Lipid Nanoparticle Acts as a Potential Adjuvant for Influenza Split Vaccine without Inducing Inflammatory Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Mice
2.3. Synthesis of LNP and LNP-CpG
2.4. Production and Purification of Recombinant HA and Neuraminidase Proteins
2.5. Preparation and Stimulation of Mouse Bone-Marrow-Derived DCs
2.6. Immunization
2.7. Neutralization Assay
2.8. Enzyme-Linked Lectin Assay (ELLA) to Determine Neuraminidase Inhibition (NI)
2.9. Cytokine Production by Splenocytes after Immunization
2.10. Influenza Virus Challenge after Immunization
2.11. Inflammation Assay
2.12. Statistical Analyses
3. Results
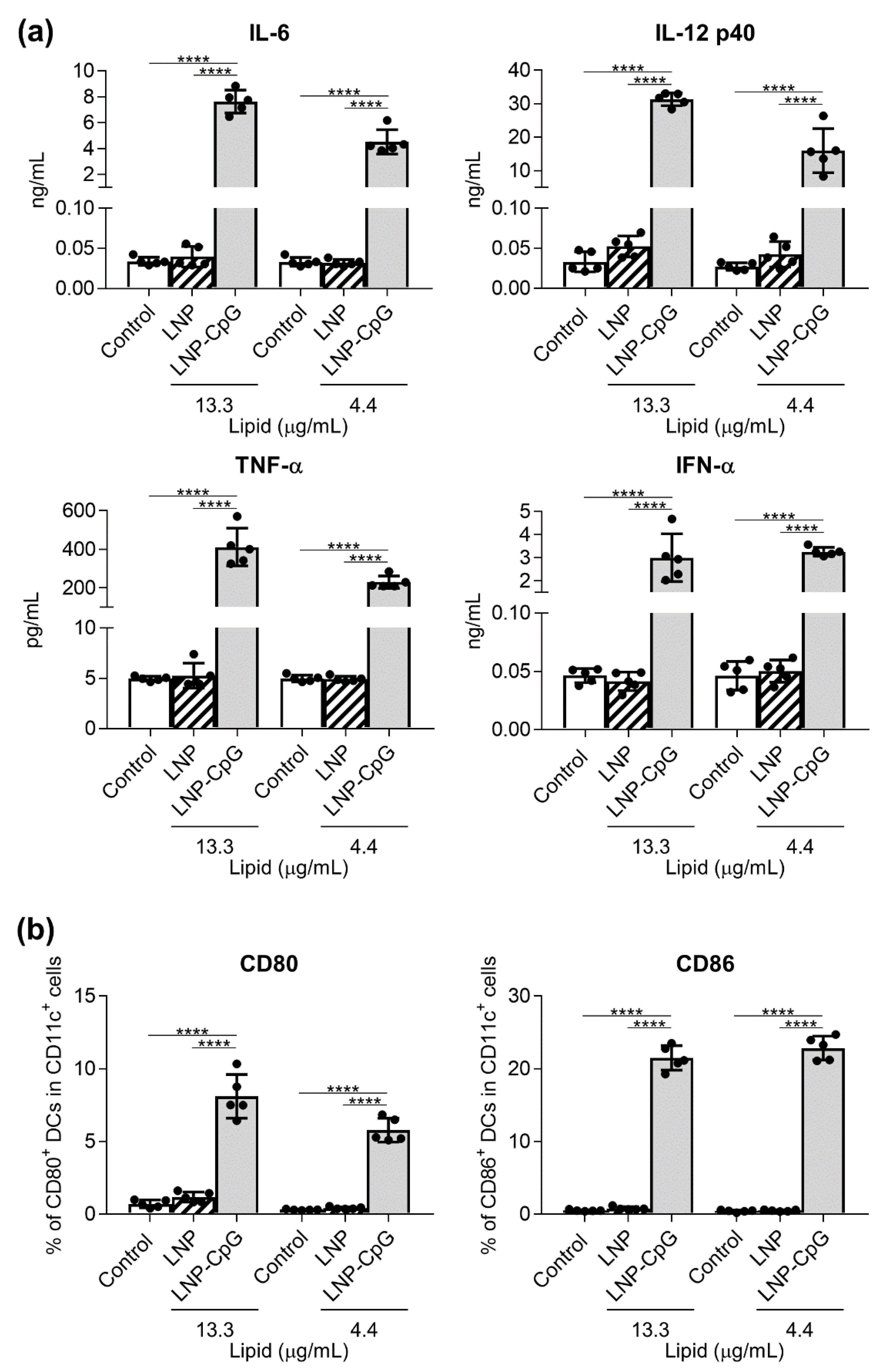
3.1. In Contrast to LNP-CpG, LNP Does Not Induce Cytokine Production or Enhance the Expression of Co-Stimulatory Molecules in Mouse DCs
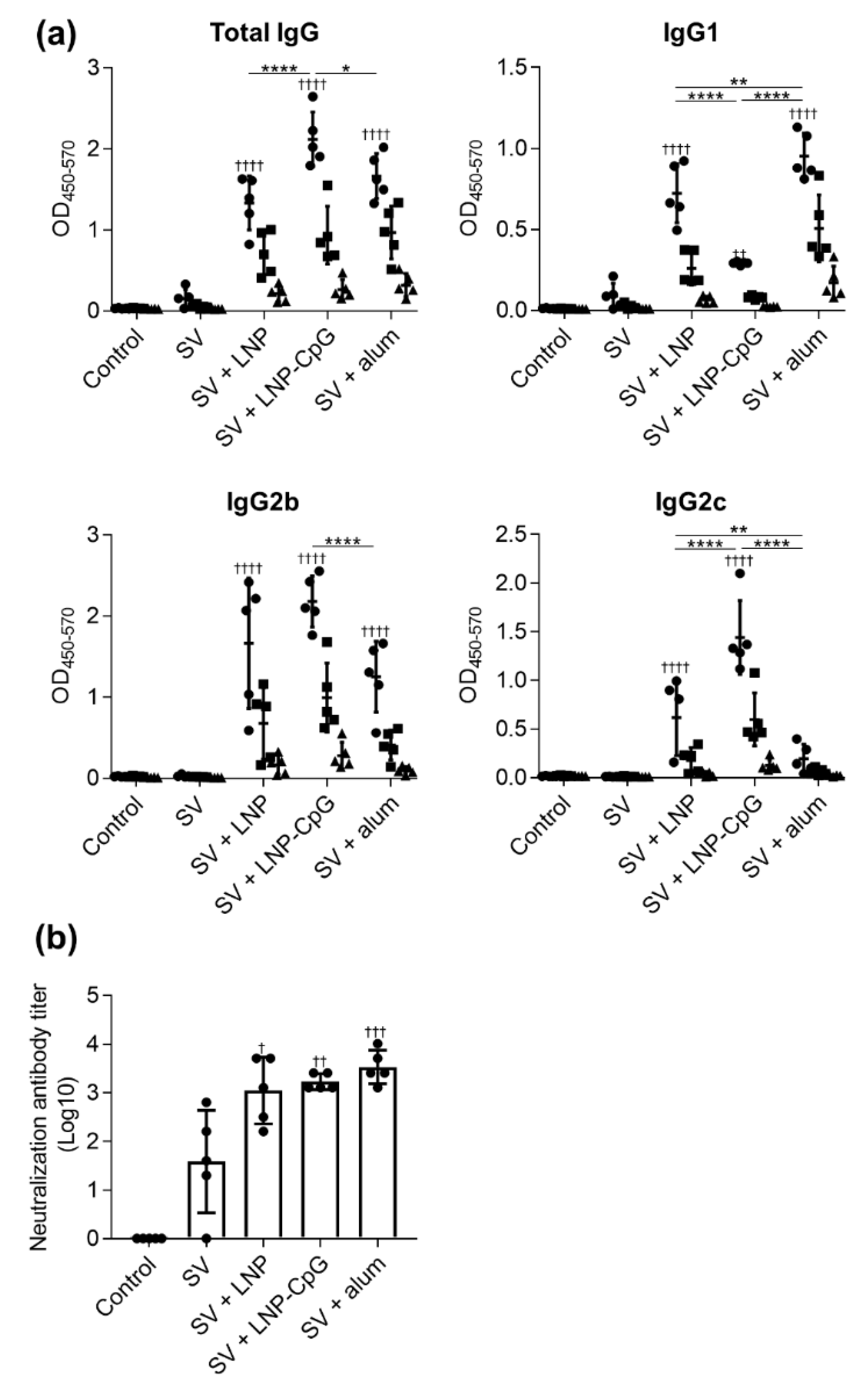
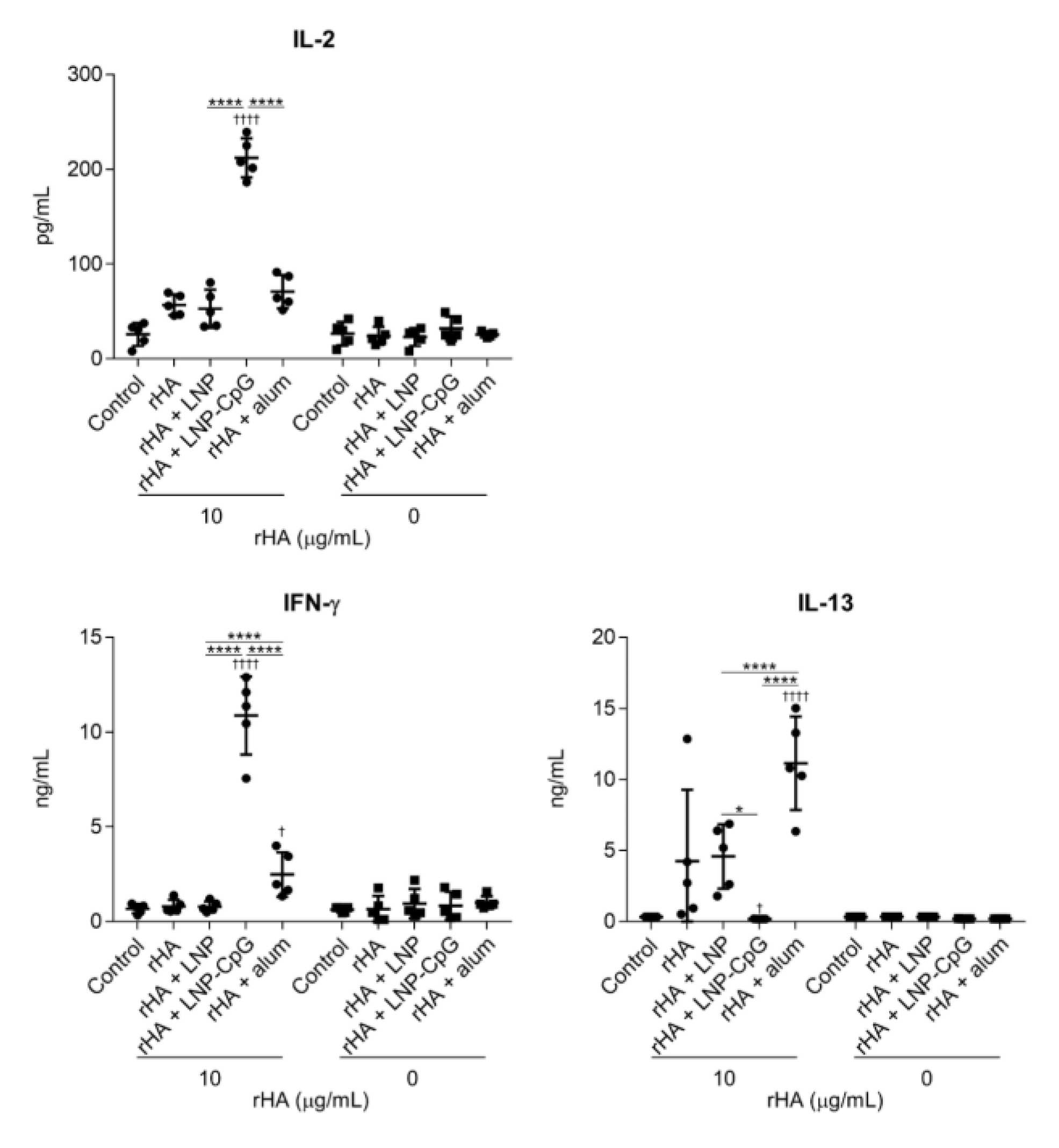
3.2. LNP Improves Antigen-Specific Antibody Responses and Th1 Responses Induced by SV
3.3. SV plus LNP has Strong Protective Effects Against Influenza Virus Challenge
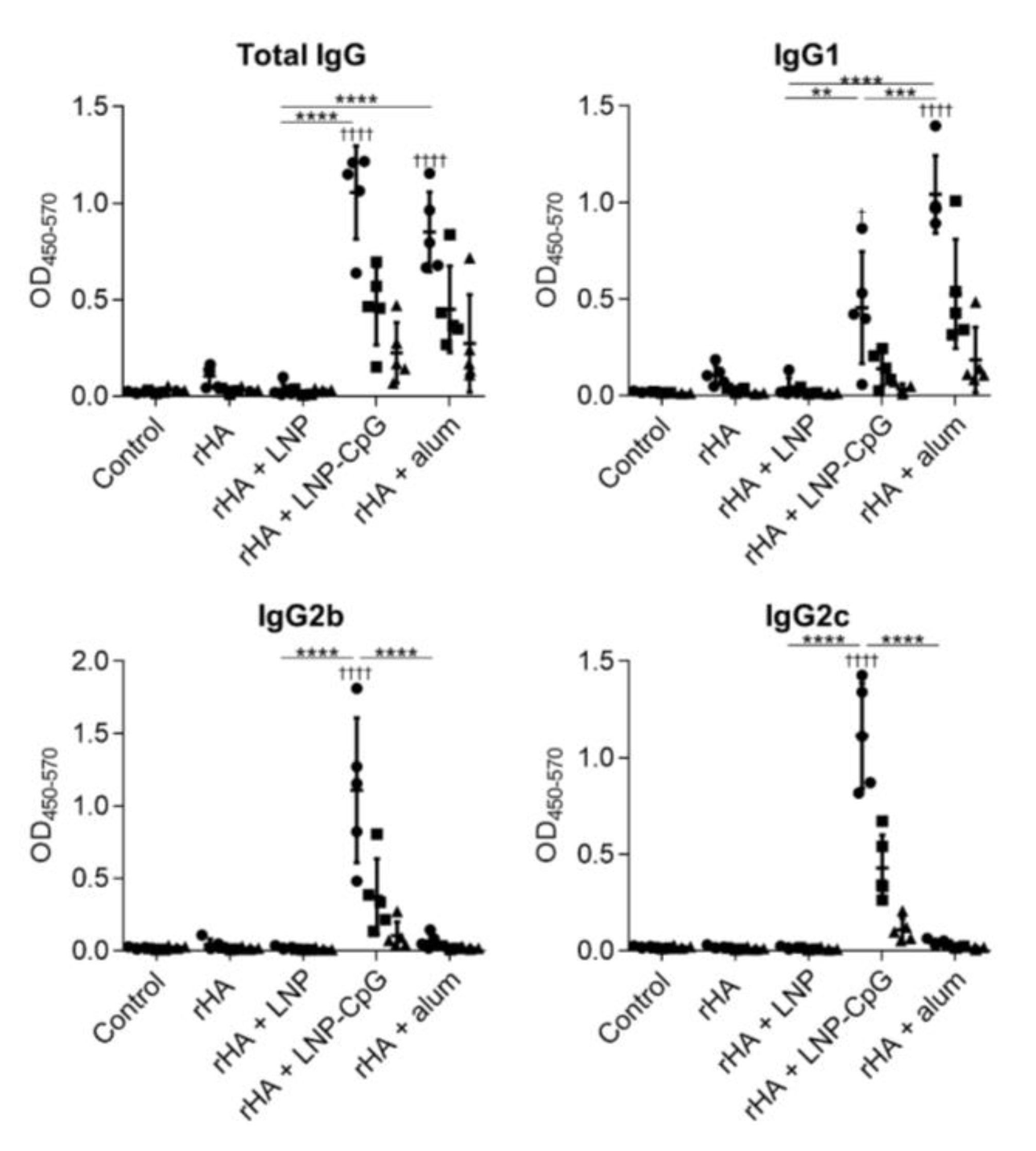
3.4. LNP Does Not Enhance Immune Responses When Used with Recombinant HA Protein as an Antigen
3.5. LNP Does Not Induce Inflammatory Responses In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krammer, F.; Palese, P. Advances in the development of influenza virus vaccines. Nat. Rev. Drug. Discov. 2015, 14, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Vaccines: The fourth century. Clin. Vaccine Immunol. 2009, 16, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Bobbala, S.; Hook, S. Is there an optimal formulation and delivery strategy for subunit vaccines? Pharm. Res. 2016, 33, 2078–2097. [Google Scholar] [CrossRef]
- Sekiya, T.; Mifsud, E.J.; Ohno, M.; Nomura, N.; Sasada, M.; Fujikura, D.; Daito, T.; Shingai, M.; Ohara, Y.; Nishimura, T.; et al. Inactivated whole virus particle vaccine with potent immunogenicity and limited il-6 induction is ideal for influenza. Vaccine 2019, 37, 2158–2166. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Russell, R.F.; Kinnear, E. Adjuvanted influenza vaccines. Hum. Vaccin Immunother. 2018, 14, 550–564. [Google Scholar] [CrossRef]
- Temizoz, B.; Kuroda, E.; Ishii, K.J. Vaccine adjuvants as potential cancer immunotherapeutics. Int. Immunol. 2016, 28, 329–338. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef]
- Shirota, H.; Klinman, D.M. Recent progress concerning cpg DNA and its use as a vaccine adjuvant. Expert Rev. Vaccines 2014, 13, 299–312. [Google Scholar] [CrossRef]
- Schillie, S.; Harris, A.; Link-Gelles, R.; Romero, J.; Ward, J.; Nelson, N. Recommendations of the advisory committee on immunization practices for use of a hepatitis b vaccine with a novel adjuvant. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 455–458. [Google Scholar] [CrossRef]
- Heikenwalder, M.; Polymenidou, M.; Junt, T.; Sigurdson, C.; Wagner, H.; Akira, S.; Zinkernagel, R.; Aguzzi, A. Lymphoid follicle destruction and immunosuppression after repeated cpg oligodeoxynucleotide administration. Nat. Med. 2004, 10, 187–192. [Google Scholar] [CrossRef]
- Seya, T.; Shime, H.; Takeda, Y.; Tatematsu, M.; Takashima, K.; Matsumoto, M. Adjuvant for vaccine immunotherapy of cancer--focusing on toll-like receptor 2 and 3 agonists for safely enhancing antitumor immunity. Cancer Sci. 2015, 106, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Irvine, D.J.; Swartz, M.A.; Szeto, G.L. Engineering synthetic vaccines using cues from natural immunity. Nat. Mater. 2013, 12, 978–990. [Google Scholar] [CrossRef]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle vaccines against infectious diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Misato, K.; Aoshi, T.; Yamamoto, Y.; Kubota, Y.; Wu, X.; Kuroda, E.; Ishii, K.J.; Yamamoto, H.; Yoshioka, Y. Carbonate apatite nanoparticles act as potent vaccine adjuvant delivery vehicles by enhancing cytokine production induced by encapsulated cytosine-phosphate-guanine oligodeoxynucleotides. Front. Immunol. 2018, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Bros, M.; Nuhn, L.; Simon, J.; Moll, L.; Mailander, V.; Landfester, K.; Grabbe, S. The protein corona as a confounding variable of nanoparticle-mediated targeted vaccine delivery. Front. Immunol. 2018, 9, 1760. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Cullis, P.R.; van der Meel, R. Lipid nanoparticles enabling gene therapies: From concepts to clinical utility. Nucleic Acid Ther. 2018, 28, 146–157. [Google Scholar] [CrossRef]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mrna vaccines protect against zika virus infection. Cell 2017, 168, 1114–1125.e10. [Google Scholar] [CrossRef]
- Liang, F.; Lindgren, G.; Lin, A.; Thompson, E.A.; Ols, S.; Rohss, J.; John, S.; Hassett, K.; Yuzhakov, O.; Bahl, K.; et al. Efficient targeting and activation of antigen-presenting cells in vivo after modified mrna vaccine administration in rhesus macaques. Mol. Ther. 2017, 25, 2635–2647. [Google Scholar] [CrossRef]
- Moyle, P.M.; Hartas, J.; Henningham, A.; Batzloff, M.R.; Good, M.F.; Toth, I. An efficient, chemically-defined semisynthetic lipid-adjuvanted nanoparticulate vaccine development system. Nanomedicine 2013, 9, 935–944. [Google Scholar] [CrossRef]
- du Plessis, L.H.; Marais, E.B.; Mohammed, F.; Kotze, A.F. Applications of lipid based formulation technologies in the delivery of biotechnology-based therapeutics. Curr. Pharm. Biotechnol. 2014, 15, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Skwarczynski, M.; Toth, I. Recent advances in peptide-based subunit nanovaccines. Nanomedicine 2014, 9, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, G.; Thoryk, E.A.; Cox, K.S.; Meschino, S.; Dubey, S.A.; Vora, K.A.; Celano, R.; Gindy, M.; Casimiro, D.R.; Bett, A.J. A novel lipid nanoparticle adjuvant significantly enhances b cell and t cell responses to sub-unit vaccine antigens. Vaccine 2016, 34, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, G.; Thoryk, E.A.; Cox, K.S.; Smith, J.S.; Wolf, J.J.; Gindy, M.E.; Casimiro, D.R.; Bett, A.J. A tetravalent sub-unit dengue vaccine formulated with ionizable cationic lipid nanoparticle induces significant immune responses in rodents and non-human primates. Sci. Rep. 2016, 6, 34215. [Google Scholar] [CrossRef]
- Thoryk, E.A.; Swaminathan, G.; Meschino, S.; Cox, K.S.; Gindy, M.; Casimiro, D.R.; Bett, A.J. Co-administration of lipid nanoparticles and sub-unit vaccine antigens is required for increase in antigen-specific immune responses in mice. Vaccines 2016, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Munakata, L.; Tanimoto, Y.; Osa, A.; Meng, J.; Haseda, Y.; Naito, Y.; Machiyama, H.; Kumanogoh, A.; Omata, D.; Maruyama, K.; et al. Lipid nanoparticles of type-a cpg d35 suppress tumor growth by changing tumor immune-microenvironment and activate cd8 t cells in mice. J. Control. Release 2019, 313, 106–119. [Google Scholar] [CrossRef]
- Shirai, S.; Shibuya, M.; Kawai, A.; Tamiya, S.; Munakata, L.; Omata, D.; Suzuki, R.; Aoshi, T.; Yoshioka, Y. Lipid nanoparticles potentiate cpg-oligodeoxynucleotide-based vaccine for influenza virus. Front. Immunol. 2019, 10, 3018. [Google Scholar] [CrossRef]
- Wei, C.J.; Xu, L.; Kong, W.P.; Shi, W.; Canis, K.; Stevens, J.; Yang, Z.Y.; Dell, A.; Haslam, S.M.; Wilson, I.A.; et al. Comparative efficacy of neutralizing antibodies elicited by recombinant hemagglutinin proteins from avian h5n1 influenza virus. J. Virol. 2008, 82, 6200–6208. [Google Scholar] [CrossRef]
- Prevato, M.; Ferlenghi, I.; Bonci, A.; Uematsu, Y.; Anselmi, G.; Giusti, F.; Bertholet, S.; Legay, F.; Telford, J.L.; Settembre, E.C.; et al. Expression and characterization of recombinant, tetrameric and enzymatically active influenza neuraminidase for the setup of an enzyme-linked lectin-based assay. PLoS ONE 2015, 10, e0135474. [Google Scholar] [CrossRef]
- Lu, F.; Hogenesch, H. Kinetics of the inflammatory response following intramuscular injection of aluminum adjuvant. Vaccine 2013, 31, 3979–3986. [Google Scholar] [CrossRef]
- Stephen, J.; Scales, H.E.; Benson, R.A.; Erben, D.; Garside, P.; Brewer, J.M. Neutrophil swarming and extracellular trap formation play a significant role in alum adjuvant activity. NPJ Vaccines 2017, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- HogenEsch, H.; O’Hagan, D.T.; Fox, C.B. Optimizing the utilization of aluminum adjuvants in vaccines: You might just get what you want. NPJ Vaccines 2018, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.; Korsholm, K.S.; Andersen, P.; Agger, E.M. Cationic liposomes as vaccine adjuvants. Expert Rev. Vaccines 2011, 10, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Vasievich, E.A.; Chen, W.; Huang, L. Enantiospecific adjuvant activity of cationic lipid dotap in cancer vaccine. Cancer Immunol. Immunother. 2011, 60, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Pizzuto, M.; Bigey, P.; Lachages, A.M.; Hoffmann, C.; Ruysschaert, J.M.; Escriou, V.; Lonez, C. Cationic lipids as one-component vaccine adjuvants: A promising alternative to alum. J. Control. Release 2018, 287, 67–77. [Google Scholar] [CrossRef]
- Lonez, C.; Bessodes, M.; Scherman, D.; Vandenbranden, M.; Escriou, V.; Ruysschaert, J.M. Cationic lipid nanocarriers activate toll-like receptor 2 and nlrp3 inflammasome pathways. Nanomedicine 2014, 10, 775–782. [Google Scholar] [CrossRef]
- Lonez, C.; Irvine, K.L.; Pizzuto, M.; Schmidt, B.I.; Gay, N.J.; Ruysschaert, J.M.; Gangloff, M.; Bryant, C.E. Critical residues involved in toll-like receptor 4 activation by cationic lipid nanocarriers are not located at the lipopolysaccharide-binding interface. Cell Mol. Life Sci. 2015, 72, 3971–3982. [Google Scholar] [CrossRef]
- Moyer, T.J.; Zmolek, A.C.; Irvine, D.J. Beyond antigens and adjuvants: Formulating future vaccines. J. Clin. Investig. 2016, 126, 799–808. [Google Scholar] [CrossRef]
- Hirai, T.; Yoshioka, Y.; Izumi, N.; Ichihashi, K.; Handa, T.; Nishijima, N.; Uemura, E.; Sagami, K.; Takahashi, H.; Yamaguchi, M.; et al. Metal nanoparticles in the presence of lipopolysaccharides trigger the onset of metal allergy in mice. Nat. Nanotechnol. 2016, 11, 808–816. [Google Scholar]
- Pockros, P.J.; Guyader, D.; Patton, H.; Tong, M.J.; Wright, T.; McHutchison, J.G.; Meng, T.C. Oral resiquimod in chronic hcv infection: Safety and efficacy in 2 placebo-controlled, double-blind phase iia studies. J. Hepatol. 2007, 47, 174–182. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vacchelli, E.; Eggermont, A.; Fridman, W.H.; Galon, J.; Sautes-Fridman, C.; Tartour, E.; Zitvogel, L.; Kroemer, G. Trial watch: Experimental toll-like receptor agonists for cancer therapy. Oncoimmunology 2012, 1, 699–716. [Google Scholar] [CrossRef] [PubMed]
- Lynn, G.M.; Laga, R.; Darrah, P.A.; Ishizuka, A.S.; Balaci, A.J.; Dulcey, A.E.; Pechar, M.; Pola, R.; Gerner, M.Y.; Yamamoto, A.; et al. In Vivo characterization of the physicochemical properties of polymer-linked tlr agonists that enhance vaccine immunogenicity. Nat. Biotechnol. 2015, 33, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Kuroda, E.; Hirai, T.; Tsutsumi, Y.; Ishii, K.J. Allergic responses induced by the immunomodulatory effects of nanomaterials upon skin exposure. Front. Immunol. 2017, 8, 169. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirai, S.; Kawai, A.; Shibuya, M.; Munakata, L.; Omata, D.; Suzuki, R.; Yoshioka, Y. Lipid Nanoparticle Acts as a Potential Adjuvant for Influenza Split Vaccine without Inducing Inflammatory Responses. Vaccines 2020, 8, 433. https://doi.org/10.3390/vaccines8030433
Shirai S, Kawai A, Shibuya M, Munakata L, Omata D, Suzuki R, Yoshioka Y. Lipid Nanoparticle Acts as a Potential Adjuvant for Influenza Split Vaccine without Inducing Inflammatory Responses. Vaccines. 2020; 8(3):433. https://doi.org/10.3390/vaccines8030433
Chicago/Turabian StyleShirai, Seiki, Atsushi Kawai, Meito Shibuya, Lisa Munakata, Daiki Omata, Ryo Suzuki, and Yasuo Yoshioka. 2020. "Lipid Nanoparticle Acts as a Potential Adjuvant for Influenza Split Vaccine without Inducing Inflammatory Responses" Vaccines 8, no. 3: 433. https://doi.org/10.3390/vaccines8030433
APA StyleShirai, S., Kawai, A., Shibuya, M., Munakata, L., Omata, D., Suzuki, R., & Yoshioka, Y. (2020). Lipid Nanoparticle Acts as a Potential Adjuvant for Influenza Split Vaccine without Inducing Inflammatory Responses. Vaccines, 8(3), 433. https://doi.org/10.3390/vaccines8030433



