Evaluation of a Pseudotyped Virus Neutralisation Test for the Measurement of Equine Influenza Virus-Neutralising Antibody Responses Induced by Vaccination and Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Serum Samples
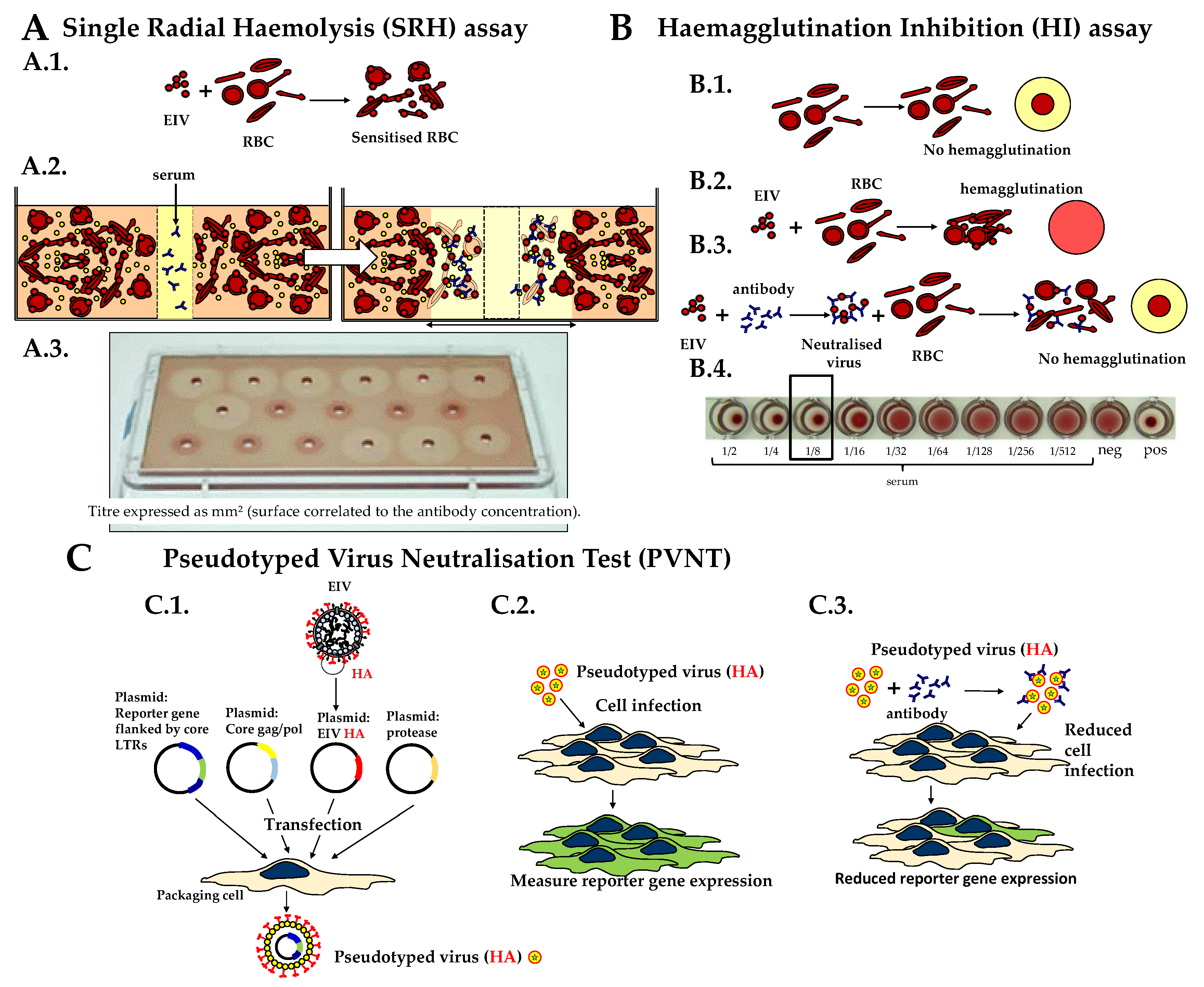
2.2. Single Radial Haemolysis (SRH) Assay
2.3. Haemagglutination Inhibition (HI) Test
2.4. Pseudotyped Virus Neutralization Test (PVNT)
2.5. Statistical Analysis
3. Results
3.1. Defining PVNT Threshold for Positivity
3.2. Post-Vaccination Antibody Responses
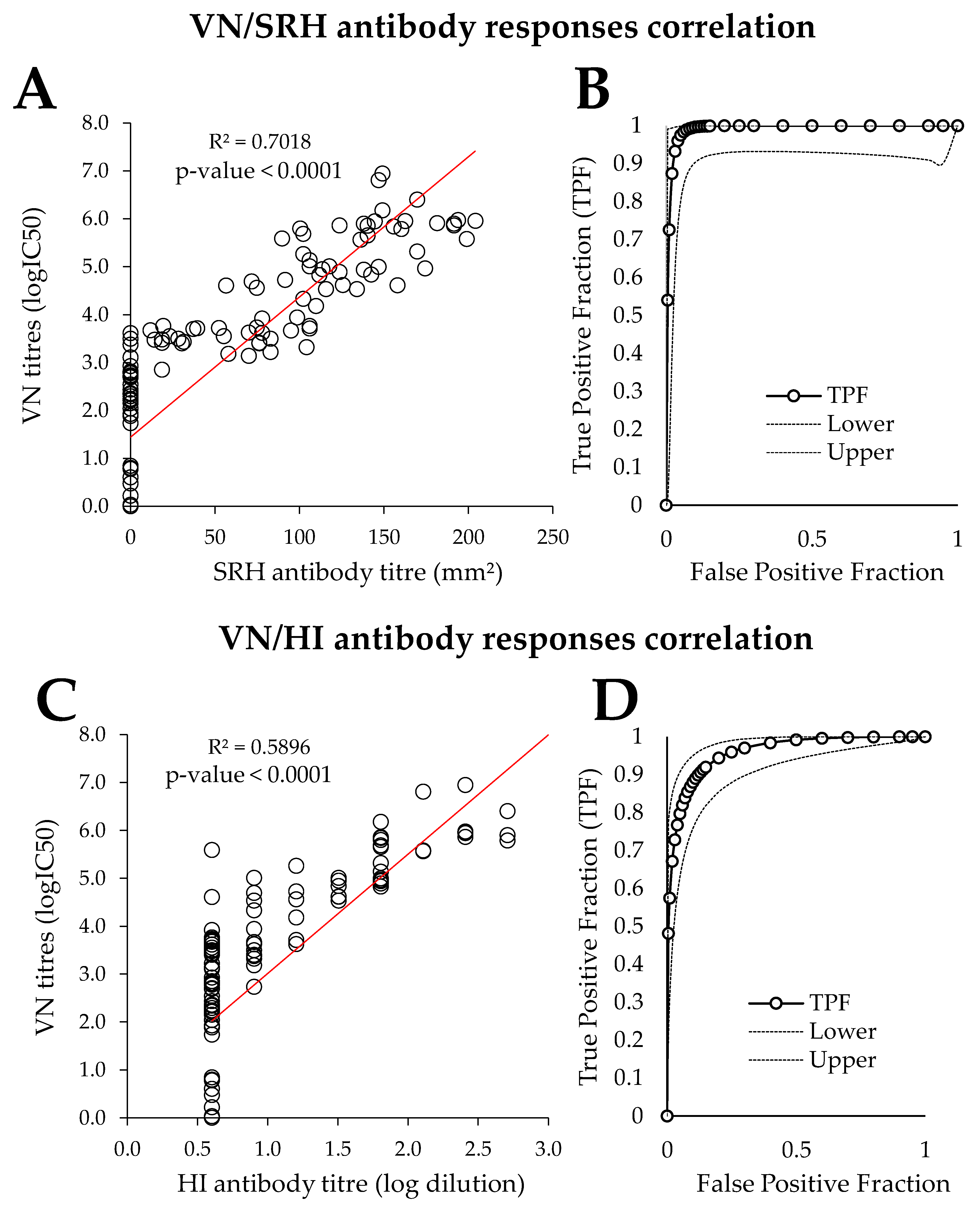
3.3. Comparison of PVNT with SRH and HI
4. Discussion
4.1. Defining PVNT Threshold for Positivity
4.2. Post-Vaccination Antibody Responses
4.3. Comparison of PVNT with SRH and HI
4.4. Comparison of Heterologous Strains in PVNT and SRH Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paillot, R. A systematic review of recent advances in equine influenza vaccination. Vaccines 2014, 2, 797–831. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.M. Equine influenza. Cold Spring Harb. Perspect. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Quirarte, H.O.; Lopez-Guerrero, D.V.; Gutierrez-Xicotencatl, L.; Esquivel-Guadarrama, F. Protective antibodies against influenza proteins. Front. Immunol. 2019, 10, 1677. [Google Scholar] [CrossRef]
- Eichelberger, M.C.; Monto, A.S. Neuraminidase, the forgotten surface antigen, emerges as an influenza vaccine target for broadened protection. J. Infect. Dis. 2019, 219, S75–S80. [Google Scholar] [CrossRef] [PubMed]
- OIE. Equine Influenza (Infection with Equine Influenza Virus): Chapter 3.5.7. 2019. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.05.07_EQ_INF.pdf (accessed on 5 August 2020).
- Mumford, J.A.; Wood, J. Establishing an acceptability threshold for equine influenza vaccines. Dev. Biol. Stand 1992, 79, 137–146. [Google Scholar] [PubMed]
- Mumford, J.A.; Jessett, D.M.; Dunleavy, U.; Wood, J.L.N.; Hannant, D.; Sundquist, B.; Cook, R.F. Antigenicity and immunogenicity of experimental equine influenza iscom vaccines. Vaccine 1994, 12, 857–863. [Google Scholar] [CrossRef]
- Chambers, T.M.; Reedy, S.E. Equine influenza serological methods. Methods Mol. Biol. 2020, 2123, 401–412. [Google Scholar]
- Scott, S.; Molesti, E.; Temperton, N.; Ferrara, F.; Bottcher-Friebertshauser, E.; Daly, J. The use of equine influenza pseudotypes for serological screening. J. Mol. Genet. Med. 2012, 6, 304–308. [Google Scholar] [CrossRef]
- Scott, S.D.; Kinsley, R.; Temperton, N.; Daly, J.M. The optimisation of pseudotyped viruses for the characterisation of immune responses to equine influenza virus. Pathogens 2016, 5, 68. [Google Scholar] [CrossRef]
- Daly, J.; Daas, A.; Behr-Gross, M.E. Collaborative study for the establishment of a candidate equine influenza subtype 2 american-like strain a/eq/south africa/4/03 - horse antiserum biological reference preparation. Pharmeuropa Bio 2007, 2007, 7–14. [Google Scholar]
- Steffen, I.; Simmons, G. Pseudotyping viral vectors with emerging virus envelope proteins. Curr. Gene Ther. 2016, 16, 47–55. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Q.; Huang, W.; Li, X.; Wang, Y. Current status on the development of pseudoviruses for enveloped viruses. Rev. Med. Virol. 2018, 28. [Google Scholar] [CrossRef]
- Katz, J.M.; Hancock, K.; Xu, X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert Rev. Anti Infect. Ther. 2011, 9, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Lagarde, N.; Ma, E.S.; de Jong, M.D.; Peiris, J.S. Optimization and evaluation of an influenza a (h5) pseudotyped lentiviral particle-based serological assay. J. Clin. Virol. 2010, 47, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Lai, J.C. Production of influenza pseudotyped lentiviral particles and their use in influenza research and diagnosis: An update. Expert Rev. Anti Infect. Ther. 2011, 9, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Temperton, N.; Wright, E.; Scott, S.D. Retroviral pseudotpes—From scientific tools to clinical utility. Encyclopedia Life Sci. 2015. [Google Scholar] [CrossRef]
- Szecsi, J.; Boson, B.; Johnsson, P.; Dupeyrot-Lacas, P.; Matrosovich, M.; Klenk, H.D.; Klatzmann, D.; Volchkov, V.; Cosset, F.L. Induction of neutralising antibodies by virus-like particles harbouring surface proteins from highly pathogenic h5n1 and h7n1 influenza viruses. Virol. J. 2006, 3, 70. [Google Scholar] [CrossRef]
- Fougerolle, S.; Legrand, L.; Garrett, D.; Birand, I.; Foursin, M.; D’Ablon, X.; Bayssat, P.; Newton, R.J.; Pronost, S.; Paillot, R. Influential factors inducing suboptimal humoral response to vector-based influenza immunisation in thoroughbred foals. Vaccine 2016. [Google Scholar] [CrossRef]
- Gildea, S.; Arkins, S.; Walsh, C.; Cullinane, A. A comparison of antibody responses to commercial equine influenza vaccines following primary vaccination of thoroughbred weanlings--a randomised blind study. Vaccine 2011, 29, 9214–9223. [Google Scholar] [CrossRef]
- Cullinane, A.; Gildea, S.; Weldon, E. Comparison of primary vaccination regimes for equine influenza: Working towards an evidence-based regime. Equine Vet. J. 2014, 46, 669–673. [Google Scholar] [CrossRef]
- Cullinane, A.; Weld, J.; Osborne, M.; Nelly, M.; McBride, C.; Walsh, C. Field studies on equine influenza vaccination regimes in thoroughbred foals and yearlings. Vet. J. 2001, 161, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Van Oirschot, J.T.; Bruin, G.; de Boer-Luytze, E.; Smolders, G. Maternal antibodies against equine influenza virus in foals and their interference with vaccination. Zentralbl. Veterinarmed. B 1991, 38, 391–396. [Google Scholar] [CrossRef] [PubMed]
- van Maanen, C.; Bruin, G.; de Boer-Luijtze, E.; Smolders, G.; de Boer, G.F. Interference of maternal antibodies with the immune response of foals after vaccination against equine influenza. Vet. Q. 1992, 14, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Regourd, E.; Behr-Gross, M.E. Establishment of a candidate equine influenza florida clade 2 strain a/eq/richmond/1/07 horse antiserum as ph. Eur. Biological reference preparation/oie international reference reagent. Pharmeur. Bio Sci. Notes 2020, 2020, 125–140. [Google Scholar]
- Morley, P.S.; Hanson, L.K.; Bogdan, J.R.; Townsend, H.G.; Appleton, J.A.; Haines, D.M. The relationship between single radial hemolysis, hemagglutination inhibition, and virus neutralization assays used to detect antibodies specific for equine influenza viruses. Vet. Microbiol. 1995, 45, 81–92. [Google Scholar] [CrossRef]
- Trombetta, C.M.; Remarque, E.J.; Mortier, D.; Montomoli, E. Comparison of hemagglutination inhibition, single radial hemolysis, virus neutralization assays, and elisa to detect antibody levels against seasonal influenza viruses. Influenza Other Respir. Viruses 2018, 12, 675–686. [Google Scholar] [CrossRef]
- Amitai, A.; Chakraborty, A.K.; Kardar, M. The low spike density of hiv may have evolved because of the effects of t helper cell depletion on affinity maturation. PLoS Comput. Biol. 2018, 14, e1006408. [Google Scholar] [CrossRef]

| Group | n (Ponies) | n (Samples) | Vaccine |
|---|---|---|---|
| 1 | 5 | 20 | A/equine/Richmond/1/2007 (FC2; 400 HAU/mL) |
| 2 | 5 | 20 | A/equine/Richmond/1/2007 (FC2; 100 HAU/mL) |
| 3 | 5 | 20 | A/equine/Kentucky/2009 (FC1; 400 HAU/mL) |
| 4 | 5 | 20 | A/equine/Kentucky/2009 (FC1; 100 HAU/mL) |
| 5 | 4 | 16 | None (control group) |
| 6 | 3 | 12 | Commercial vaccine 1 |
| Total: | 27 | 108 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinsley, R.; Pronost, S.; De Bock, M.; Temperton, N.; Daly, J.M.; Paillot, R.; Scott, S. Evaluation of a Pseudotyped Virus Neutralisation Test for the Measurement of Equine Influenza Virus-Neutralising Antibody Responses Induced by Vaccination and Infection. Vaccines 2020, 8, 466. https://doi.org/10.3390/vaccines8030466
Kinsley R, Pronost S, De Bock M, Temperton N, Daly JM, Paillot R, Scott S. Evaluation of a Pseudotyped Virus Neutralisation Test for the Measurement of Equine Influenza Virus-Neutralising Antibody Responses Induced by Vaccination and Infection. Vaccines. 2020; 8(3):466. https://doi.org/10.3390/vaccines8030466
Chicago/Turabian StyleKinsley, Rebecca, Stéphane Pronost, Manuelle De Bock, Nigel Temperton, Janet M. Daly, Romain Paillot, and Simon Scott. 2020. "Evaluation of a Pseudotyped Virus Neutralisation Test for the Measurement of Equine Influenza Virus-Neutralising Antibody Responses Induced by Vaccination and Infection" Vaccines 8, no. 3: 466. https://doi.org/10.3390/vaccines8030466
APA StyleKinsley, R., Pronost, S., De Bock, M., Temperton, N., Daly, J. M., Paillot, R., & Scott, S. (2020). Evaluation of a Pseudotyped Virus Neutralisation Test for the Measurement of Equine Influenza Virus-Neutralising Antibody Responses Induced by Vaccination and Infection. Vaccines, 8(3), 466. https://doi.org/10.3390/vaccines8030466









