Porcine Reproductive and Respiratory Syndrome Virus Interferes with Swine Influenza A Virus Infection of Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Newborn Pig Tracheal Epithelial Cell Line
2.2. Alveolar Macrophages
2.3. Virus Propagation, Purification and Titration
2.4. Real-Time Monitoring of SwIAV Cytopathic Effects Using Real-Time Cell Analysis
2.5. Virus Inactivation
2.6. Precision-Cut Lung Slices
2.7. Virus Infection and Stimulation of Newborn Pig Tracheal Epithelial Cells and Precision-Cut Lung Slices
2.8. Immune Gene Expression Analysis and Virus Detection by Quantitative Real-Time PCR
2.9. Western Blotting
2.10. Immunofluorescence Analyses
2.11. Statistical Analyses
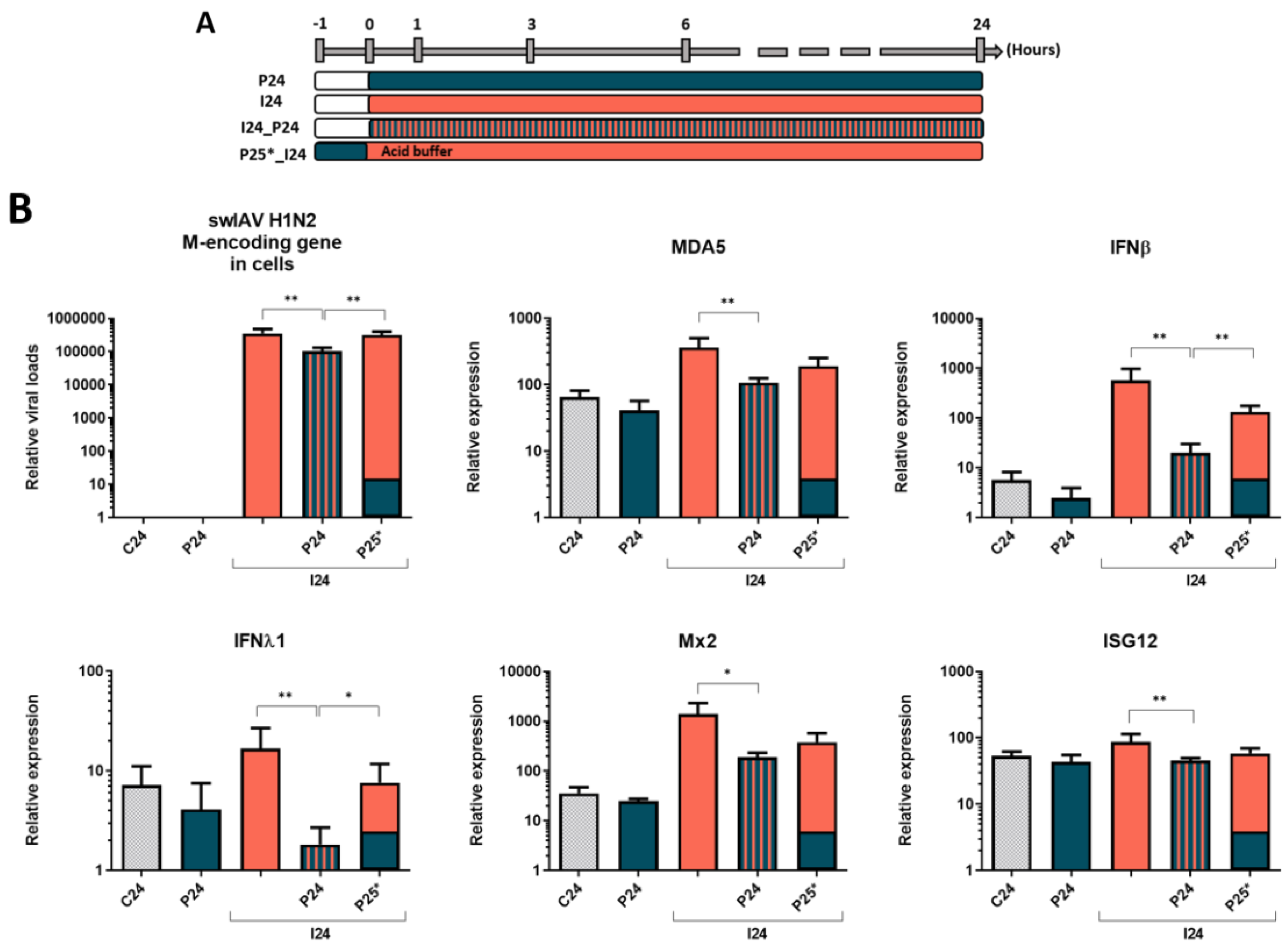
3. Results
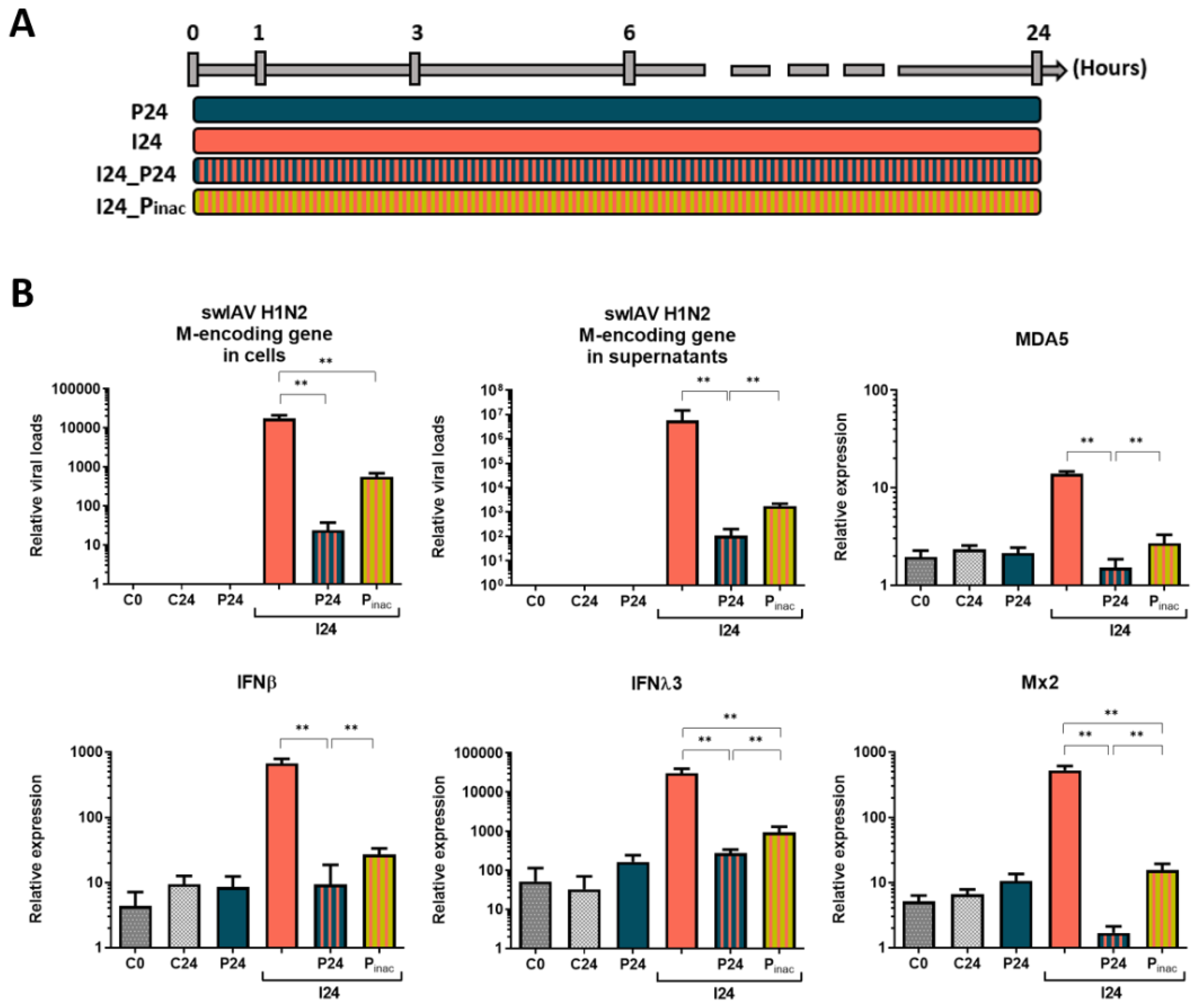
3.1. Simultaneous PRRSV-1 Contact with NPTr Epithelial Cells Strongly Decreases swIAV H1N2 Replication and Immune Gene Expression
3.2. The Effect of PRRSV-1 Is till Observed if PRRSV-1 Is Added Shortly before swIAV H1N2 to NPTr Cells
3.3. Inactivation of Non-Internalized Viruses by Acid Wash Abrogates PRRSV-1 Effect on swIAV H1N2 Infection in NPTr Cells
3.4. Inactivated PRRSV-1 still Impacts swIAV H1N2 Infection of Tracheal Epithelial Cells
3.5. PRRSV-1 Impacts swIAV H1N2 Infection in Primary Tissue Lung Slices
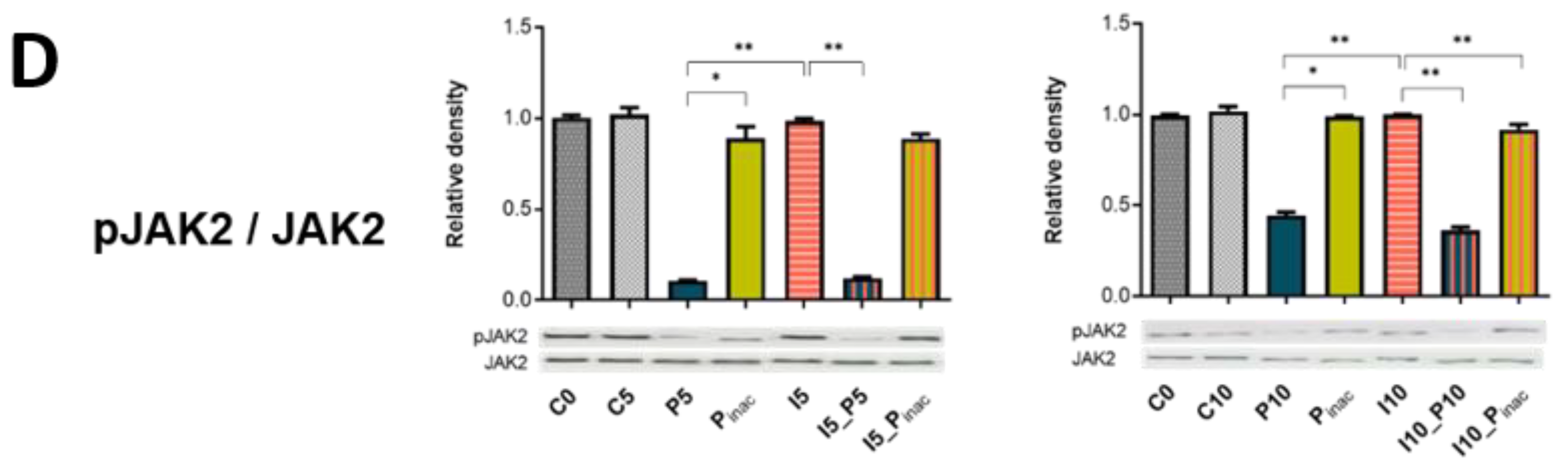
3.6. PRRSV-1 and swIAV H1N2-Induced Signaling Pathways in NPTr Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yaeger, M.; Van Alstine, W.G. Respiratory System. In Diseases of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley-Blackwell: Chichester, West Sussex UK, 2019; pp. 393–407. ISBN 978-1-119-35085-9. [Google Scholar]
- Saade, G.; Deblanc, C.; Bougon, J.; Marois-Créhan, C.; Fablet, C.; Auray, G.; Belloc, C.; Leblanc-Maridor, M.; Gagnon, C.A.; Zhu, J.; et al. Coinfections and their molecular consequences in the porcine respiratory tract. Vet. Res. 2020, 51, 80. [Google Scholar] [CrossRef] [PubMed]
- Meurens, F.; Schynts, F.; Keil, G.M.M.; Muylkens, B.; Vanderplasschen, A.; Gallego, P.; Thiry, E. Superinfection prevents recombination of the alphaherpesvirus bovine herpesvirus 1. J. Virol. 2004, 78, 3872–3879. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Goyal, S.M.; Joo, H.S. Retrospective analysis of etiologic agents associated with respiratory diseases in pigs. Can. Vet. J. 2003, 44, 735–737. [Google Scholar] [PubMed]
- Fablet, C.; Marois-Crehan, C.; Simon, G.; Grasland, B.; Jestin, A.; Kobisch, M.; Madec, F.; Rose, N. Infectious agents associated with respiratory diseases in 125 farrow-to-finish pig herds: A cross-sectional study. Vet. Microbiol. 2012, 157, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Fablet, C.; Marois, C.; Kuntz-Simon, G.; Rose, N.; Dorenlor, V.; Eono, F.; Eveno, E.; Jolly, J.P.; Le Devendec, L.; Tocqueville, V.; et al. Longitudinal study of respiratory infection patterns of breeding sows in five farrow-to-finish herds. Vet. Microbiol. 2011, 147, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Jung, J.-Y.; Kim, J.-H.; Kang, S.-C.; Hwang, E.-K.; Park, B.-K.; Kim, D.-Y.; Kim, J.-H. Epidemiological characteristics of pulmonary pneumocystosis and concurrent infections in pigs in Jeju Island, Korea. J. Vet. Sc. 2011, 12, 15. [Google Scholar] [CrossRef]
- Fablet, C.; Marois-Créhan, C.; Grasland, B.; Simon, G.; Rose, N. Factors associated with herd-level PRRSV infection and age-time to seroconversion in farrow-to-finish herds. Vet. Microbiol. 2016, 192, 10–20. [Google Scholar] [CrossRef]
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef]
- Crisci, E.; Mussa, T.; Fraile, L.; Montoya, M. Review: Influenza virus in pigs. Mol. Immunol. 2013, 55, 200–211. [Google Scholar] [CrossRef]
- Kuhn, J.H.; Lauck, M.; Bailey, A.L.; Shchetinin, A.M.; Vishnevskaya, T.V.; Bào, Y.; Ng, T.F.F.; LeBreton, M.; Schneider, B.S.; Gillis, A.; et al. Reorganization and expansion of the nidoviral family Arteriviridae. Arch. Virol. 2016, 161, 755–768. [Google Scholar] [CrossRef]
- Van Reeth, K.; Nauwynck, H.; Pensaert, M. Dual infections of feeder pigs with porcine reproductive and respiratory syndrome virus followed by porcine respiratory coronavirus or swine influenza virus: A clinical and virological study. Vet. Microbiol. 1996, 48, 325–335. [Google Scholar] [CrossRef]
- Van Reeth, K.; Nauwynck, H.; Pensaert, M. Clinical effects of experimental dual infections with porcine reproductive and respiratory syndrome virus followed by swine influenza virus in conventional and colostrum-deprived pigs. J. Vet. Med. B. Infect. Dis. Vet. Public Health 2001, 48, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Dobrescu, I.; Levast, B.; Lai, K.; Delgado-Ortega, M.; Walker, S.; Banman, S.; Townsend, H.; Simon, G.; Zhou, Y.; Gerdts, V.; et al. In vitro and ex vivo analyses of co-infections with swine influenza and porcine reproductive and respiratory syndrome viruses. Vet. Microbiol. 2014, 169, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Provost, C.; Hamonic, G.; Gagnon, C.A.; Meurens, F. Dual infections of CD163 expressing NPTr epithelial cells with influenza A virus and PRRSV. Vet. Microbiol. 2017, 207, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sharma, S.; Barua, S.; Tripathi, B.N.; Rouse, B.T. Virological and Immunological Outcomes of Coinfections. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Scalvini, A.; Losio, M.N.; Corradi, A.; Soncini, M.; Bignotti, E.; Milanesi, E.; Ajmone-Marsan, P.; Barlati, S.; Bellotti, D.; et al. Establishment and characterization of two new pig cell lines for use in virological diagnostic laboratories. J. Virol. Methods 2003, 107, 205–212. [Google Scholar] [CrossRef]
- Kojnok, J. The role of pigs in the spreading of Aujeszky’s disease among cattle and sheep. Acta. Vet. Acad. Sci. Hung. 1961, 12, 53–58. [Google Scholar]
- Thieulent, C.J.; Hue, E.S.; Fortier, C.I.; Dallemagne, P.; Zientara, S.; Munier-Lehmann, H.; Hans, A.; Fortier, G.D.; Pitel, P.-H.; Vidalain, P.-O.; et al. Screening and evaluation of antiviral compounds against Equid alpha-herpesviruses using an impedance-based cellular assay. Virology 2019, 526, 105–116. [Google Scholar] [CrossRef]
- Fang, Y.; Ye, P.; Wang, X.; Xu, X.; Reisen, W. Real-time monitoring of flavivirus induced cytopathogenesis using cell electric impedance technology. J. Virol. Meth. 2011, 173, 251–258. [Google Scholar] [CrossRef]
- Limame, R.; Wouters, A.; Pauwels, B.; Fransen, E.; Peeters, M.; Lardon, F.; De Wever, O.; Pauwels, P. Comparative analysis of dynamic cell viability, migration and invasion assessments by novel real-time technology and classic endpoint assays. PLoS ONE 2012, 7, e46536. [Google Scholar] [CrossRef]
- Mettenleiter, T.C. Glycoprotein gIII deletion mutants of pseudorabies virus are impaired in virus entry. Virology 1989, 171, 623–625. [Google Scholar] [CrossRef]
- Meurens, F.; Keil, G.M.; Muylkens, B.; Gogev, S.; Schynts, F.; Negro, S.; Wiggers, L.; Thiry, E. Interspecific recombination between two ruminant alphaherpesviruses, bovine herpesviruses 1 and 5. J. Virol. 2004, 78, 9828–9836. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Gao, P.; Kong, C.; Zhou, L.; Ge, X.; Guo, X.; Han, J.; Yang, H. The nsp2 Hypervariable Region of Porcine Reproductive and Respiratory Syndrome Virus Strain JXwn06 Is Associated with Viral Cellular Tropism to Primary Porcine Alveolar Macrophages. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Provost, C.; Jia, J.J.; Music, N.; Lévesque, C.; Lebel, M.-È.; del Castillo, J.R.; Jacques, M.; Gagnon, C.A. Identification of a new cell line permissive to porcine reproductive and respiratory syndrome virus infection and replication which is phenotypically distinct from MARC-145 cell line. Virol. J. 2012, 9, 1–14. [Google Scholar] [CrossRef][Green Version]
- Delgado-Ortega, M.; Melo, S.; Punyadarsaniya, D.; Ramé, C.; Olivier, M.; Soubieux, D.; Marc, D.; Simon, G.G.; Herrler, G.; Berri, M.; et al. Innate immune response to a H3N2 subtype swine influenza virus in newborn porcine trachea cells, alveolar macrophages, and precision-cut lung slices. Vet. Res. 2014, 45, 42. [Google Scholar] [CrossRef]
- Meurens, F.; Berri, M.; Auray, G.; Melo, S.; Levast, B.; Virlogeux-Payant, I.; Chevaleyre, C.; Gerdts, V.; Salmon, H. Early immune response following Salmonella enterica subspecies enterica serovar Typhimurium infection in porcine jejunal gut loops. Vet. Res. 2009, 40, 5. [Google Scholar] [CrossRef]
- Gourbeyre, P.; Berri, M.; Lippi, Y.; Meurens, F.; Vincent-Naulleau, S.; Laffitte, J.; Rogel-Gaillard, C.; Pinton, P.; Oswald, I.P. Pattern recognition receptors in the gut: Analysis of their expression along the intestinal tract and the crypt/villus axis. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome. Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Delgado-Ortega, M.; Melo, S.; Meurens, F. Expression of SOCS1-7 and CIS mRNA in porcine tissues. Vet. Immunol. Immunopathol. 2011, 144, 493–498. [Google Scholar] [CrossRef]
- Bruel, T.; Guibon, R.; Melo, S.; Guillén, N.; Salmon, H.; Girard-Misguich, F.; Meurens, F.; Guillen, N.; Salmon, H.; Girard-Misguich, F.; et al. Epithelial induction of porcine suppressor of cytokine signaling 2 (SOCS2) gene expression in response to Entamoeba. Histolytica. Dev. Comp. Immunol. 2010, 34, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Richt, J.A.; Lager, K.M.; Clouser, D.F.; Spackman, E.; Suarez, D.L.; Yoon, K.J. Real-time reverse transcription-polymerase chain reaction assays for the detection and differentiation of North American swine influenza viruses. J. Vet. Diagn. Investig. 2004, 16, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.; Renson, P.; Andraud, M.; Paboeuf, F.; Le Potier, M.F.; Bourry, O. Porcine reproductive and respiratory syndrome virus (PRRSv) modified-live vaccine reduces virus transmission in experimental conditions. Vaccine 2015, 33, 2493–2499. [Google Scholar] [CrossRef] [PubMed]
- Thanawongnuwech, R.; Young, T.F.; Thacker, B.; Thacker, E.L. Differential production of proinfammatory cytokines: In vitro PRRSV and Mycoplasma hyopneumoniae co-infection model. Vet. Immunol. Immunopathol. 2001, 79, 115–127. [Google Scholar] [CrossRef]
- Dufour, V.; Chevallier, S.; Cariolet, R.; Somasundaram, S.; Lefevre, F.; Jestin, A.; Albina, E. Induction of porcine cytokine mRNA expression after DNA immunization and pseudorabies virus infection. J. Interferon. Cytokine. Res. 2000, 20, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Silversides, D.W.; Music, N.; Jacques, M.; Gagnon, C.A.; Webby, R. Investigation of the species origin of the St. Jude Porcine Lung epithelial cell line (SJPL) made available to researchers. J. Virol. 2010, 84, 5454–5455. [Google Scholar] [CrossRef]
- Zhang, Q.; Yoo, D. PRRS virus receptors and their role for pathogenesis. Vet. Microbiol. 2015, 177, 229–241. [Google Scholar] [CrossRef]
- Xie, J.; Christiaens, I.; Yang, B.; Van Breedam, W.; Cui, T.; Nauwynck, H.J. Molecular cloning of porcine Siglec-3, Siglec-5 and Siglec-10, and identification of Siglec-10 as an alternative receptor for porcine reproductive and respiratory syndrome virus (PRRSV). J. Gen. Virol. 2017, 98, 2030–2042. [Google Scholar] [CrossRef]
- Hou, G.; Xue, B.; Li, L.; Nan, Y.; Zhang, L.; Li, K.; Zhao, Q.; Hiscox, J.A.; Stewart, J.P.; Wu, C.; et al. Direct Interaction Between CD163 N-Terminal Domain and MYH9 C-Terminal Domain Contributes to Porcine Reproductive and Respiratory Syndrome Virus Internalization by Permissive Cells. Front. Microbiol. 2019, 10, 1815. [Google Scholar] [CrossRef]
- Van Gorp, H.; Van Breedam, W.; Van Doorsselaere, J.; Delputte, P.L.; Nauwynck, H.J. Identification of the CD163 protein domains involved in infection of the porcine reproductive and respiratory syndrome virus. J. Virol. 2010, 84, 3101–3105. [Google Scholar] [CrossRef]
- Whitworth, K.M.; Rowland, R.R.R.; Ewen, C.L.; Trible, B.R.; Kerrigan, M.A.; Cino-Ozuna, A.G.; Samuel, M.S.; Lightner, J.E.; McLaren, D.G.; Mileham, A.J.; et al. Gene-edited pigs are protected from porcine reproductive and respiratory syndrome virus. Nat. Biotechnol. 2016, 34, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Murtaugh, M.P. Functional analysis of porcine reproductive and respiratory syndrome virus N-glycans in infection of permissive cells. Virology 2015, 477, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Delputte, P.L.; Nauwynck, H.J. Porcine arterivirus infection of alveolar macrophages is mediated by sialic acid on the virus. J. Virol. 2004, 78, 8094–8101. [Google Scholar] [CrossRef] [PubMed]
- Cássaro, C.M.; Dietrich, C.P. Distribution of sulfated mucopolysaccharides in invertebrates. J. Biol. Chem. 1977, 252, 2254–2261. [Google Scholar] [PubMed]
- Dietrich, C.P.; Sampaio, L.O.; Toledo, O.M.; Cássaro, C.M. Cell recognition and adhesiveness: A possible biological role for the sulfated mucopolysaccharides. Biochem. Biophys. Res. Commun. 1977, 75, 329–336. [Google Scholar] [CrossRef]
- Simon Davis, D.A.; Parish, C.R. Heparan Sulfate: A Ubiquitous Glycosaminoglycan with Multiple Roles in Immunity. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, X. A dual effect of porcine reproductive and respiratory syndrome virus replication on the phosphatidylinositol-3-kinase-dependent Akt pathway. Arch. Virol. 2010, 155, 571–575. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, S.; Tong, W.; Zhu, J.; Yu, H.; Zhou, Y.; Morrison, R.B.; Tong, G. Control of the PI3K/Akt pathway by porcine reproductive and respiratory syndrome virus. Arch. Virol. 2013, 158, 1227–1234. [Google Scholar] [CrossRef]
- Long, S.; Zhou, Y.; Bai, D.; Hao, W.; Zheng, B.; Xiao, S.; Fang, L. Fatty Acids Regulate Porcine Reproductive and Respiratory Syndrome Virus Infection via the AMPK-ACC1 Signaling Pathway. Viruses 2019, 11, 1145. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, C. Porcine reproductive and respiratory syndrome virus replication is suppressed by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. Virus Res. 2010, 152, 50–58. [Google Scholar] [CrossRef]
- Sur, J.H.; Cooper, V.L.; Galeota, J.A.; Hesse, R.A.; Doster, A.R.; Osorio, F.A. In vivo detection of porcine reproductive and respiratory syndrome virus RNA by in situ hybridization at different times postinfection. J. Clin. Microbiol. 1996, 34, 2280–2286. [Google Scholar] [CrossRef] [PubMed]
- Sur, J.H.; Doster, A.R.; Christian, J.S.; Galeota, J.A.; Wills, R.W.; Zimmerman, J.J.; Osorio, F.A. Porcine reproductive and respiratory syndrome virus replicates in testicular germ cells, alters spermatogenesis, and induces germ cell death by apoptosis. J. Virol. 1997, 71, 9170–9179. [Google Scholar] [CrossRef] [PubMed]
- Marjuki, H.; Gornitzky, A.; Marathe, B.M.; Ilyushina, N.A.; Aldridge, J.R.; Desai, G.; Webby, R.J.; Webster, R.G. Influenza A virus-induced early activation of ERK and PI3K mediates V-ATPase-dependent intracellular pH change required for fusion. Cell. Microbiol. 2011, 13, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chen, J.; Cheng, L.; Xu, K.; Yang, Y.; Su, X. Deficiency of HIF-1α enhances influenza A virus replication by promoting autophagy in alveolar type II epithelial cells. Emerg. Microbes. Infect. 2020, 9, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Mukaigawa, J.; Nayak, D.P. Two signals mediate nuclear localization of influenza virus (A/WSN/33) polymerase basic protein 2. J. Virol. 1991, 65, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Goraya, M.U.; Wang, S.; Munir, M.; Chen, J.-L. Induction of innate immunity and its perturbation by influenza viruses. Protein. Cell. 2015, 6, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, Y.-J. Antagonizing Interferon-Mediated Immune Response by Porcine Reproductive and Respiratory Syndrome Virus. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.-J. Antagonizing cytokine-mediated JAK-STAT signaling by porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 2017, 209, 57–65. [Google Scholar] [CrossRef]
- Patel, D.; Nan, Y.; Shen, M.; Ritthipichai, K.; Zhu, X.; Zhang, Y.-J. Porcine reproductive and respiratory syndrome virus inhibits type I interferon signaling by blocking STAT1/STAT2 nuclear translocation. J. Virol. 2010, 84, 11045–11055. [Google Scholar] [CrossRef]
- Meischel, T.; Villalon-Letelier, F.; Saunders, P.M.; Reading, P.C.; Londrigan, S.L. Influenza A virus interactions with macrophages: Lessons from epithelial cells. Cell. Microbiol. 2020, 22, e13170. [Google Scholar] [CrossRef]


| Primer Abbreviation and Full Name | Primer Sequences: Sense (S) and Anti-Sense (AS) | Amplicon Size (bp) | Annealing Temperature (°C) | Accession Number or PMIDs |
|---|---|---|---|---|
| Viruses | ||||
| SwIAV H1N2 M-encoding gene | (S) AGATGAGTCTTCTAACCGAGGTCG (AS) TGCAAAAACATCTTCAAGTCTCTG (P) (6FAM)-TCAGGCCCCCTCAAAGCCGA-(TAM) | 100 | 60 | 15460317 |
| PRRSV Finistère ORF5 | (S) AGAACCAGCGCCAATTCAGA (AS) TCTTTTTCGCCTGTCCTCCC (P) (HEX)-AAACACAGCTCCAATGGGGAATGGC-(TAM) | 135 | 60 | 28241868 |
| ADV gB-encoding gene | (S) GCGGGTACGTGTACTACGAG (AS) GAGGCCCTGGAAGAAGTTGG (P) (6FAM)-ACTACAGCTACGTGCGCATGGTGGAG-(TAM) | 287 | 63 | NC_006151 |
| Reference genes | ||||
| B2MI Beta-2-microgobulin | (S) CAAGATAGTTAAGTGGGATCGAGAC (AS) TGGTAACATCAATACGATTTCTGA | 161 | 58 | 17697375 |
| HPRT1 Hypoxanthine phosphoribosyltransferase 1 | (S) GGACTTGAATCATGTTTGTG (AS) CAGATGTTTCCAAACTCAAC | 91 | 60 | 17697375 |
| GAPDH Glyceraldehyde 3-phosphate dehydrogenase | (S) CTTCACGACCATGGAGAAGG (AS) CCAAGCAGTTGGTGGTACAG | 170 | 63 | AF017079 |
| Pattern Recognition Receptors | ||||
| MDA5 Melanoma differentiation-associated protein 5 | (S) AGCCCACCATCTGATTGGAG (AS) TTCTTCTGCCACCGTGGTAG | 133 | 60 | MF358967.1 |
| RIGI Retinoic acid-inducible gene I | (S) CGACATTGCTCAGTGCAATC (AS) TCAGCGTTAGCAGTCAGAAG | 126 | 60 | NM_213804 |
| TLR2 Toll-like receptor 2 | (S) ACGGACTGTGGTGCATGAAG (AS) GGACACGAAAGCGTCATAGC | 101 | 62 | NM_213761.1 |
| TLR3 Toll-like receptor 3 | (S) GACCTCCCGGCAAATATAAC (AS) GGGAGACTTTGGCACAATTC | 155 | 60 | NM_001097444 |
| TLR4 Toll-like receptor 4 | (S) TGTGCGTGTGAACACCAGAC (AS) AGGTGGCGTTCCTGAAACTC | 136 | 62 | NM_001293316.1 |
| TLR6 Toll-like receptor 6 | (S) TCCCAGGATCAAGGTTCTTG (AS) GAGCAGAGTCCCCTTATAAC | 370 | 60 | NM_213760.2 |
| TLR7 Toll-like receptor 7 | (S) CGGTGTTTGTGATGACAGAC (AS) AACTCCCACAGAGCCTCTTC | 174 | 62 | NM_001097434.1 |
| TLR8 Toll-like receptor 8 | (S) CACATTTGCCCGGTATCAAG (AS) TGTGTCACTCCTGCTATTCG | 145 | 58 | NM_214187.1 |
| TLR9 Toll-like receptor 9 | (S) GGCCTTCAGCTTCACCTTGG (AS) GGTCAGCGGCACAAACTGAG | 151 | 64 | NM_213958.1 |
| TLR10 Toll-like receptor 10 | (S) CTTTGATCTGCCCTGGTATCTCA (AS) CATGTCCGTGCCCACTGAC | 51 | 60 | AB_208699,1 |
| Interferons | ||||
| IFNα Interferon alpha | (S) GGCTCTGGTGCATGAGATGC (AS) CAGCCAGGATGGAGTCCTCC | 197 | 62 | JQ839262.1 |
| IFNβ Interferon beta | (S) AGTTGCCTGGGACTCCTCAA (AS) CCTCAGGGACCTCAAAGTTCAT | 70 | 60 | 21645029 |
| IFNλ1 Swine interferon lamba 1 | (S) GAGGCTGAGCTAGACTTGAC (AS) CCTGAAGTTCGACGTGGATG | 115 | 60 | NM_001142837 |
| IFNλ3 Swine interferon lamba 3 | (S) CCTGGAAGCCTCTGTCATGT (AS) TCTCCACTGGCGACACATT | 72 | 60 | 29677213 |
| Interferon-stimulated genes | ||||
| PKR Protein kinase R | (S) CACATCGGCTTCAGAGTCAG (AS) GGGCGAGGTAAATGTAGGTG | 166 | 61 | NM_214319 |
| OAS1 2’-5’-Oligoadenylate Synthetase 1 | (S) CCCTGTTCGCGTCTCCAAAG (AS) GCGGGCAGGACATCAAACTC | 303 | 64 | NM_214303 |
| MX1 Myxovirus resistance protein 1 | (S) AGTGTCGGCTGTTTACCAAG (AS) TTCACAAACCCTGGCAACTC | 151 | 60 | NM_214061 |
| MX2 Myxovirus resistance protein 2 | (S) CCGACTTCAGTTCAGGATGG (AS) ACAGGAGACGGTCCGTTTAC | 156 | 62 | AB258432 |
| ISG12 Interferon-stimulated gene 12 | (S) GTACTTCCTCCCTGATAGG (AS) ACAGCTACAGAAGCCTTG | 76 | 54 | NM_001198921.1 |
| ISG15 Interferon stimulated gene 15 | (S) GATCGGTGTGCCTGCCTTC (AS) CGTTGCTGCGACCCTTGT | 176 | 58 | NM_001128469.3 |
| Targeted Protein | Specific Antibody |
|---|---|
| -Phospho-AKT | -Rabbit polyclonal anti-phospho-AKT (Ser473) #9271 (Ozyme) |
| -AKT | -Rabbit monoclonal anti-AKT (11E7) #4685 (Ozyme) |
| -Phospho-AMPK -AMPK | -Rabbit monoclonal anti-phospho-AMPK alpha (Thr172) (40H9) #2535L (Ozyme) -Rabbit polyclonal anti-Total AMPK #2532L (Ozyme) |
| -Phospho-ERK1/2 -ERK2 | -Rabbit monoclonal anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (D13.14.4E) #4370 (Ozyme) -Rabbit polyclonal anti-ERK2 (GTX27948) (Tebu-bio) |
| -Phospho-JAK2 -JAK2 | -Rabbit polyclonal anti-phospho-JAK2 (Tyr1007/1008) #3771 (Ozyme) -Rabbit monoclonal anti-JAK2 (D2E12) #3230 (Ozyme) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saade, G.; Ménard, D.; Hervet, C.; Renson, P.; Hue, E.; Zhu, J.; Dubreil, L.; Paillot, R.; Pronost, S.; Bourry, O.; et al. Porcine Reproductive and Respiratory Syndrome Virus Interferes with Swine Influenza A Virus Infection of Epithelial Cells. Vaccines 2020, 8, 508. https://doi.org/10.3390/vaccines8030508
Saade G, Ménard D, Hervet C, Renson P, Hue E, Zhu J, Dubreil L, Paillot R, Pronost S, Bourry O, et al. Porcine Reproductive and Respiratory Syndrome Virus Interferes with Swine Influenza A Virus Infection of Epithelial Cells. Vaccines. 2020; 8(3):508. https://doi.org/10.3390/vaccines8030508
Chicago/Turabian StyleSaade, Georges, Déborah Ménard, Caroline Hervet, Patricia Renson, Erika Hue, Jianzhong Zhu, Laurence Dubreil, Romain Paillot, Stéphane Pronost, Olivier Bourry, and et al. 2020. "Porcine Reproductive and Respiratory Syndrome Virus Interferes with Swine Influenza A Virus Infection of Epithelial Cells" Vaccines 8, no. 3: 508. https://doi.org/10.3390/vaccines8030508
APA StyleSaade, G., Ménard, D., Hervet, C., Renson, P., Hue, E., Zhu, J., Dubreil, L., Paillot, R., Pronost, S., Bourry, O., Simon, G., Dupont, J., Bertho, N., & Meurens, F. (2020). Porcine Reproductive and Respiratory Syndrome Virus Interferes with Swine Influenza A Virus Infection of Epithelial Cells. Vaccines, 8(3), 508. https://doi.org/10.3390/vaccines8030508











