P.M.; Bettinger, G.; Watts, D.M. Evaluation of a needle-free vaccine delivery device for vaccinating rats with rift valley fever vaccine candidates. Int. J. Vet. Sci. 2018, 7, 140–144. [Google Scholar]
- Williamson, D.E.; Marshall, J.R. Intradermal Injection Device 2018. U.S. Patent 10,045,911, 14 August 2018. [Google Scholar]
- Chang, C.; Sun, J.; Hayashi, H.; Suzuki, A.; Sakaguchi, Y.; Miyazaki, H.; Nishikawa, T.; Nakagami, H.; Yamashita, K.; Kaneda, Y. Stable Immune Response Induced by Intradermal DNA Vaccination by a Novel Needleless Pyro-Drive Jet Injector. AAPS PharmSciTech 2019, 21, 19. [Google Scholar] [CrossRef]
- Bavdekar, A.; Oswal, J.; Ramanan, P.V.; Aundhkar, C.; Venugopal, P.; Kapse, D.; Miller, T.; McGray, S.; Zehrung, D.; Kulkarni, P.S.; et al. Immunogenicity and safety of measles-mumps-rubella vaccine delivered by disposable-syringe jet injector in India: A randomized, parallel group, non-inferiority trial. Vaccine 2018, 36, 1220–1226. [Google Scholar] [CrossRef]
- Bavdekar, A.; Malshe, N.; Ravichandran, L.; Sapru, A.; Kawade, A.; Lalwani, S.; Palkar, S.; Hanumante, N.; Gunale, B.; Kapse, D.; et al. Clinical study of safety and immunogenicity of pentavalent DTP-HB-Hib vaccine administered by disposable-syringe jet injector in India. Contemp. Clin. Trials Commun. 2019, 14, 100321. [Google Scholar] [CrossRef]
- Hannaman, D. Electroporation based TriGrid™ delivery system (TDS) for DNA vaccine administration. In Gene Vaccines; Springer: Vienna, Austria, 2014; ISBN 9783709104392. [Google Scholar]
- Ichor Medical Systems, Inc. Ichor Medical Systems’ TriGrid® to be Used with a COVID-19 DNA Vaccine in Development by the Naval Medical Research Center. Businesswire 2020. Available online: https://www.businesswire.com/news/home/20200427005071/en/Ichor-Medical-Systems%E2%80%99-TriGrid%C2%AE-COVID-19-DNA-Vaccine (accessed on 27 April 2020).
- Viegas, E.O.; Kroidl, A.; Munseri, P.J.; Missanga, M.; Nilsson, C.; Tembe, N.; Bauer, A.; Joachim, A.; Joseph, S.; Mann, P.; et al. Optimizing the immunogenicity of HIV prime-boost DNA-MVA-rgp140/GLA vaccines in a phase II randomized factorial trial design. PLoS ONE 2018, 13, e0206838. [Google Scholar] [CrossRef]
- Hasegawa, T.; Nakagami, H.; Akiyama, Y.; Nishijima, S. Fundamental study on gene transfer utilizing magnetic force and jet injector. Prog. Supercond. Cryog. 2017, 19, 9–12. [Google Scholar] [CrossRef]
- Mitragotri, S. Current status and future prospects of needle-free liquid jet injectors. Nat. Rev. Drug Discov. 2006, 5, 543–548. [Google Scholar] [CrossRef]
- Mohizin, A.; Kim, J.K. Current engineering and clinical aspects of needle-free injectors: A review. J. Mech. Sci. Technol. 2018, 32, 5737–5747. [Google Scholar] [CrossRef]
- Weiss, R.; Hessenberger, M.; Kitzmüller, S.; Bach, D.; Weinberger, E.E.; Krautgartner, W.D.; Hauser-Kronberger, C.; Malissen, B.; Boehler, C.; Kalia, Y.N.; et al. Transcutaneous vaccination via laser microporation. J. Control. Release 2012, 162, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Bay, C.; Lerche, C.M.; Ferrick, B.; Philipsen, P.A.; Togsverd-Bo, K.; Haedersdal, M. Comparison of physical pretreatment regimens to Enhance Protoporphyrin IX uptake in photodynamic therapy: A randomized clinical trial. JAMA Dermatol. 2017, 153, 270–278. [Google Scholar] [CrossRef]
- Kashiwagi, S. Laser adjuvant for vaccination. FASEB J. 2020, 34, 3485–3500. [Google Scholar] [CrossRef]
- Chen, X.; Shah, D.; Kositratna, G.; Manstein, D.; Anderson, R.R.; Wu, M.X. Facilitation of transcutaneous drug delivery and vaccine immunization by a safe laser technology. J. Control. Release 2012, 159, 43–51. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, H.; Park, Y.; Kong, W.H.; Lee, S.W.; Kwok, S.J.J.; Hahn, S.K.; Yun, S.H. Noninvasive Transdermal Vaccination Using Hyaluronan Nanocarriers and Laser Adjuvant. Adv. Funct. Mater. 2016, 26, 2512–2522. [Google Scholar] [CrossRef]
- Kashiwagi, S.; Brauns BS, T. Classification of Laser Vaccine Adjuvants. J. Vaccines Vaccin. 2016. [Google Scholar] [CrossRef]
- Chen, X.; Kim, P.; Farinelli, B.; Doukas, A.; Yun, S.H.; Gelfand, J.A.; Anderson, R.R.; Wu, M.X. A novel laser vaccine adjuvant increases the motility of antigen presenting cells. PLoS ONE 2010, 5, e13776. [Google Scholar] [CrossRef]
- Kimizuka, Y.; Katagiri, W.; Locascio, J.J.; Shigeta, A.; Sasaki, Y.; Shibata, M.; Morse, K.; Sîrbulescu, R.F.; Miyatake, M.; Reeves, P.; et al. Brief Exposure of Skin to Near-Infrared Laser Modulates Mast Cell Function and Augments the Immune Response. J. Immunol. 2018, 201, 3587–3603. [Google Scholar] [CrossRef]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016, 23, 564–578. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Matsuo, K.; Ishii, Y.; Quan, Y.S.; Kamiyama, F.; Mukai, Y.; Yoshioka, Y.; Okada, N.; Nakagawa, S. Transcutaneous vaccination using a hydrogel patch induces effective immune responses to tetanus and diphtheria toxoid in hairless rat. J. Control. Release 2011, 149, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Hirobe, S.; Matsuo, K.; Quan, Y.S.; Kamiyama, F.; Morito, H.; Asada, H.; Takaya, Y.; Mukai, Y.; Okada, N.; Nakagawa, S. Clinical study of transcutaneous vaccination using a hydrogel patch for tetanus and diphtheria. Vaccine 2012, 30, 1847–1854. [Google Scholar] [CrossRef]
- Dey, S.; Mazumder, B.; Patel, J.R. Enhanced Percutaneous Permeability of Acyclovir by DMSO from Topical Gel Formulation. Int. J. Pharm. Sci. Drug Res. 2009, 1, 13–18. [Google Scholar] [CrossRef]
- Mohammadi-Samani, S.; Jamshidzadeh, A.; Montaseri, H.; Rangbar-Zahedani, M.; Kianrad, R. The effects of some permeability enhancers on the percutaneous absorption of lidocaine. Pak. J. Pharm. Sci. 2010, 23, 83–86. [Google Scholar]
- Zhang, Y.; Li, J.; Liu, C.Y.; Zhou, X.K.; Qiu, J.; Zhang, Y.B.; Huang, N.Y.; Li, Y.; Chen, X.J.; Li, X.L.; et al. A novel transdermal plasmid-dimethylsulfoxide delivery technique for treatment of psoriasis. Dermatology 2010, 221, 84–92. [Google Scholar] [CrossRef]
- Southwell, D.; Barry, B.W. Penetration enhancers for human skin: Mode of action of 2-pyrrolidone and dimethylformamide on partition and diffusion of model compounds water, n-alcohols, and caffeine. J. Investig. Dermatol. 1983, 80, 507–514. [Google Scholar] [CrossRef]
- Stoughton, R.R.; Mcclure, W.O. Azone®: A new non-toxic enhancer of cu’taneous penetration. Drug Dev. Ind. Pharm. 1983, 9, 725–744. [Google Scholar] [CrossRef]
- Haq, A.; Michniak-Kohn, B. Effects of solvents and penetration enhancers on transdermal delivery of thymoquinone: Permeability and skin deposition study. Drug Deliv. 2018, 25, 1943–1949. [Google Scholar] [CrossRef]
- Akrill, P.; Cocker, J.; Dixon, S. Dermal exposure to aqueous solutions of N-methyl pyrrolidone. Toxicol. Lett. 2002, 134, 265–269. [Google Scholar] [CrossRef]
- Keener, S.A.; Wrbitzky, R.; Bader, M. Human volunteer study on the influence of exposure duration and dilution of dermally applied N-methyl-2-pyrrolidone (NMP) on the urinary elimination of NMP metabolites. Int. Arch. Occup. Environ. Health 2007, 80, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, F.H.W.; Coenraads, P.J.; Kardaun, S.H. Toxic hygroscopic contact reaction to N-methyl-2-pyrrolidone. Contact Dermat. 2001, 45, 303–304. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.F.; Wang, Z.X.; Xiang, S.J.; Chen, H.J.; Shen, Q.; Liu, L.; Wu, W.F.; Cao, S.W.; Wang, Z.W.; Yang, Z.J.; et al. Mechanisms of white mustard seed (Sinapis alba L.) volatile oils as transdermal penetration enhancers. Fitoterapia 2019, 138, 104195. [Google Scholar] [CrossRef]
- Tamayo, I.; Gamazo, C.; de Souza Rebouças, J.; Irache, J.M. Topical immunization using a nanoemulsion containing bacterial membrane antigens. J. Drug Deliv. Sci. Technol. 2017, 42, 207–214. [Google Scholar] [CrossRef]
- Li, N.; Peng, L.H.; Chen, X.; Nakagawa, S.; Gao, J.Q. Effective transcutaneous immunization by antigen-loaded flexible liposome in vivo. Int. J. Nanomed. 2011, 6, 3241. [Google Scholar]
- Jin, S.E.; Kim, C.K. Charge-mediated topical delivery of plasmid DNA with cationic lipid nanoparticles to the skin. Colloids Surfaces B Biointerfaces 2014, 116, 582–590. [Google Scholar] [CrossRef]
- Asadian-Birjand, M.; Bergueiro, J.; Rancan, F.; Cuggino, J.C.; Mutihac, R.C.; Achazi, K.; Dernedde, J.; Blume-Peytayi, U.; Vogt, A.; Calderón, M. Engineering thermoresponsive polyether-based nanogels for temperature dependent skin penetration. Polym. Chem. 2015, 6, 5827–5831. [Google Scholar] [CrossRef]
- Lai, J.C.Y.; Cheng, W.K.; Hopkins, P.D.; Komba, M.; Carlow, D.A.; Dutz, J.P. Topical Adjuvant Application during Subcutaneous Vaccination Promotes Resident Memory T Cell Generation. J. Immunol. 2019, 203, 2443–2450. [Google Scholar] [CrossRef]
- Van Kampen, K.R.; Shi, Z.; Gao, P.; Zhang, J.; Foster, K.W.; Chen, D.T.; Marks, D.; Elmets, C.A.; Tang, D.C.C. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine 2005, 23, 1029–1036. [Google Scholar] [CrossRef]
- Andrews, S.N.; Zarnitsyn, V.; Bondy, B.; Prausnitz, M.R. Optimization of microdermabrasion for controlled removal of stratum corneum. Int. J. Pharm. 2011, 407, 95–104. [Google Scholar] [CrossRef][Green Version]
- Gill, H.S.; Andrews, S.N.; Sakthivel, S.K.; Fedanov, A.; Williams, I.R.; Garber, D.A.; Priddy, F.H.; Yellin, S.; Feinberg, M.B.; Staprans, S.I.; et al. Selective removal of stratum corneum by microdermabrasion to increase skin permeability. Eur. J. Pharm. Sci. 2009, 38, 95–103. [Google Scholar] [CrossRef]
- Routhu, N.K.; Gangadhara, S.; Cheedarla, N.; Shiferaw, A.; Rahman, S.A.; Sahoo, A.; Shi, P.-Y.; Menachery, V.D.; Floyd, K.; Fischinger, S.; et al. Modified Vaccinia Ankara Based SARS-CoV-2 Vaccine Expressing Full-Length Spike Induces Strong Neutralizing Antibody Response. bioRxiv 2020. [Google Scholar] [CrossRef]
- Tadros, A.R.; Romanyuk, A.; Miller, I.C.; Santiago, A.; Noel, R.K.; O’Farrell, L.; Kwong, G.A.; Prausnitz, M.R. STAR particles for enhanced topical drug and vaccine delivery. Nat. Med. 2020, 26, 341–347. [Google Scholar] [CrossRef]
- Schulze, K.; Ebensen, T.; Riese, P.; Prochnow, B.; Lehr, C.M.; Guzmán, C.A. New horizons in the development of novel needle-free immunization strategies to increase vaccination efficacy. Curr. Top. Microbiol. Immunol. 2016. [Google Scholar] [CrossRef]
- Scheuplein, R.J. Mechanism of percutaneous absorption. II. Transient diffusion and the relative importance of various routes of skin penetration. J. Investig. Dermatol. 1967. [Google Scholar] [CrossRef]
- Lademann, J.; Richter, H.; Schanzer, S.; Meinke, M.; Darvin, M.; Schleusener, J.; Carrer, V.; Breuckmann, P.; Patzelt, A. Importance of the follicular penetration path for drug delivery by means of nanocarriers. Dermatologist 2019, 70, 185–192. [Google Scholar]
- Fan, H.; Lin, Q.; Morrissey, G.R.; Khavari, P.A. Immunization via hair follicles by topical application of naked DNA to normal skin. Nat. Biotechnol. 1999, 17, 870–872. [Google Scholar] [CrossRef]
- Mahe, B.; Vogt, A.; Liard, C.; Duffy, D.; Abadie, V.; Bonduelle, O.; Boissonnas, A.; Sterry, W.; Verrier, B.; Blume-Peytavi, U.; et al. Nanoparticle-based targeting of vaccine compounds to skin antigen-presenting cells by hair follicles and their transport in mice. J. Investig. Dermatol. 2009, 129, 1156–1164. [Google Scholar] [CrossRef]
- Vogt, A.; Combadiere, B.; Hadam, S.; Stieler, K.M.; Lademann, J.; Schaefer, H.; Autran, B.; Sterry, W.; Blume-Peytavi, U. 40 nm, but not 750 or 1500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. J. Investig. Dermatol. 2006, 126, 1316–1322. [Google Scholar] [CrossRef]
- Combadière, B.; Vogt, A.; Mahé, B.; Costagliola, D.; Hadam, S.; Bonduelle, O.; Sterry, W.; Staszewski, S.; Schaefer, H.; van der Werf, S.; et al. Preferential amplification of CD8 effector-T cells after transcutaneous application of an inactivated influenza vaccine: A randomized phase I trial. PLoS ONE 2010, 5, e10818. [Google Scholar] [CrossRef]
- Haidari, G.; Cope, A.; Miller, A.; Venables, S.; Yan, C.; Ridgers, H.; Reijonen, K.; Hannaman, D.; Spentzou, A.; Hayes, P.; et al. Combined skin and muscle vaccination differentially impact the quality of effector T cell functions: The CUTHIVAC-001 randomized trial. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Vogt, A.; Mahé, B.; Costagliola, D.; Bonduelle, O.; Hadam, S.; Schaefer, G.; Schaefer, H.; Katlama, C.; Sterry, W.; Autran, B.; et al. Transcutaneous Anti-Influenza Vaccination Promotes Both CD4 and CD8 T Cell Immune Responses in Humans. J. Immunol. 2008, 180, 1482–1489. [Google Scholar] [CrossRef]
- Mittal, A.; Schulze, K.; Ebensen, T.; Weissmann, S.; Hansen, S.; Guzmán, C.A.; Lehr, C.M. Inverse micellar sugar glass (IMSG) nanoparticles for transfollicular vaccination. J. Control. Release 2015, 206, 140–152. [Google Scholar] [CrossRef]
- Mittal, A.; Raber, A.S.; Schaefer, U.F.; Weissmann, S.; Ebensen, T.; Schulze, K.; Guzmán, C.A.; Lehr, C.M.; Hansen, S. Non-invasive delivery of nanoparticles to hair follicles: A perspective for transcutaneous immunization. Vaccine 2013, 31, 3442–3451. [Google Scholar] [CrossRef]
- Mittal, A.; Schulze, K.; Ebensen, T.; Weißmann, S.; Hansen, S.; Lehr, C.M.; Guzmán, C.A. Efficient nanoparticle-mediated needle-free transcutaneous vaccination via hair follicles requires adjuvantation. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 147–154. [Google Scholar] [CrossRef]
- Pollard, H.; Remy, J.S.; Loussouarn, G.; Demolombe, S.; Behr, J.P.; Escande, D. Polyethylenimine but not cationic lipids promotes transgene delivery to the nucleus in mammalian cells. J. Biol. Chem. 1998, 273, 7507–7511. [Google Scholar] [CrossRef]
- Denet, A.R.; Vanbever, R.; Préat, V. Skin electroporation for transdermal and topical delivery. Adv. Drug Deliv. Rev. 2004, 56, 659–674. [Google Scholar] [CrossRef]
- Wolff, J.A.; Dowty, M.E.; Jiao, S.; Repetto, G.; Berg, R.K.; Ludtke, J.J.; Williams, P.; Slautterback, D.B. Expression of naked plasmids by cultured myotubes and entry of plasmids into T tubules and caveolae of mammalian skeletal muscle. J. Cell Sci. 1992, 103, 1249–1259. [Google Scholar]
- Hirao, L.A.; Wu, L.; Khan, A.S.; Satishchandran, A.; Draghia-Akli, R.; Weiner, D.B. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine 2008, 26, 440–448. [Google Scholar] [CrossRef]
- Dolter, K.E.; Evans, C.F.; Ellefsen, B.; Song, J.; Boente-Carrera, M.; Vittorino, R.; Rosenberg, T.J.; Hannaman, D.; Vasan, S. Immunogenicity, safety, biodistribution and persistence of ADVAX, a prophylactic DNA vaccine for HIV-1, delivered by in vivo electroporation. Vaccine 2011, 29, 795–803. [Google Scholar] [CrossRef]
- Martinon, F.; Kaldma, K.; Sikut, R.; Çulina, S.; Romain, G.; Tuomela, M.; Adojaan, M.; Männik, A.; Toots, U.; Kivisild, T.; et al. Persistent immune responses induced by a human immunodeficiency virus dna vaccine delivered in association with electroporation in the skin of nonhuman primates. Hum. Gene Ther. 2009, 20, 1291–1307. [Google Scholar] [CrossRef]
- Hannaman, D.; Dupuy, L.C.; Ellefsen, B.; Schmaljohn, C.S. A Phase 1 clinical trial of a DNA vaccine for Venezuelan equine encephalitis delivered by intramuscular or intradermal electroporation. Vaccine 2016, 34, 3607–3612. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Electroporation, DNA. Available online: https://www.clinicaltrials.gov/ct2/results?cond=&term=electroporation+dna&cntry=&state=&city=&dist= (accessed on 25 June 2020).
- Beckett, C.G.; Tjaden, J.; Burgess, T.; Danko, J.R.; Tamminga, C.; Simmons, M.; Wu, S.J.; Sun, P.; Kochel, T.; Raviprakash, K.; et al. Evaluation of a prototype dengue-1 DNA vaccine in a Phase 1 clinical trial. Vaccine 2011, 29, 960–968. [Google Scholar] [CrossRef]
- Cashman, K.A.; Wilkinson, E.R.; Shaia, C.I.; Facemire, P.R.; Bell, T.M.; Bearss, J.J.; Shamblin, J.D.; Wollen, S.E.; Broderick, K.E.; Sardesai, N.Y.; et al. A DNA vaccine delivered by dermal electroporation fully protects cynomolgus macaques against Lassa fever. Hum. Vaccines Immunother. 2017, 13, 2902–2911. [Google Scholar] [CrossRef]
- Jiang, J.; Banglore, P.; Cashman, K.A.; Schmaljohn, C.S.; Schultheis, K.; Pugh, H.; Nguyen, J.; Humeau, L.M.; Broderick, K.E.; Ramos, S.J. Immunogenicity of a protective intradermal DNA vaccine against lassa virus in cynomolgus macaques. Hum. Vaccines Immunother. 2019, 15, 2066–2074. [Google Scholar] [CrossRef]
- Smith, T.R.F.; Ramos, S.; Yang, M.; Gary, E.N.; Schultheis, K.; Walker, S.N.; Doan, A.; Vasquez, M. Rapid development of a synthetic DNA vaccine for COVID-19. Nat. Res. 2020. [Google Scholar] [CrossRef]
- Modjarrad, K.; Roberts, C.C.; Mills, K.T.; Castellano, A.R.; Paolino, K.; Muthumani, K.; Reuschel, E.L.; Robb, M.L.; Racine, T.; Oh, M.; et al. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: A phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect. Dis. 2019, 19, 1013–1022. [Google Scholar] [CrossRef]
- Patel, A.; Walters, J.; Reuschel, E.L.; Schultheis, K.; Parzych, E.; Gary, E.N.; Maricic, I.; Purwar, M.; Eblimit, Z.; Walker, S.N.; et al. Intradermal-delivered DNA vaccine provides anamnestic protection in a rhesus macaque SARS-CoV-2 challenge model. bioRxiv 2020. [Google Scholar] [CrossRef]
- World Health Organization. Draft of the Landscape of COVID-19 Candidate Vaccines—13 August 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Wallace, M.; Evans, B.; Woods, S.; Mogg, R.; Zhang, L.; Finnefrock, A.C.; Rabussay, D.; Fons, M.; Mallee, J.; Mehrotra, D.; et al. Tolerability of two sequential electroporation treatments using MedPulser DNA delivery system (DDS) in healthy adults. Mol. Ther. 2009, 17, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Weiland, O.; Ahlén, G.; Diepolder, H.; Jung, M.C.; Levander, S.; Fons, M.; Mathiesen, I.; Sardesai, N.Y.; Vahlne, A.; Frelin, L.; et al. Therapeutic DNA vaccination using in vivo electroporation followed by standard of care therapy in patients with genotype 1 chronic hepatitis C. Mol. Ther. 2013, 21, 1796–1805. [Google Scholar] [CrossRef]
- El-Kamary, S.S.; Billington, M.; Deitz, S.; Colby, E.; Rhinehart, H.; Wu, Y.; Blackwelder, W.; Edelman, R.; Lee, A.; King, A. Safety and tolerability of the Easy Vax clinical epidermal electroporation system in healthy adults. Mol. Ther. 2012, 20, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Arun Kumar, S.; Jhan, Y.Y.; Bishop, C.J. Engineering DNA vaccines against infectious diseases. Acta Biomater. 2018, 80, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Kutzler, M.A.; Weiner, D.B. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef]
- Verganti, S.; Berlato, D.; Blackwood, L.; Amores-Fuster, I.; Polton, G.A.; Elders, R.; Doyle, R.; Taylor, A.; Murphy, S. Use of Oncept melanoma vaccine in 69 canine oral malignant melanomas in the UK. J. Small Anim. Pract. 2017, 58, 10–16. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Erasmus, J.H.; Khandhar, A.P.; Walls, A.C.; Hemann, E.A.; O’Connor, M.A.; Murapa, P.; Archer, J.; Leventhal, S.; Fuller, J.; Lewis, T.; et al. Single-dose replicating RNA vaccine induces neutralizing antibodies against SARS-CoV-2 in nonhuman primates. bioRxiv Prepr. Serv. Biol. 2020. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Garland, M.J.; Alkilani, A.Z. Microneedle-iontophoresis combinations for enhanced transdermal drug delivery. In Drug Delivery System; Methods in Molecular Biology Book Series; Humana Press: New York, NY, USA, 2014; ISBN 9781493903627. [Google Scholar]
- Ita, K. Transdermal delivery of vaccines—Recent progress and critical issues. Biomed. Pharmacother. 2016, 83, 1080–1088. [Google Scholar] [CrossRef]
- Banga, A.K.; Bose, S.; Ghosh, T.K. Iontophoresis and electroporation: Comparisons and contrasts. Int. J. Pharm. 1999, 179, 1–19. [Google Scholar] [CrossRef]
- Guy, R.H.; Kalia, Y.N.; Delgado-Charro, M.B.; Merino, V.; López, A.; Marro, D. Iontophoresis: Electrorepulsion and electroosmosis. J. Control. Release 2000, 64, 129–132. [Google Scholar] [CrossRef]
- Xu, T.; Xu, Y.H.; Wei, M.Y.; Deng, L.H.; Wu, C. Bin In vitro study of transdermal penetration and iontophoresis of hepatitis B vaccines through rat skin. Yaoxue Xuebao 2011, 46, 713–719. [Google Scholar]
- Fukuta, T.; Oshima, Y.; Michiue, K.; Tanaka, D.; Kogure, K. Non-invasive delivery of biological macromolecular drugs into the skin by iontophoresis and its application to psoriasis treatment. J. Control. Release 2020. [Google Scholar] [CrossRef]
- Bernardi, D.S.; Bitencourt, C.; da Silveira, D.S.C.; da Cruz, E.L.C.M.; Pereira-da-Silva, M.A.; Faccioli, L.H.; Lopez, R.F.V. Effective transcutaneous immunization using a combination of iontophoresis and nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2439–2448. [Google Scholar] [CrossRef] [PubMed]
- Taveira, S.F.; De Santana, D.C.A.S.; Araújo, L.M.P.C.; Marquele-Oliveira, F.; Nomizo, A.; Lopez, R.F.V. Effect of iontophoresis on topical delivery of doxorubicin-loaded solid lipid nanoparticles. J. Biomed. Nanotechnol. 2014, 10, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Hama, S.; Ikeda, Y.; Nagasaki, Y.; Kogure, K. Anti-cancer vaccination by transdermal delivery of antigen peptide-loaded nanogels via iontophoresis. Int. J. Pharm. 2015, 483, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Kigasawa, K.; Kajimoto, K.; Nakamura, T.; Hama, S.; Kanamura, K.; Harashima, H.; Kogure, K. Noninvasive and efficient transdermal delivery of CpG-oligodeoxynucleotide for cancer immunotherapy. J. Control. Release 2011, 150, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Venuganti, V.V.K.; Saraswathy, M.; Dwivedi, C.; Kaushik, R.S.; Perumal, O.P. Topical gene silencing by iontophoretic delivery of an antisense oligonucleotide-dendrimer nanocomplex: The proof of concept in a skin cancer mouse model. Nanoscale 2015, 7, 3903–3914. [Google Scholar] [CrossRef] [PubMed]
- Loder, E.W.; Rayhill, M.; Burch, R.C. Safety Problems With a Transdermal Patch for Migraine: Lessons From the Development, Approval, and Marketing Process. Headache 2018, 58, 1639–1657. [Google Scholar] [CrossRef]
- Talbi, Y.; Campo, E.; Brulin, D.; Fourniols, J.Y. Controllable and re-usable patch for transdermal iontophoresis drug delivery. Electron. Lett. 2018, 54, 739–740. [Google Scholar] [CrossRef]
- Talbi, Y.; Brulin, D.; Campo, E.; Fourniols, J.Y. Controlled permeation of lidocaine hydrochloride using a smart drug delivery system. In Proceedings of the Proceedings of the 13th IASTED International Conference on Biomedical Engineering (BioMed), Innsbruck, Austria, 20–21 February 2017. [Google Scholar]
- Ohl, C.D.; Arora, M.; Ikink, R.; De Jong, N.; Versluis, M.; Delius, M.; Lohse, D. Sonoporation from jetting cavitation bubbles. Biophys. J. 2006, 91, 4285–4295. [Google Scholar] [CrossRef]
- Tomizawa, M.; Shinozaki, F.; Motoyoshi, Y.; Sugiyama, T.; Yamamoto, S.; Sueishi, M. Sonoporation: Gene transfer using ultrasound. World J. Methodol. 2013, 3, 39. [Google Scholar] [CrossRef]
- Kwan, J.J.; Myers, R.; Coviello, C.M.; Graham, S.M.; Shah, A.R.; Stride, E.; Carlisle, R.C.; Coussios, C.C. Ultrasound-Propelled Nanocups for Drug Delivery. Small 2015, 11, 5305–5314. [Google Scholar] [CrossRef]
- Nwokeoha, S.; Carlisle, R.; Cleveland, R.O. The Application of Clinical Lithotripter Shock Waves to RNA Nucleotide Delivery to Cells. Ultrasound Med. Biol. 2016, 42, 2478–2492. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ita, K. Recent progress in transdermal sonophoresis. Pharm. Dev. Technol. 2017, 22, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Katz, N.P.; Shapiro, D.E.; Herrmann, T.E.; Kost, J.; Custer, L.M. Rapid Onset of Cutaneous Anesthesia with EMLA Cream after Pretreatment with a New Ultrasound-Emitting Device. Anesth. Analg. 2004, 98, 371–376. [Google Scholar] [CrossRef]
- Mitragotri, S.; Kost, J. Low-frequency sonophoresis: A noninvasive method of drug delivery and diagnostics. Biotechnol. Prog. 2000, 16, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Menon, J.U.; Owen, J.; Skaripa-Koukelli, I.; Wallington, S.; Gray, M.; Mannaris, C.; Kersemans, V.; Allen, D.; Kinchesh, P.; et al. Ultrasound-mediated cavitation enhances the delivery of an EGFR-targeting liposomal formulation designed for chemo-radionuclide therapy. Theranostics 2019, 9, 5595. [Google Scholar] [CrossRef] [PubMed]
- Mullick Chowdhury, S.; Wang, T.Y.; Bachawal, S.; Devulapally, R.; Choe, J.W.; Abou Elkacem, L.; Yakub, B.K.; Wang, D.S.; Tian, L.; Paulmurugan, R.; et al. Ultrasound-guided therapeutic modulation of hepatocellular carcinoma using complementary microRNAs. J. Control. Release 2016, 238, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Kost, J.; Leong, K.; Langer, R. Ultrasound-enhanced polymer degradation and release of incorporated substances. Proc. Natl. Acad. Sci. USA 1989, 86, 7663–7666. [Google Scholar] [CrossRef]
- Myers, R.; Grundy, M.; Rowe, C.; Coviello, C.M.; Bau, L.; Erbs, P.; Foloppe, J.; Balloul, J.M.; Story, C.; Coussios, C.C.; et al. Ultrasound-mediated cavitation does not decrease the activity of small molecule, antibody or viral-based medicines. Int. J. Nanomed. 2018, 13, 337–349. [Google Scholar] [CrossRef]
- Van Blokland, A.C. Instigating and Monitoring Transdermal Drug Delivery Using Ultrasound-Mediated Cavitation. Ph.D. Thesis, University of Oxford, Oxford, UK, 2018. [Google Scholar]
- Bhatnagar, S.; Kwan, J.J.; Shah, A.R.; Coussios, C.C.; Carlisle, R.C. Exploitation of sub-micron cavitation nuclei to enhance ultrasound-mediated transdermal transport and penetration of vaccines. J. Control. Release 2016, 238, 22–30. [Google Scholar] [CrossRef]
- Tezel, A.; Paliwal, S.; Shen, Z.; Mitragotri, S. Low-frequency ultrasound as a transcutaneous immunization adjuvant. Vaccine 2005, 23, 3800–3807. [Google Scholar] [CrossRef] [PubMed]
- Teo, B.M.; Cheong-Qi Seah, B. Recent advances in ultrasound-based transdermal drug delivery. Int. J. Nanomed. 2018, 13, 7749–7763. [Google Scholar]
- Ryu, Y.C.; Kim, D.I.; Kim, S.H.; Wang, H.M.D.; Hwang, B.H. Synergistic Transdermal Delivery of Biomacromolecules Using Sonophoresis after Microneedle Treatment. Biotechnol. Bioprocess Eng. 2018, 23, 286–292. [Google Scholar] [CrossRef]
| Technique | Devices | Vaccine Target | Development Stage | Advantages/Disadvantages |
|---|---|---|---|---|
| Needle adapter | ID Adapter (West Pharmaceutical Services, Inc., USA) | Poliovirus | Commercially available | Advantages:
|
| Microinjection |
| Hepatitis B Influenza |
| Advantages:
|
| Microneedles | 0 | Hepatitis B Hepatitis C Influenza Poliovirus SARS-Cov-2 Shigellosis | Phase 1 clinical trials | Advantages:
|
| Tattooing | Multiple needle tattoo device or permanent make-up device | HPV HIV Lyme disease Melioidosis Tuberculosis | Phase 1 clinical trials | Advantages:
|
| Jet and ballistic delivery |
| Dengue Diphtheria, tetanus and pertussis Hepatitis B HPV Influenza Measles, Mumps and Rubella Poliovirus SARS-CoV-2 Rift Valley Fever virus |
| Advantages:
|
| Transfollicular | 0 | Influenza | Clinical/preclinical trials | Advantages:
|
| Thermal ablation |
| Influenza [188] |
| Advantages:
|
| Chemical enhancer | 0 | Diphtheria Tetanus | Preclinical trials | Advantages:
|
| Abrasion |
| HIV Tetanus Vaccinia |
| Advantages:
|
| Electroporation |
| Dengue Lassa Virus Hepatitis C SARS-CoV-2 |
| Advantages:
|
| Iontophoresis |
| Cancer in preclinical studies |
| Advantages:
|
| Ultrasound | 0 | 0 | Preclinical trials | Advantages:
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hettinga, J.; Carlisle, R. Vaccination into the Dermal Compartment: Techniques, Challenges, and Prospects. Vaccines 2020, 8, 534. https://doi.org/10.3390/vaccines8030534
Hettinga J, Carlisle R. Vaccination into the Dermal Compartment: Techniques, Challenges, and Prospects. Vaccines. 2020; 8(3):534. https://doi.org/10.3390/vaccines8030534
Chicago/Turabian StyleHettinga, Johanna, and Robert Carlisle. 2020. "Vaccination into the Dermal Compartment: Techniques, Challenges, and Prospects" Vaccines 8, no. 3: 534. https://doi.org/10.3390/vaccines8030534
APA StyleHettinga, J., & Carlisle, R. (2020). Vaccination into the Dermal Compartment: Techniques, Challenges, and Prospects. Vaccines, 8(3), 534. https://doi.org/10.3390/vaccines8030534





