Kinetics of Anti-Hepatitis B Surface Antigen Titers in Nurse Students after a Two-Year Follow-Up
Abstract
1. Introduction
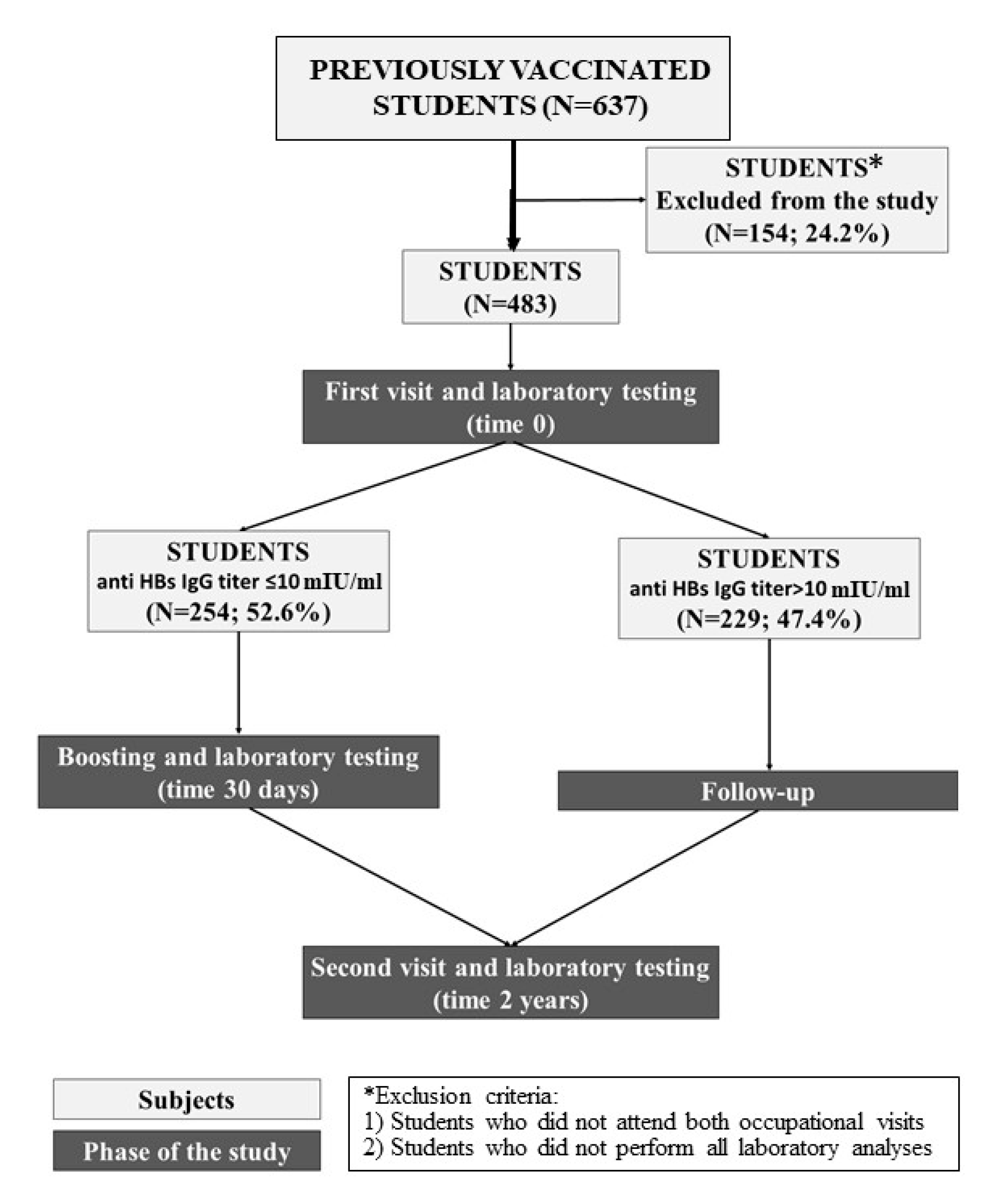
2. Materials and Methods
- Those who attended both occupational visits in the first and third years.
- Those who underwent all laboratory analyses (in the first year, after HBV booster if required, and in the third year).
2.1. Serological Tests
2.2. Statistical Analysis
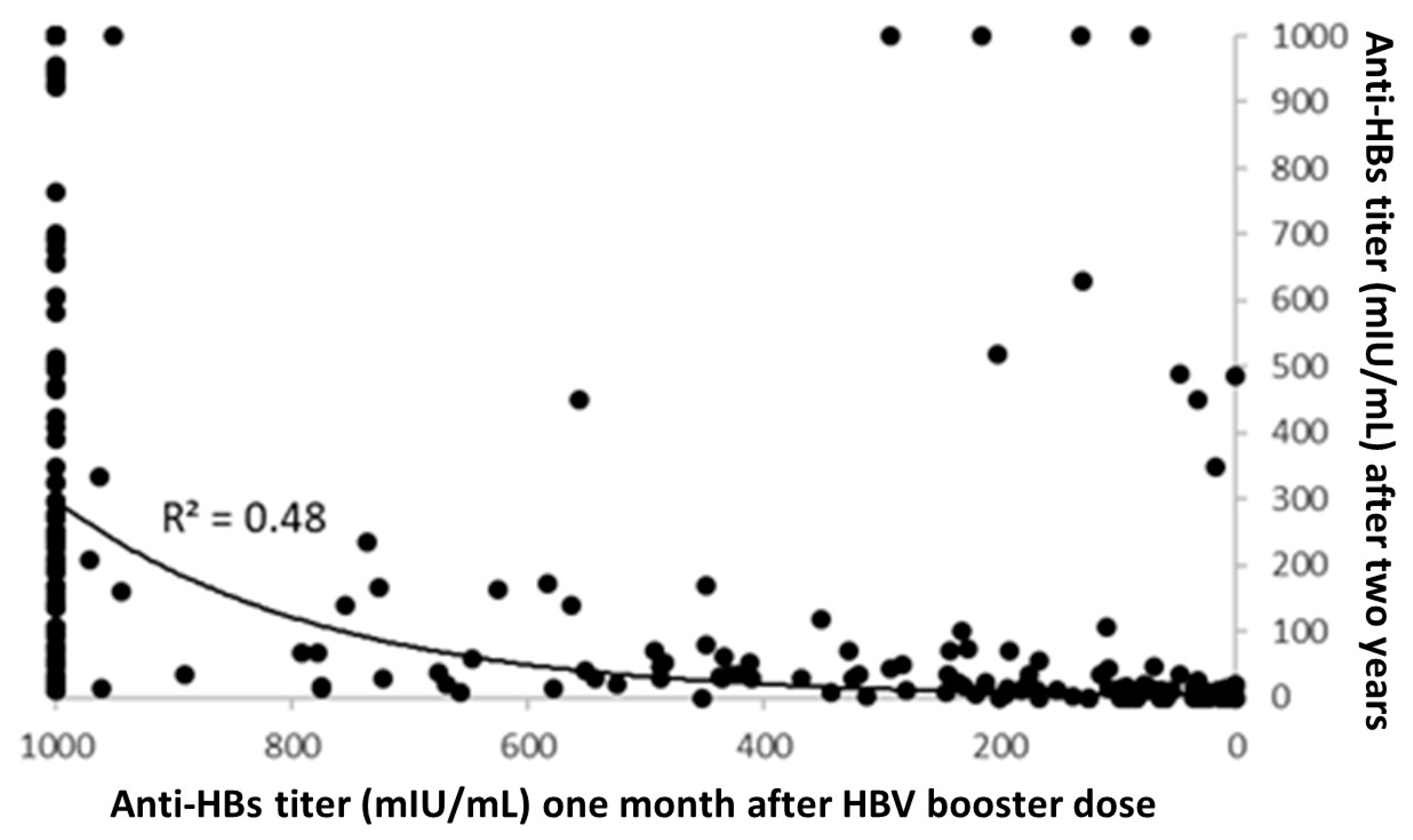
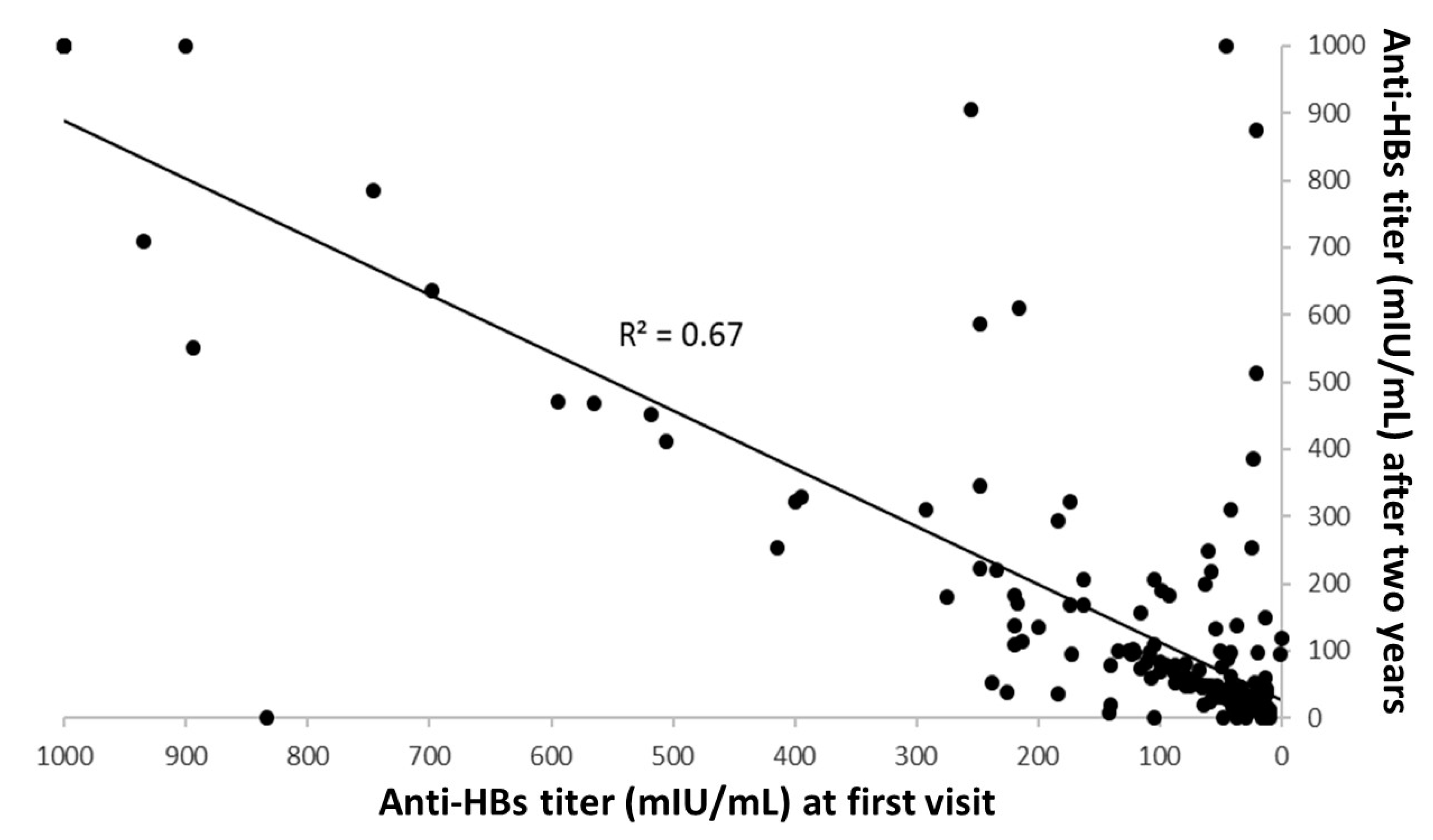
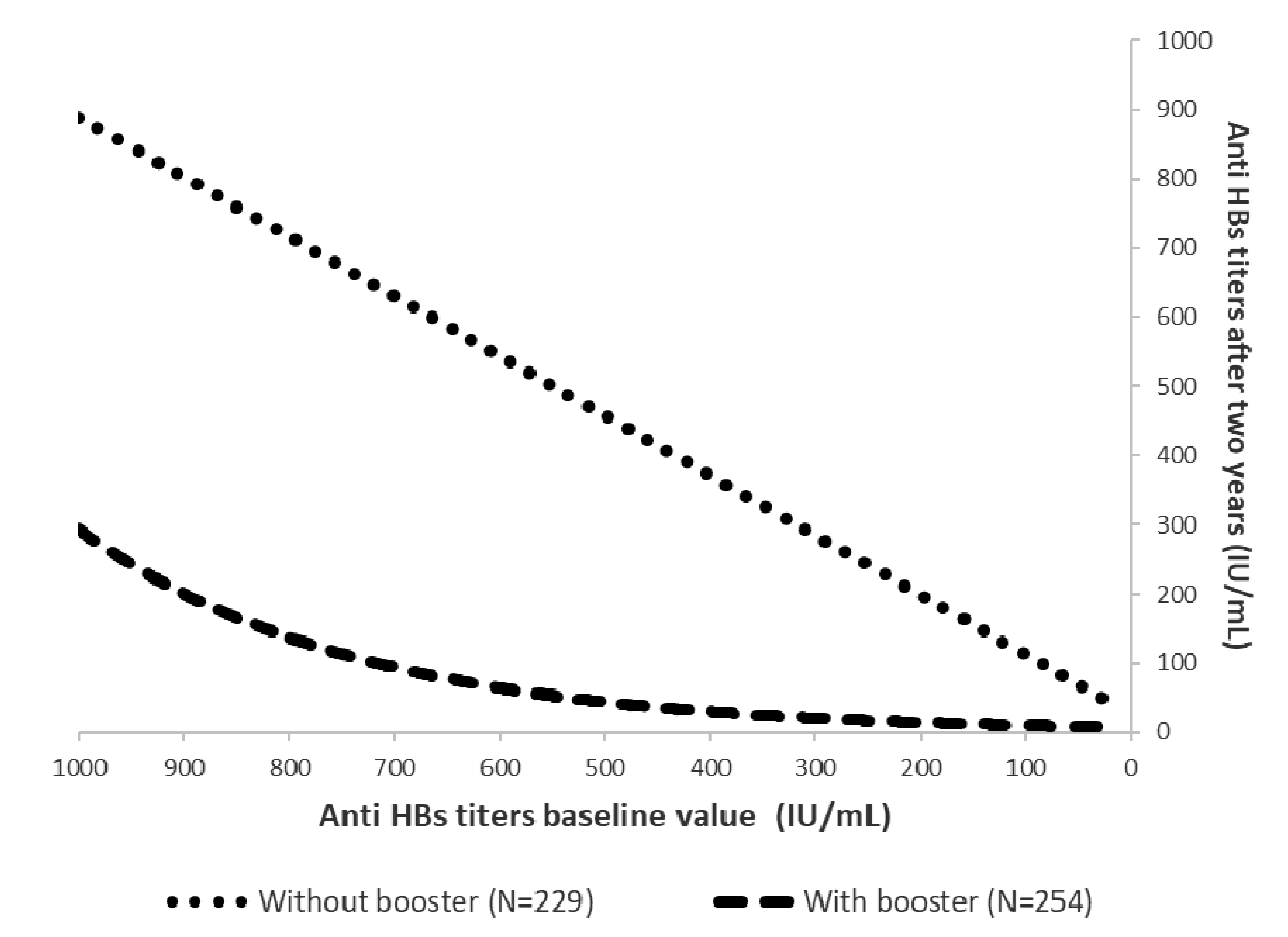
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kay, A.; Zoulim, F. Hepatitis B virus genetic variability and evolution. Virus Res. 2007, 127, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Locarnini, S.; Hatzakis, A.; Chen, D.S.; Lok, A. Strategies to control hepatitis B: Public policy, epidemiology, vaccine and drugs. J. Hepatol. 2015, 62, S76–S86. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.P.; Easterbrook, P.J.; McMahon, B.J. Epidemiology of Hepatitis B Virus Infection and Impact of Vaccination on Disease. Clin. Liver Dis. 2016, 20, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Hepatitis B in the WHO European Region. Available online: https://www.euro.who.int/__data/assets/pdf_file/0007/377251/Fact-Sheet-Hepatitis-B_2019-ENG.pdf (accessed on 24 June 2020).
- Hepatitis B—Annual Epidemiological Report for 2017. Available online: https://www.ecdc.europa.eu/en/publications-data/hepatitis-b-annual-epidemiological-report-2017 (accessed on 22 June 2020).
- Bonanni, P.; Bonaccorsi, G. Vaccination against hepatitis B in health care workers. Vaccine 2001, 19, 2389–2394. [Google Scholar] [CrossRef]
- Williams, I.T.; Perz, J.F.; Bell, B.P. Viral Hepatitis Transmission in Ambulatory Health Care Settings. Clin. Infect. Dis. 2004, 38, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Hou, J. Epidemiology and Prevention of Hepatitis B Virus Infection. Int. J. Med. Sci. 2005, 2, 50–57. [Google Scholar] [CrossRef]
- Seto, W.-K.; Lo, Y.-R.; Pawlotsky, J.M.; Yuen, M.-F. Chronic hepatitis B virus infection. Lancet 2018, 392, 2313–2324. [Google Scholar] [CrossRef]
- Mysore, K.; Leung, D.H. Hepatitis B and C. Clin. Liver Dis. 2018, 22, 703–722. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Gallone, M.S.; LaRocca, A.M.V.; Vimercati, L.; Quarto, M.; Tafuri, S. HBV seroprevalence after 25 years of universal mass vaccination and management of non-responders to the anti-Hepatitis B vaccine: An Italian study among medical students. J. Viral Hepat. 2018, 26, 136–144. [Google Scholar] [CrossRef]
- Grazzini, M.; Arcangeli, G.; Mucci, N.; Bonanni, P.; Bini, C.; Bechini, A.; Boccalini, S.; Tiscione, E.; Paolini, D. High chance to overcome the non-responder status to hepatitis B vaccine after a further full vaccination course: Results from the extended study on healthcare students and workers in Florence, Italy. Hum. Vaccines Immunother. 2019, 16, 949–954. [Google Scholar] [CrossRef]
- Rice, B.; Tomkins, S.E.; Ncube, F.M. Sharp truth: Health care workers remain at risk of bloodborne infection. Occup. Med. 2015, 65, 210–214. [Google Scholar] [CrossRef]
- Italian Ministry of Health. Ministerial Circular n.20, 4 October 1991. Disposizioni relative all’applicazione della legge 27 maggio 1991, n.165. Gazz.Uff. n. 251, 25 ottobre 1991. Available online: https://www.gazzettaufficiale.it/eli/id/1991/10/25/091A4650/sg (accessed on 22 June 2020).
- Law n. 165, 27 May 1991.Obbligatorietà della vaccinazione contro l’epatite virale B. Gazz. Uff. n. 127, 1 giugno 1991. Available online: https://www.gazzettaufficiale.it/eli/id/1991/06/01/091G0201/sg#:~:text=La%20Camera%20dei%20deputati%20ed,nel%20primo%20anno%20di%20vita (accessed on 8 June 2020).
- European Consensus Group on Hepatitis B Immunity. Are booster immunisations needed for lifelong hepatitis B immunity? Lancet 2000, 355, 561–565. [Google Scholar]
- Gabbuti, A.; Romano’, L.; Blanc, P.; Meacci, F.; Amendola, A.; Mele, A.; Mazzotta, F.; Zanetti, A.R. Long-term immunogenicity of hepatitis B vaccination in a cohort of Italian healthy adolescents. Vaccine 2007, 25, 3129–3132. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Klenerman, P.; Thimme, R. Discordance of hepatitis B vaccination policies for healthcare workers between the USA, the UK, and Germany. Hepatol. Res. 2020, 50, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Coppola, N.; Corvino, A.R.; De Pascalis, S.; Signoriello, G.; Di Fiore, E.; Nienhaus, A.; Sagnelli, E.; Lamberti, M. The long-term immunogenicity of recombinant hepatitis B virus (HBV) vaccine: Contribution of universal HBV vaccination in Italy. BMC Infect. Dis. 2015, 15, 149. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Verso, M.G.; Costantino, C.; Vitale, F.; Amodio, E. Immunization against Hepatitis B Surface Antigen (HBsAg) in a Cohort of Nursing Students Two Decades after Vaccination: Surprising Feedback. Vaccines 2019, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Leuridan, E.; Van Damme, P. Hepatitis B and the Need for a Booster Dose. Clin. Infect. Dis. 2011, 53, 68–75. [Google Scholar] [CrossRef]
- Pileggi, C.; Papadopoli, R.; Bianco, A.; Pavia, M. Hepatitis B vaccine and the need for a booster dose after primary vaccination. Vaccine 2017, 35, 6302–6307. [Google Scholar] [CrossRef]
- Dini, G.; Toletone, A.; Barberis, I.; DeBarbieri, N.; Massa, E.; Paganino, C.; Bersi, F.; Montecucco, A.; Alicino, C.; Durando, P. Persistence of protective anti-HBs antibody levels and anamnestic response to HBV booster vaccination: A cross-sectional study among healthcare students 20 years following the universal immunization campaign in Italy. Hum. Vaccines Immunother. 2017, 13, 440–444. [Google Scholar] [CrossRef]
- Coppeta, L.; Pietroiusti, A.; Balbi, O.; De Zordo, L.M.; Mormone, F.; Policardo, S.; Lieto, P.; Pietroiusti, A.; Magrini, A. Zordo Persistence of Immunity for Hepatitis B Virus among Heathcare Workers and Italian Medical Students 20 Years after Vaccination. Int. J. Environ. Res. Public Health 2019, 16, 1515. [Google Scholar] [CrossRef]
- Othman, S.N.; Rashid, Z.Z.; Wahab, A.A.; Samat, M.N.A.; Ding, C.H.; Ali, U.K. Hepatitis B seroepidemiology and booster vaccination in pre-clinical medical students in a Malaysian university. Malays. J. Pathol. 2018, 40, 295–302. [Google Scholar] [PubMed]
- Hess, L.; Riesenberg, K.; Rolston, K.V.I.; Nesher, L. Administering an additional hepatitis B vaccination dose after 18 years maintains adequate long-term protection levels in healthcare workers. Infect. Dis. 2020, 52, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Stroffolini, T.; Sersale’s Study Group; Guadagnino, V.; Caroleo, B.; De Sarro, G.; Focà, A.; Liberto, M.C.; Giancotti, A.; Barreca, G.S.; Marascio, N.; et al. Long-term immunogenicity of hepatitis B vaccination in children and adolescents in a southern Italian town. Infection 2011, 40, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Chiara, F.; Bartolucci, G.B.; Mongillo, M.; Ferretto, L.; Nicolli, A.; Trevisan, A. Hepatitis B vaccination at three months of age: A successful strategy? Vaccine 2013, 31, 1696–1700. [Google Scholar] [CrossRef]
- Francis, D.P.; Hadler, S.C.; Thompson, S.E.; Maynard, J.E.; Ostrow, D.G.; Altman, N.; Braff, E.H.; O’Malley, P.; Hawkins, D.; Judson, F.N.; et al. The Prevention of Hepatitis B with Vaccine: Report of the Centers for Disease Control Multi-Center Efficacy Trial among Homosexual Men. Ann. Intern. Med. 1982, 97, 362–366. [Google Scholar] [CrossRef]
- Immunization Action Coalition. Hepatitis B and the Health Care Worker: CDC Answers Frequently Asked Questions about How to Protect Health Care Workers. Available online: https://www.who.int/occupational_health/activities/3hepatiti.pdf (accessed on 12 May 2020).
- Poovorawan, Y.; Chongsrisawat, V.; Theamboonlers, A.; Crasta, P.D.; Messier, M.; Hardt, K. Long-term anti-HBs antibody persistence following infant vaccination against hepatitis B and evaluation of anamnestic response: A 20-year follow-up study in Thailand. Hum. Vaccines Immunother. 2013, 9, 1679–1684. [Google Scholar] [CrossRef]
- Spada, E.; Romano, L.; Tosti, M.E.; Zuccaro, O.; Paladini, S.; Chironna, M.; Coppola, R.C.; Cuccia, M.; Mangione, R.; Marrone, F.; et al. Hepatitis B immunity in teenagers vaccinated as infants: An Italian 17-year follow-up study. Clin. Microbiol. Infect. 2014, 20, 680–686. [Google Scholar] [CrossRef]
- Zhu, C.-L.; Liu, P.; Chen, T.; Ni, Z.; Lu, L.-L.; Huang, F.; Lü, J.; Sun, Z.; Qu, C. Presence of immune memory and immunity to hepatitis B virus in adults after neonatal hepatitis B vaccination. Vaccine 2011, 29, 7835–7841. [Google Scholar] [CrossRef]
- Ni, Y.-H.; Chang, M.-H.; Wu, J.-F.; Hsu, H.-Y.; Chen, H.-L.; Chen, D.S. Minimization of hepatitis B infection by a 25-year universal vaccination program. J. Hepatol. 2012, 57, 730–735. [Google Scholar] [CrossRef]
- Mendy, M.; Peterson, I.; Hossin, S.; Peto, T.J.; Jobarteh, M.L.; Jeng-Barry, A.; Sidibeh, M.; Jatta, A.; Moore, S.E.; Hall, A.J.; et al. Observational Study of Vaccine Efficacy 24 Years after the Start of Hepatitis B Vaccination in Two Gambian Villages: No Need for a Booster Dose. PLoS ONE 2013, 8, e58029. [Google Scholar] [CrossRef]
- Guidance for Evaluating Health-Care Personnel for Hepatitis B Virus Protection and for Administer- Ing Postexposure Management. CDC n.d. Available online: http://www.cdc.gov/mmwr/pdf/rr/rr6210.pdf (accessed on 8 June 2020).
- Fitzsimons, D.; François, G.; Hall, A.; McMahon, B.J.; Meheus, A.; Zanetti, A.; Duval, B.; Jilg, W.; Böcher, W.O.; Lu, S.-N.; et al. Long-term efficacy of hepatitis B vaccine, booster policy, and impact of hepatitis B virus mutants. Vaccine 2005, 23, 4158–4166. [Google Scholar] [CrossRef] [PubMed]
- Mahallawi, W. Persistence of hepatitis B surface antibody and immune memory to hepatitis B vaccine among medical college students in Madinah. Ann. Saudi Med. 2018, 38, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Jilg, W.; Schmidt, M.; Deinhardt, F. Vaccination against Hepatitis B: Comparison of Three Different Vaccination Schedules. J. Infect. Dis. 1989, 160, 766–769. [Google Scholar] [CrossRef] [PubMed]
| Variable | N (%) | Median Anti HBs IgG Titer at First Visit (mIU/mL) (IQR) |
|---|---|---|
| Sex | ||
| Male | 160 (33.1) | 8 (0–43) |
| Female | 323 (66.9) | 0 (0–28) |
| Birth cohort | ||
| 1992 or less | 101 (20.9) | 38 (3–174) |
| 1993–1994 | 84 (17.4) | 0 (0–22) |
| 1995–1996 | 198 (41.0) | 0 (0–17) |
| >1997 | 100 (20.7) | 6 (0–18) |
| Vaccination timing | ||
| At adolescence | 85 (17.6) | 50 (8–219) |
| At infancy | 398 (82.4) | 0 (0–19) |
| Booster after first visit | ||
| Yes | 254 (52.6) | 0 (0–0) |
| No | 229 (47.4) | 36 (16–105) |
| Total | 483 (100) | 6 (0–34) |
| Starting Anti-HBs Titers Baseline * Value (mIU/mL) | HBs Titers <10 mIU/mL after Two Years | ||
|---|---|---|---|
| Boosted Students, N (%) | Not Boosted Students, N (%) | p-Value | |
| 0–100 | 34/61 (55.7%) | 30/168 (17.9%) | <0.001 |
| 101–200 | 5/20 (25%) | 2/24 (8.3%) | 0.24 |
| 201–300 | 2/16 (12.5%) | 0/15 (0%) | 0.49 |
| 301–400 | 2/5 (40%) | 0/2 (0%) | 1 |
| >400 | 3/149 (2%) | 1/17 (5.9%) | 0.34 |
| Total | 46/254 (18.1%) | 33/229 (14.4%) | 0.39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verso, M.G.; Costantino, C.; Marrella, A.; Immordino, P.; Vitale, F.; Amodio, E. Kinetics of Anti-Hepatitis B Surface Antigen Titers in Nurse Students after a Two-Year Follow-Up. Vaccines 2020, 8, 467. https://doi.org/10.3390/vaccines8030467
Verso MG, Costantino C, Marrella A, Immordino P, Vitale F, Amodio E. Kinetics of Anti-Hepatitis B Surface Antigen Titers in Nurse Students after a Two-Year Follow-Up. Vaccines. 2020; 8(3):467. https://doi.org/10.3390/vaccines8030467
Chicago/Turabian StyleVerso, Maria Gabriella, Claudio Costantino, Alessandro Marrella, Palmira Immordino, Francesco Vitale, and Emanuele Amodio. 2020. "Kinetics of Anti-Hepatitis B Surface Antigen Titers in Nurse Students after a Two-Year Follow-Up" Vaccines 8, no. 3: 467. https://doi.org/10.3390/vaccines8030467
APA StyleVerso, M. G., Costantino, C., Marrella, A., Immordino, P., Vitale, F., & Amodio, E. (2020). Kinetics of Anti-Hepatitis B Surface Antigen Titers in Nurse Students after a Two-Year Follow-Up. Vaccines, 8(3), 467. https://doi.org/10.3390/vaccines8030467









