Influenza Neuraminidase: A Neglected Protein and Its Potential for a Better Influenza Vaccine
Abstract
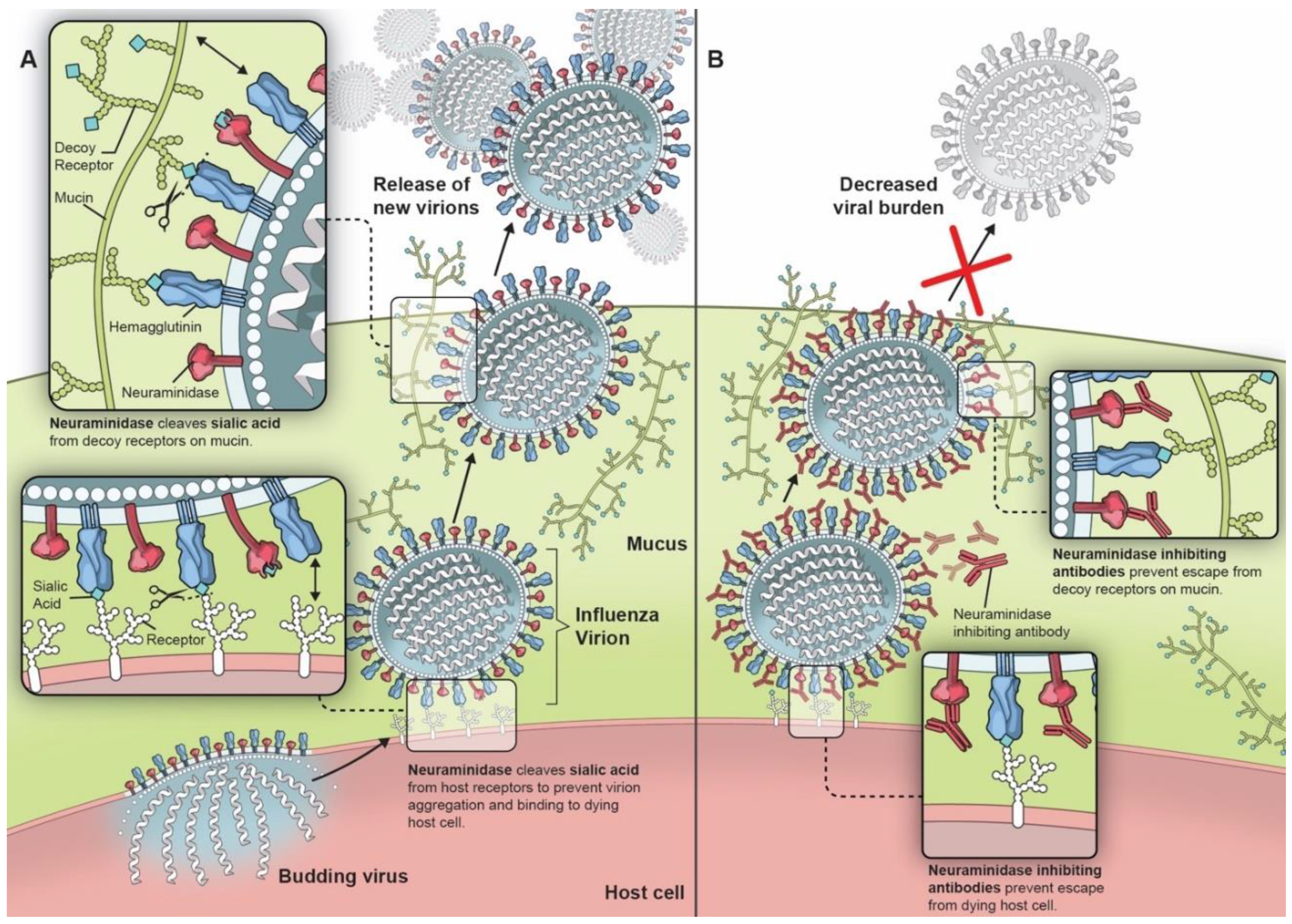
1. Introduction
2. Neuraminidase and Its Potential as a Vaccine Target
3. In Vitro and Animal Studies
4. Importance of NA Immunity in Humans
5. NA-Based Vaccine Strategies
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Both, G.W.; Sleigh, M.J.; Cox, N.J.; Kendal, A.P. Antigenic drift in influenza virus H3 hemagglutinin from 1968 to 1980: Multiple evolutionary pathways and sequential amino acid changes at key antigenic sites. J. Virol. 1983, 48, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Taubenberger, J.K. Making universal influenza vaccines: Lessons from the 1918 pandemic. J. Infect. Dis. 2019, 219, S5–S13. [Google Scholar] [CrossRef] [PubMed]
- Ping, J.; Li, C.; Deng, G.; Jiang, Y.; Tian, G.; Zhang, S.; Bu, Z.; Chen, H. Single-amino-acid mutation in the HA alters the recognition of H9N2 influenza virus by a monoclonal antibody. Biochem. Biophys. Res. Commun. 2008, 371, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Sandbulte, M.R.; Westgeest, K.B.; Gao, J.; Xu, X.; Klimov, A.I.; Russell, C.A.; Burke, D.F.; Smith, D.J.; Fouchier, R.A.M.; Eichelberger, M.C. Discordant antigenic drift of neuraminidase and hemagglutinin in H1N1 and H3N2 influenza viruses. Proc. Natl. Acad. Sci. USA 2011, 108, 20748–20753. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K.; Morens, D.M. 1918 Influenza: The Mother of All Pandemics. Emerg. Infect. Dis. 2006, 12, 15–22. [Google Scholar] [CrossRef]
- Rambaut, A.; Pybus, O.G.; Nelson, M.I.; Viboud, C.; Taubenberger, J.K.; Holmes, E. The genomic and epidemiological dynamics of human influenza A virus. Nature 2008, 453, 615–619. [Google Scholar] [CrossRef]
- Memoli, M.J.; Jagger, B.W.; Dugan, V.G.; Qi, L.; Jackson, J.P.; Taubenberger, J.K. Recent Human Influenza A/H3N2 Virus Evolution Driven by Novel Selection Factors in Addition to Antigenic Drift. J. Infect. Dis. 2009, 200, 1232–1241. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Past Seasons Vaccine Effectiveness Estimates. Available online: https://www.cdc.gov/flu/vaccines-work/past-seasons-estimates.html (accessed on 13 December 2019).
- Jefferson, T.; Rivetti, D.; Rivetti, A.; Rudin, M.; Di Pietrantonj, C.; Demicheli, V. Efficacy and effectiveness of influenza vaccines in elderly people: A systematic review. Lancet 2005, 366, 1165–1174. [Google Scholar] [CrossRef]
- Osterholm, M.T.; Kelley, N.S.; Sommer, A.; Belongia, E.A. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 36–44. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Janjua, N.Z.; De Serres, G.; Sabaiduc, S.; Eshaghi, A.; Dickinson, J.A.; Fonseca, K.; Winter, A.-L.; Gubbay, J.B.; Krajden, M.; et al. Low 2012–13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS ONE 2014, 9, e92153. [Google Scholar] [CrossRef]
- Paules, C.I.; Marston, H.D.; Eisinger, R.W.; Baltimore, D.; Fauci, A.S. The pathway to a universal influenza vaccine. Immunity 2017, 47, 599–603. [Google Scholar] [CrossRef]
- Johansson, B.E.; Eichelberger, M.C. Influenza: A unique problem in vaccination. Future Virol. 2010, 5, 651–664. [Google Scholar] [CrossRef]
- Hobson, D.; Curry, R.L.; Beare, A.S.; Ward-Gardner, A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. Epidemiol. Infect. 1972, 70, 767–777. [Google Scholar] [CrossRef]
- Eichelberger, M.C.; Monto, A.S. Neuraminidase, the forgotten surface antigen, emerges as an influenza vaccine target for broadened protection. J. Infect. Dis. 2019, 219, S75–S80. [Google Scholar] [CrossRef] [PubMed]
- Sultana, I.; Yang, K.; Getie-Kebtie, M.; Couzens, L.; Markoff, L.; Alterman, M.; Eichelberger, M.C. Stability of neuraminidase in inactivated influenza vaccines. Vaccine 2014, 32, 2225–2230. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Sultana, I.; Couzens, L.; Mindaye, S.; Eichelberger, M.C. Assessment of influenza a neuraminidase (subtype N1) potency by ELISA. J. Virol. Methods 2017, 244, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Getie-Kebtie, M.; Sultana, I.; Eichelberger, M.; Alterman, M. Label-free mass spectrometry-based quantification of hemagglutinin and neuraminidase in influenza virus preparations and vaccines. Influenza Other Respir. Viruses 2012, 7, 521–530. [Google Scholar] [CrossRef]
- Gottschalk, A. Neuraminic acid: The functional group of some biologically active mucoproteins. Yale J. Boil. Med. 1956, 28, 525–537. [Google Scholar]
- Gottschalk, A. Neuraminidase: The specific enzyme of influenza virus and Vibrio cholerae. Biochim. Biophys. Acta (BBA) Bioenerg. 1957, 23, 645–646. [Google Scholar] [CrossRef]
- Heimer, R.; Meyer, K. Studies on sialic acid of submaxillary mucoid. Proc. Natl. Acad. Sci. USA 1956, 42, 728–734. [Google Scholar] [CrossRef]
- Blix, F.G.; Gottschalk, A.; Klenk, E. Proposed nomenclature in the field of neuraminic and sialic acids. Nature 1957, 179, 1088. [Google Scholar] [CrossRef] [PubMed]
- Air, G.; Laver, W.G. The neuraminidase of influenza virus. Proteins Struct. Funct. Bioinform. 1989, 6, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Dugan, V.G.; Chen, R.; Spiro, D.J.; Sengamalay, N.; Zaborsky, J.; Ghedin, E.; Nolting, J.; Swayne, D.E.; Runstadler, J.A.; Happ, G.M.; et al. The evolutionary genetics and emergence of avian influenza viruses in wild birds. PLoS Pathog. 2008, 4, e1000076. [Google Scholar] [CrossRef] [PubMed]
- Air, G. Influenza neuraminidase. Influenza Other Respir. Viruses 2011, 6, 245–256. [Google Scholar] [CrossRef]
- Tweed, S.A.; Skowronski, D.M.; David, S.T.; Larder, A.; Petric, M.; Lees, W.; Li, Y.; Katz, J.; Krajden, M.; Tellier, R.; et al. Human illness from avian influenza H7N3, British Columbia. Emerg. Infect. Dis. 2004, 10, 2196–2199. [Google Scholar] [CrossRef]
- Yang, Z.F.; Mok, C.K.P.; Peiris, J.S.M.; Zhong, N.S. Human infection with a novel avian influenza A(H5N6) virus. N. Engl. J. Med. 2015, 373, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, M.P.; Wilbrink, B.; Conyn, M.; Natrop, G.; Van Der Nat, H.; Vennema, H.; Meijer, A.; Van Steenbergen, J.; Fouchier, R.; Osterhaus, A.D.; et al. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet 2004, 363, 587–593. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, H.; Gao, R.; Zhang, J.; Wang, D.-Y.; Xiong, Y.; Fan, G.; Yang, F.; Li, X.; Zhou, J.; et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: A descriptive study. Lancet 2014, 383, 714–721. [Google Scholar] [CrossRef]
- Gao, R.; Cao, B.; Hu, Y.; Feng, Z.; Wang, D.; Hu, W.; Chen, J.; Jie, Z.; Qiu, H.; Xu, K.; et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 2013, 368, 1888–1897. [Google Scholar] [CrossRef]
- Ciminski, K.; Schwemmle, M. Bat-Borne Influenza A Viruses: An awakening. Cold Spring Harb. Perspect. Med. 2019, a038612. [Google Scholar] [CrossRef]
- Li, Y.; Cao, H.; Dao, N.; Luo, Z.; Yu, H.; Chen, Y.; Xing, Z.; Baumgarth, N.; Cardona, C.; Chen, X. High-throughput neuraminidase substrate specificity study of human and avian influenza A viruses. Virology 2011, 415, 12–19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Compans, R.W.; Dimmock, N.J.; Meier-Ewert, H. Effect of Antibody to Neuraminidase on the Maturation and Hemagglutinating Activity of an Influenza A2 Virus. J. Virol. 1969, 4, 528–534. [Google Scholar] [CrossRef]
- Liu, C.; Eichelberger, M.C.; Compans, R.W.; Air, G. Influenza type A virus neuraminidase does not play a role in viral entry, replication, assembly, or budding. J. Virol. 1995, 69, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-L.; Liang, C.-H.; Wu, C.-Y.; Forrest, H.L.; Ferguson, A.; Choy, K.-T.; Jones, J.; Wong, D.D.-Y.; Cheung, P.-H.; Hsu, C.-H.; et al. Hemagglutinin-neuraminidase balance confers respiratory-droplet transmissibility of the pandemic H1N1 influenza virus in ferrets. Proc. Natl. Acad. Sci. USA 2011, 108, 14264–14269. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Wolff, T.; Herwig, A.; Pleschka, S.; Klenk, H.-D. Interdependence of hemagglutinin glycosylation and neuraminidase as regulators of influenza virus growth: A study by reverse genetics. J. Virol. 2000, 74, 6316–6323. [Google Scholar] [CrossRef]
- Matrosovich, M.N.; Matrosovich, T.Y.; Gray, T.; Roberts, N.A.; Klenk, H.-D. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J. Virol. 2004, 78, 12665–12667. [Google Scholar] [CrossRef]
- Bar-On, Y.; Glasner, A.; Meningher, T.; Achdout, H.; Gur, C.; Lankry, D.; Vitenshtein, A.; Meyers, A.F.; Mandelboim, M.; Mandelboim, O. Neuraminidase-mediated, NKp46-dependent immune-evasion mechanism of influenza viruses. Cell Rep. 2013, 3, 1044–1050. [Google Scholar] [CrossRef]
- Bar-On, Y.; Seidel, E.; Tsukerman, P.; Mandelboim, M.; Mandelboim, O. Influenza virus uses its neuraminidase protein to evade the recognition of two activating NK cell receptors. J. Infect. Dis. 2014, 210, 410–418. [Google Scholar] [CrossRef]
- Lin, Y.P.; Gregory, V.; Collins, P.; Kloess, J.; Wharton, S.; Cattle, N.; Lackenby, A.; Daniels, R.; Hay, A. Neuraminidase receptor binding variants of human influenza A(H3N2) viruses resulting from substitution of aspartic acid 151 in the catalytic site: A role in virus attachment? J. Virol. 2010, 84, 6769–6781. [Google Scholar] [CrossRef]
- Mohr, P.G.; Deng, Y.-M.; Mckimm-Breschkin, J.L. The neuraminidases of MDCK grown human influenza A(H3N2) viruses isolated since 1994 can demonstrate receptor binding. Virol. J. 2015, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- McCullers, J.A.; Bartmess, K.C. Role of neuraminidase in lethal synergism between influenza virus and streptococcus pneumoniae. J. Infect. Dis. 2003, 187, 1000–1009. [Google Scholar] [CrossRef]
- Peltola, V.T.; Murti, K.G.; McCullers, J.A. Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J. Infect. Dis. 2005, 192, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Hayden, F.G.; Treanor, J.J.; Fritz, R.S.; Lobo, M.; Betts, R.F.; Miller, M.; Kinnersley, N.; Mills, R.G.; Ward, P.; Straus, S.E. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza. JAMA 1999, 282, 1240–1246. [Google Scholar] [CrossRef]
- Nicholson, K.; Aoki, F.; Osterhaus, A.; Trottier, S.; Carewicz, O.; Mercier, C.; Rode, A.; Kinnersley, N.; Ward, P. Efficacy and safety of oseltamivir in treatment of acute influenza: A randomised controlled trial. Lancet 2000, 355, 1845–1850. [Google Scholar] [CrossRef]
- Dobson, J.; Whitley, R.; Pocock, S.; Monto, A.S. Oseltamivir treatment for influenza in adults: A meta-analysis of randomised controlled trials. Lancet 2015, 385, 1729–1737. [Google Scholar] [CrossRef]
- Petrie, J.G.; Ohmit, S.E.; Johnson, E.; Truscon, R.; Monto, A.S. Persistence of antibodies to influenza hemagglutinin and neuraminidase following one or two years of influenza vaccination. J. Infect. Dis. 2015, 212, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.E.; Bucher, D.J.; Kilbourne, E.D. Purified influenza virus hemagglutinin and neuraminidase are equivalent in stimulation of antibody response but induce contrasting types of immunity to infection. J. Virol. 1989, 63, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Memoli, M.J.; Shaw, P.A.; Han, A.; Czajkowski, L.; Reed, S.; Athota, R.; Bristol, T.; Fargis, S.; Risos, K.; Powers, J.H.; et al. Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1N1 virus healthy human challenge model. mBio 2016, 7, e00417-16. [Google Scholar] [CrossRef]
- Gao, J.; Couzens, L.; Burke, D.F.; Wan, H.; Wilson, P.; Memoli, M.J.; Xu, X.; Harvey, R.; Wrammert, J.; Ahmed, R.; et al. Antigenic drift of the influenza A(H1N1)pdm09 virus neuraminidase results in reduced effectiveness of A/California/7/2009 (H1N1pdm09)-specific antibodies. mBio 2019, 10. [Google Scholar] [CrossRef]
- Westgeest, K.B.; De Graaf, M.; Fourment, M.; Bestebroer, T.M.; Van Beek, R.; Spronken, M.I.J.; De Jong, J.C.; Rimmelzwaan, G.F.; Russell, C.A.; Osterhaus, A.D.; et al. Genetic evolution of the neuraminidase of influenza A (H3N2) viruses from 1968 to 2009 and its correspondence to haemagglutinin evolution. J. Gen. Virol. 2012, 93, 1996–2007. [Google Scholar] [CrossRef]
- Kilbourne, E.D.; Johansson, B.E.; Grajower, B. Independent and disparate evolution in nature of influenza A virus hemagglutinin and neuraminidase glycoproteins. Proc. Natl. Acad. Sci. USA 1990, 87, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Schulman, J.L.; Kilbourne, E.D. Independent variation in nature of hemagglutinin and neuraminidase antigens of influenza virus: Distinctiveness of hemagglutinin antigen of Hong Kong/68 virus. Proc. Natl. Acad. Sci. USA 1969, 63, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Wohlbold, T.J.; Podolsky, K.A.; Chromikova, V.; Kirkpatrick, E.; Falconieri, V.; Meade, P.; Amanat, F.; Tan, J.; Tenoever, B.R.; Tan, G.S.; et al. Broadly protective murine monoclonal antibodies against influenza B virus target highly conserved neuraminidase epitopes. Nat. Microbiol. 2017, 2, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Kilbourne, E.D.; Laver, W.G.; Schulman, J.L.; Webster, R.G. Antiviral activity of antiserum specific for an influenza virus neuraminidase. J. Virol. 1968, 2, 281–288. [Google Scholar] [CrossRef]
- Madeley, C.R.; Kendal, A.P. Flocculation of influenza virus by specific anti-neuraminidase antibody. Arch. Virol. 1970, 31, 219–229. [Google Scholar] [CrossRef][Green Version]
- Schulman, J.L.; Khakpour, M.; Kilbourne, E.D. Protective effects of specific immunity to viral neuraminidase on influenza virus infection of mice. J. Virol. 1968, 2, 778–786. [Google Scholar] [CrossRef]
- Johansson, B. Immunization with influenza A virus hemagglutinin and neuraminidase produced in recombinant baculovirus results in a balanced and broadened immune response superior to conventional vaccine. Vaccine 1999, 17, 2073–2080. [Google Scholar] [CrossRef]
- Johansson, B.E.; Kilbourne, E.D. Comparative long-term effects in a mouse model system of influenza whole virus and purified neuraminidase vaccines followed by sequential infections. J. Infect. Dis. 1990, 162, 800–809. [Google Scholar] [CrossRef]
- Johansson, B.E.; Kilbourne, E.D. Programmed antigenic stimulation: Kinetics of the immune response to challenge infections of mice primed with influenza inactivated whole virus or neuraminidase vaccine. Vaccine 1991, 9, 330–333. [Google Scholar] [CrossRef]
- Johansson, B.E.; Grajower, B.; Kilbourne, E.D. Infection-permissive immunization with influenza virus neuraminidase prevents weight loss in infected mice. Vaccine 1993, 11, 1037–1039. [Google Scholar] [CrossRef]
- Johansson, B.E.; Kilbourne, E.D. Immunization with purified N1 and N2 influenza virus neuraminidases demonstrates cross-reactivity without antigenic competition. Proc. Natl. Acad. Sci. USA 1994, 91, 2358–2361. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.E.; Matthews, J.; Kilbourne, E.D. Supplementation of conventional influenza A vaccine with purified viral neuraminidase results in a balanced and broadened immune response. Vaccine 1998, 16, 1009–1015. [Google Scholar] [CrossRef]
- Webster, R.; Reay, P.; Laver, W. Protection against lethal influenza with neuraminidase. Virology 1988, 164, 230–237. [Google Scholar] [CrossRef]
- Bosch, B.J.; Bodewes, R.; De Vries, R.P.; Kreijtz, J.H.C.M.; Bartelink, W.; Van Amerongen, G.; Rimmelzwaan, G.F.; De Haan, C.A.M.; Osterhaus, A.D.; Rottier, P.J. Recombinant soluble, multimeric HA and NA exhibit distinctive types of protection against pandemic swine-origin 2009 A(H1N1) influenza virus infection in ferrets. J. Virol. 2010, 84, 10366–10374. [Google Scholar] [CrossRef]
- McMahon, M.; Kirkpatrick, E.; Stadlbauer, D.; Strohmeier, S.; Bouvier, N.M.; Krammer, F. Mucosal immunity against neuraminidase prevents influenza B virus transmission in guinea pigs. mBio 2019, 10, e00560-19. [Google Scholar] [CrossRef]
- Schulman, J.L. Effects of immunity on transmission of influenza: Experimental studies. Prog. Med. Virol. 1970, 12, 128. [Google Scholar]
- DeRoo, T.; Jou, W.M.; Fiers, W. Recombinant neuraminidase vaccine protects against lethal influenza. Vaccine 1996, 14, 561–569. [Google Scholar] [CrossRef]
- Walz, L.; Kays, S.-K.; Zimmer, G.; Von Messling, V. Neuraminidase-inhibiting antibody titers correlate with protection from heterologous influenza virus strains of the same neuraminidase subtype. J. Virol. 2018, 92, JVI.01006. [Google Scholar] [CrossRef]
- Johansson, B.E.; Kilbourne, E.D. Dissociation of influenza virus hemagglutinin and neuraminidase eliminates their intravirionic antigenic competition. J. Virol. 1993, 67, 5721–5723. [Google Scholar] [CrossRef]
- Johansson, B.E.; Price, P.M.; Kilbourne, E.D. Immunogenicity of influenza A virus N2 neuraminidase produced in insect larvae by baculovirus recombinants. Vaccine 1995, 13, 841–845. [Google Scholar] [CrossRef]
- Johansson, B.E.; Pokorny, B.A.; Tiso, V.A. Supplementation of conventional trivalent influenza vaccine with purified viral N1 and N2 neuraminidases induces a balanced immune response without antigenic competition. Vaccine 2002, 20, 1670–1674. [Google Scholar] [CrossRef]
- Johansson, B.E.; Brett, I.C. Recombinant influenza B virus HA and NA antigens administered in equivalent amounts are immunogenically equivalent and induce equivalent homotypic and broader heterovariant protection in mice than conventional and live influenza vaccines. Hum. Vaccines 2008, 4, 420–424. [Google Scholar] [CrossRef]
- Brett, I.C.; Johansson, B.E. Immunization against influenza A virus: Comparison of conventional inactivated, live-attenuated and recombinant baculovirus produced purified hemagglutinin and neuraminidase vaccines in a murine model system. Virology 2005, 339, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Ko, E.-J.; Kim, M.-C.; Lee, Y.-N.; Kim, K.-H.; Jung, Y.-J.; Kang, S.-M. Roles of antibodies to influenza A virus hemagglutinin, neuraminidase, and M2e in conferring cross protection. Biochem. Biophys. Res. Commun. 2017, 493, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Martinet, W.; Saelens, X.; DeRoo, T.; Neirynck, S.; Contreras, R.; Jou, W.M.; Fiers, W. Protection of mice against a lethal influenza challenge by immunization with yeast-derived recombinant influenza neuraminidase. JBIC J. Boil. Inorg. Chem. 1997, 247, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Hocart, M.; Grajower, B.; Donabedian, A.; Pokorny, B.; Whitaker, C.; Kilbourne, E.D. Preparation and characterization of a purified influenza virus neuraminidase vaccine. Vaccine 1995, 13, 1793–1798. [Google Scholar] [CrossRef]
- Lu, X.; Liu, F.; Zeng, H.; Sheu, T.; Achenbach, J.E.; Veguilla, V.; Gubareva, L.; Garten, R.; Smith, C.; Yang, H.; et al. Evaluation of the antigenic relatedness and cross-protective immunity of the neuraminidase between human influenza A (H1N1) virus and highly pathogenic avian influenza A (H5N1) virus. Virology 2014, 454, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Kilbourne, E.D.; Couch, R.B.; Kasel, J.A.; Keitel, W.A.; Cate, T.R.; Quarles, J.H.; Grajower, B.; Pokorny, B.A.; Johansson, B.E. Purified influenza A virus N2 neuraminidase vaccine is immunogenic and non-toxic in humans. Vaccine 1995, 13, 1799–1803. [Google Scholar] [CrossRef]
- Marcelin, G.; Dubois, R.M.; Rubrum, A.; Russell, C.J.; McElhaney, J.E.; Webby, R.J. A contributing role for anti-neuraminidase antibodies on immunity to pandemic H1N1 2009 influenza A virus. PLoS ONE 2011, 6, e26335. [Google Scholar] [CrossRef]
- Rott, R.; Becht, H.; Orlich, M. The significance of influenza virus neuraminidase in immunity. J. Gen. Virol. 1974, 22, 35–41. [Google Scholar] [CrossRef]
- Sandbulte, M.R.; Jimenez, G.S.; Boon, A.C.M.; Smith, L.R.; Treanor, J.J.; Webby, R.J. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 2007, 4, e59. [Google Scholar] [CrossRef] [PubMed]
- Easterbrook, J.D.; Schwartzman, L.M.; Gao, J.; Kash, J.C.; Morens, D.M.; Couzens, L.; Wan, H.; Eichelberger, M.C.; Taubenberger, J.K. Protection against a lethal H5N1 influenza challenge by intranasal immunization with virus-like particles containing 2009 pandemic H1N1 neuraminidase in mice. Virology 2012, 432, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Lee, Y.-T.; Park, S.; Jung, Y.-J.; Lee, Y.; Ko, E.-J.; Kim, Y.-J.; Li, X. (Sean); Kang, S.-M. Neuraminidase expressing virus-like particle vaccine provides effective cross protection against influenza virus. Virology 2019, 535, 179–188. [Google Scholar] [CrossRef]
- Rockman, S.; Brown, L.E.; Barr, I.G.; Gilbertson, B.; Lowther, S.; Kachurin, A.; Kachurina, O.; Klippel, J.; Bodle, J.; Pearse, M.; et al. Neuraminidase-inhibiting antibody is a correlate of cross-protection against lethal H5N1 influenza virus in ferrets immunized with seasonal influenza vaccine. J. Virol. 2013, 87, 3053–3061. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.E.; Sun, X.; Bai, Y.; Liu, Y.V.; Massare, M.J.; Pearce, M.B.; Belser, J.A.; Maines, T.R.; Creager, H.M.; Glenn, G.M.; et al. Neuraminidase-based recombinant virus-like particles protect against lethal avian influenza A(H5N1) virus infection in ferrets. Virology 2017, 509, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Van Reeth, K.; Braeckmans, D.; Cox, E.; Van Borm, S.; Berg, T.V.D.; Goddeeris, B.; De Vleeschauwer, A. Prior infection with an H1N1 swine influenza virus partially protects pigs against a low pathogenic H5N1 avian influenza virus. Vaccine 2009, 27, 6330–6339. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Kadowaki, S.-E.; Hagiwara, Y.; Yoshikawa, T.; Matsuo, K.; Kurata, T.; Tamura, S. Cross-protection against a lethal influenza virus infection by DNA vaccine to neuraminidase. Vaccine 2000, 18, 3214–3222. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Yeh, Y.-C.; Chan, J.-T.; Yang, Y.-C.; Yang, J.-R.; Liu, M.-T.; Wu, H.-S.; Hsiao, P.-W. A VLP vaccine induces broad-spectrum cross-protective antibody immunity against H5N1 and H1N1 subtypes of influenza A virus. PLoS ONE 2012, 7, e42363. [Google Scholar] [CrossRef]
- Quan, F.-S.; Kim, M.-C.; Lee, B.-J.; Song, J.-M.; Compans, R.W.; Kang, S.-M. Influenza M1 VLPs containing neuraminidase induce heterosubtypic cross-protection. Virology 2012, 430, 127–135. [Google Scholar] [CrossRef]
- Wan, H.; Gao, J.; Xu, K.; Chen, H.; Couzens, L.K.; Rivers, K.H.; Easterbrook, J.D.; Yang, K.; Zhong, L.; Rajabi, M.; et al. Molecular basis for broad neuraminidase immunity: Conserved epitopes in seasonal and pandemic H1N1 as well as H5N1 influenza viruses. J. Virol. 2013, 87, 9290–9300. [Google Scholar] [CrossRef]
- Job, E.R.; Schotsaert, M.; Ibañez, L.I.; Smet, A.; Ysenbaert, T.; Roose, K.; Dai, M.; De Haan, C.A.M.; Kleanthous, H.; Vogel, T.U.; et al. Antibodies directed towards neuraminidase N1 control disease in a mouse model of influenza. J. Virol. 2017, 92, JVI.01584-17. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Q.; Wohlbold, T.J.; Zheng, N.-Y.; Huang, M.; Huang, Y.; Neu, K.E.; Lee, J.; Wan, H.; Rojas, K.T.; Kirkpatrick, E.; et al. Influenza infection in humans induces broadly cross-reactive and protective neuraminidase-reactive antibodies. Cell 2018, 173, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-C.; Lin, C.-Y.; Tsou, Y.; Jan, J.-T.; Wu, S.-C. Cross-reactive neuraminidase-inhibiting antibodies elicited by immunization with recombinant neuraminidase proteins of H5N1 and pandemic H1N1 influenza A viruses. J. Virol. 2015, 89, 7224–7234. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.M.; Hashem, A.M.; Li, C.; Van Domselaar, G.; Larocque, L.; Wang, J.; Smith, D.; Cyr, T.; Farnsworth, A.; He, R.; et al. Universal anti-neuraminidase antibody inhibiting all influenza A subtypes. Antivir. Res. 2013, 100, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, D.; Zhu, X.; McMahon, M.; Turner, J.S.; Wohlbold, T.J.; Schmitz, A.J.; Strohmeier, S.; Yu, W.; Nachbagauer, R.; Mudd, P.A.; et al. Broadly protective human antibodies that target the active site of influenza virus neuraminidase. Science 2019, 366, 499–504. [Google Scholar] [CrossRef]
- Watanabe, Y.; Shiratsuchi, A.; Shimizu, K.; Takizawa, T.; Nakanishi, Y. Stimulation of phagocytosis of influenza virus-infected cells through surface desialylation of macrophages by viral neuraminidase. Microbiol. Immunol. 2004, 48, 875–881. [Google Scholar] [CrossRef]
- Wysocka, M.; Eisenlohr, L.; Otvos, L.; Horowitz, D.; Yewdell, J.; Bennink, J.R.; Hackett, C. Identification of overlapping class I and class II H-2d-restricted T cell determinants of influenza virus N1 neuraminidase that require infectious virus for presentation. Virology 1994, 201, 86–94. [Google Scholar] [CrossRef]
- Arora, D.J.; Houde, M. Purified glycoproteins of influenza virus stimulate cell-mediated cytotoxicity In Vivo. Nat. Immun. Cell Growth Regul. 1988, 7, 287–296. [Google Scholar]
- Arora, D.J.; Houde, M.; Justewicz, D.M.; Mandeville, R. In Vitro enhancement of human natural cell-mediated cytotoxicity by purified influenza virus glycoproteins. J. Virol. 1984, 52, 839–845. [Google Scholar] [CrossRef]
- Sinkovics, J.G. Neuraminidases and hemagglutinins of influenza viruses augment cell-mediated cytotoxicity by activating NK cells. Nat. Immun. Cell Growth Regul. 1989, 8, 187–188. [Google Scholar]
- Oh, S.; Belz, G.T.; Eichelberger, M.C. Viral neuraminidase treatment of dendritic cells enhances antigen-specific CD8+ T cell proliferation, but does not account for the CD4+ T cell independence of the CD8+ T cell response during influenza virus infection. Virology 2001, 286, 403–411. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schotsaert, M.; Ysenbaert, T.; Smet, A.; Schepens, B.; Vanderschaeghe, D.; Stegalkina, S.; Vogel, T.U.; Callewaert, N.; Fiers, W.; Saelens, X. Long-lasting cross-protection against influenza A by neuraminidase and M2e-based immunization strategies. Sci. Rep. 2016, 6, 24402. [Google Scholar] [CrossRef] [PubMed]
- Scholtissek, C.; Rohde, W.; Von Hoyningen, V.; Rott, R. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology 1978, 87, 13–20. [Google Scholar] [CrossRef]
- Monto, A.; Kendal, A. Effect of neuraminidase antibody on Hong Kong influenza. Lancet 1973, 301, 623–625. [Google Scholar] [CrossRef]
- Yamane, N.; Odagiri, T.; Arikawa, J.; Kumasaka, M.; Ishida, N. Effect of specific immunity to viral neuraminidase on subsequent influenza virus infection in man. Microbiol. Immunol. 1979, 23, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.R.; Kasel, J.A.; Chanock, R.M. Association of Serum Anti-Neuraminidase Antibody with resistance to influenza in man. N. Engl. J. Med. 1972, 286, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Arrobio, J.O.; Brandt, C.D.; Parrott, R.H.; Murphy, B.R.; Richman, U.D.; Chanock, R.M. Temperature-sensitive mutants of influenza A virus: Response of children to the influenza A/Hong Kong/68-ts-1[E] (H3N2) and influenza A/Udorn/72-ts-1[E] (H3N2) candidate vaccine viruses and significance of immunity to neuraminidase antigen. Pediatr. Res. 1976, 10, 238–242. [Google Scholar] [CrossRef]
- Park, J.-K.; Han, A.; Czajkowski, L.; Reed, S.; Athota, R.; Bristol, T.; Rosas, L.A.; Cervantes-Medina, A.; Taubenberger, J.K.; Memoli, M.J. Evaluation of preexisting anti-hemagglutinin stalk antibody as a correlate of protection in a healthy volunteer challenge with influenza A/H1N1pdm virus. mBio 2018, 9, e02284-17. [Google Scholar] [CrossRef]
- Couch, R.B.; Atmar, R.L.; Franco, L.M.; Quarles, J.M.; Wells, J.; Arden, N.; Niño, D.; Belmont, J.W. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J. Infect. Dis. 2013, 207, 974–981. [Google Scholar] [CrossRef]
- Maier, H.E.; Nachbagauer, R.; Kuan, G.; Ng, S.; Lopez, R.; Sanchez, N.; Stadlbauer, D.; Gresh, L.; Schiller, A.; Rajabhathor, A.; et al. Pre-existing antineuraminidase antibodies are associated with shortened duration of influenza A(H1N1)pdm virus shedding and illness in naturally infected adults. Clin. Infect. Dis. 2019, 70, 2290–2297. [Google Scholar] [CrossRef]
- Petrie, J.G.; Malosh, R.E.; Cheng, C.K.; Ohmit, S.E.; Martin, E.T.; Johnson, E.; Truscon, R.; Eichelberger, M.C.; Gubareva, L.V.; Fry, A.M.; et al. The household influenza vaccine effectiveness study: Lack of antibody response and protection following receipt of 2014–2015 influenza vaccine. Clin. Infect. Dis. 2017, 65, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Monto, A.S.; Petrie, J.G.; Cross, R.T.; Johnson, E.; Liu, M.; Zhong, W.; Levine, M.; Katz, J.M.; Ohmit, S.E. Antibody to influenza virus neuraminidase: An independent correlate of protection. J. Infect. Dis. 2015, 212, 1191–1199. [Google Scholar] [CrossRef]
- Slepushkin, A.N.; Schild, G.C.; Beare, A.S.; Chinn, S.; Tyrrell, D.A.J. Neuraminidase and resistance to vaccination with live influenza A2 Hong Kong vaccines. Epidemiol. Infect. 1971, 69, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Beutner, K.R.; Chow, T.; Rubi, E.; Strussenberg, J.; Clément, J.; Ogra, P.L. Evaluation of a neuraminidase-specific influenza A virus vaccine in children: Antibody responses and effects on two successive outbreaks of natural infection. J. Infect. Dis. 1979, 140, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, T.C.; Meiklejohn, G. Protection against Hong Kong influneza by adjuvant vaccine containing A2-Ann Arbor-67. Bull. World Health Organ. 1969, 41, 562–563. [Google Scholar]
- Dowdle, W.R.; Coleman, M.T.; Mostow, S.R.; Kaye, H.S.; Schoenbaum, S.C. Inactivated vaccines. 2. Laboratory indices of protection. Postgrad. Med. J. 1973, 49, 159–163. [Google Scholar] [CrossRef]
- Kendal, A.P.; Noble, G.R.; Dowdle, W.R. Neuraminidase content of influenza vaccines and neuraminidase antibody responses after vaccination of immunologically primed and unprimed populations. J. Infect. Dis. 1977, 136, S415–S424. [Google Scholar] [CrossRef]
- Gravel, C.; Li, C.; Wang, J.; Hashem, A.; Jaentschke, B.; Xu, K.-W.; Lorbetskie, B.; Gingras, G.; Aubin, Y.; Van Domselaar, G.; et al. Qualitative and quantitative analyses of virtually all subtypes of influenza A and B viral neuraminidases using antibodies targeting the universally conserved sequences. Vaccine 2010, 28, 5774–5784. [Google Scholar] [CrossRef]
- Wohlbold, T.J.; Nachbagauer, R.; Xu, H.; Tan, G.; Hirsh, A.; Brokstad, K.A.; Cox, R.J.; Palese, P.; Krammer, F. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. mBio 2015, 6, e02556-14. [Google Scholar] [CrossRef]
- Aymard, M.; Ferraris, O.; Gerentes, L.; Jolly, J.; Kessler, N. Neuraminidase assays. Dev. Biol. (Basel) 2003, 115, 75–83. [Google Scholar]
- Kendal, A.P.; Bozeman, F.M.; Ennis, F.A. Further studies of the neuraminidase content of inactivated influenza vaccines and the neuraminidase antibody responses after vaccination of immunologically primed and unprimed populations. Infect. Immun. 1980, 29, 966–971. [Google Scholar] [PubMed]
- Couch, R.B.; Atmar, R.L.; Keitel, W.; Quarles, J.M.; Wells, J.; Arden, N.; Niño, D. Randomized comparative study of the serum antihemagglutinin and antineuraminidase antibody responses to six licensed trivalent influenza vaccines. Vaccine 2012, 31, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Fedson, D.S.; Fulk, R.V.; Huber, M.A.; Reisberg, M.A.; Kasel, J.A. Anti-neuraminidase antibody response in serum and nasal secretions following intranasal or subcutaneous inactivated A2-Hong Kong-68 influenza virus vaccine. J. Immunol. 1971, 107, 730–737. [Google Scholar] [PubMed]
- Couch, R.B.; Douglas, R.G.; Fedson, D.S.; Kasel, J.A. Correlated studies of a recombinant influenza-virus vaccine. III. Protection against experimental influenza in man. J. Infect. Dis. 1971, 124, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Cate, T.R.; Rayford, Y.; Niño, D.; Winokur, P.; Brady, R.; Belshe, R.; Chen, W.; Atmar, R.L.; Couch, R.B. A high dosage influenza vaccine induced significantly more neuraminidase antibody than standard vaccine among elderly subjects. Vaccine 2010, 28, 2076–2079. [Google Scholar] [CrossRef]
- Gao, Z.; Robinson, K.; Skowronski, D.M.; De Serres, G.; Withers, S.G. Quantification of the total neuraminidase content of recent commercially-available influenza vaccines: Introducing a neuraminidase titration reagent. Vaccine 2020, 38, 715–718. [Google Scholar] [CrossRef]
- Fritz, R.; Sabarth, N.; Kiermayr, S.; Hohenadl, C.; Howard, M.K.; Ilk, R.; Kistner, O.; Ehrlich, H.J.; Barrett, P.N.; Kreil, T.R. A vero cell–derived whole-virus H5N1 vaccine effectively induces neuraminidase-inhibiting antibodies. J. Infect. Dis. 2011, 205, 28–34. [Google Scholar] [CrossRef]
- Aminoff, D. The determination of free sialic acid in the presence of the bound compound. Virology 1959, 7, 355–357. [Google Scholar] [CrossRef]
- Warren, L. The thiobarbituric acid assay of sialic acids. J. Boil. Chem. 1959, 234, 1971–1975. [Google Scholar]
- Gérentes, L.; Kessler, N.; Aymard, M. Difficulties in standardizing the neuraminidase content of influenza vaccines. Dev. Boil. Stand. 1999, 98, 189–196. [Google Scholar]
- Sandbulte, M.R.; Gao, J.; Straight, T.M.; Eichelberger, M.C. A miniaturized assay for influenza neuraminidase-inhibiting antibodies utilizing reverse genetics-derived antigens. Influenza Other Respir. Viruses 2009, 3, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Couzens, L.; Gao, J.; Westgeest, K.; Sandbulte, M.; Lugovtsev, V.Y.; Fouchier, R.; Eichelberger, M.C. An optimized enzyme-linked lectin assay to measure influenza A virus neuraminidase inhibition antibody titers in human sera. J. Virol. Methods 2014, 210, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, M.; Nachbagauer, R.; Ermler, M.E.; Bunduc, P.; Amanat, F.; Izikson, R.; Cox, M.; Palese, P.; Eichelberger, M.; Krammer, F. Analysis of anti-influenza virus neuraminidase antibodies in children, adults, and the elderly by ELISA and enzyme inhibition: Evidence for original antigenic sin. mBio 2017, 8, e02281-16. [Google Scholar] [CrossRef] [PubMed]
- Kon, T.C.; Onu, A.; Berbecila, L.; Lupulescu, E.; Ghiorgisor, A.; Kersten, G.F.; Cui, Y.-Q.; Amorij, J.-P.; Van Der Pol, L. Influenza vaccine manufacturing: Effect of inactivation, splitting and site of manufacturing. Comparison of influenza vaccine production processes. PLoS ONE 2016, 11, e0150700. [Google Scholar] [CrossRef]
- Gerdil, C. The annual production cycle for influenza vaccine. Vaccine 2003, 21, 1776–1779. [Google Scholar] [CrossRef]
- Harris, A.; Cardone, G.; Winkler, D.C.; Heymann, J.B.; Brecher, M.; White, J.M.; Steven, A.C. Influenza virus pleiomorphy characterized by cryoelectron tomography. Proc. Natl. Acad. Sci. USA 2006, 103, 19123–19127. [Google Scholar] [CrossRef]
- Wang, H.; Dou, D.; Östbye, H.; Revol, R.; Daniels, R. Structural restrictions for influenza neuraminidase activity promote adaptation and diversification. Nat. Microbiol. 2019, 4, 2565–2577. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Chang, S.-H.; Gong, X.; Wu, J.; Liu, B. Expression, purification and characterization of low-glycosylation influenza neuraminidase in α-1,6-mannosyltransferase defective Pichia pastoris. Mol. Boil. Rep. 2011, 39, 857–864. [Google Scholar] [CrossRef]
- Couch, R.B.; Kasel, J.A.; Gerin, J.L.; Schulman, J.L.; Kilbourne, E.D. Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J. Infect. Dis. 1974, 129, 411–420. [Google Scholar] [CrossRef]
- Fries, L.F.; Smith, G.E.; Glenn, G.M. A recombinant viruslike particle influenza A (H7N9) vaccine. N. Engl. J. Med. 2013, 369, 2564–2566. [Google Scholar] [CrossRef]
- Kilbourne, E.D. Comparative efficacy of neuraminidase-specific and conventional influenza virus vaccines in induction of antibody to neuraminidase in humans. J. Infect. Dis. 1976, 134, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Gostic, K.M.; Ambrose, M.; Worobey, M.; Lloyd-Smith, J.O. Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science 2016, 354, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.E.; Moran, T.M.; Bona, C.A.; Popple, S.W.; Kilbourne, E.D. Immunologic response to influenza virus neuraminidase is influenced by prior experience with the associated viral hemagglutinin. II. Sequential infection of mice simulates human experience. J. Immunol. 1987, 139, 2010–2014. [Google Scholar] [PubMed]
- Kilbourne, E.D.; Cerini, C.P.; Khan, M.W.; Mitchell, J.W.; Ogra, P.L. Immunologic response to the influenza virus neuraminidase is influenced by prior experience with the associated viral hemagglutinin. I. Studies in human vaccinees. J. Immunol. 1987, 138, 3010–3013. [Google Scholar] [PubMed]
| Subject | Neuraminidase Manufacturing Strategy | Outcome in NA-Vaccinated Subjects | Reference |
|---|---|---|---|
| White leghorn chickens | Electrophoresis-purified influenza A N2 | Increased NAI titers, decreased tracheal and cloacal viral titers | [64] |
| Guinea pigs | Baculovirus expression system influenza B NA | NAI titers, increased ELISA antibody titers, decreased nasal wash virus titers, decreased transmission | [66] |
| Manor Farm (MF-1) mice | Electrophoresis-purified influenza A N2 | Increased NAI titers, decreased pulmonary virus titers, diminished lung lesions | [57] |
| BALB/c mice | Chromatography-purified influenza A N2 | Increased NAI titers, increased ELISA antibody titers, decreased weight loss, decreased pulmonary virus titers with homotypic and heterotypic challenge | [48,59,60,61,62,63,70] |
| BALB/c mice | Baculovirus expression system influenza A N1, N2 and influenza B NA | Increased NAI titers, increased ELISA antibody titers, decreased pulmonary virus titers with homotypic and heterotypic challenge | [58,68,71,72,73,74,75] |
| BALB/c mice | Yeast expression system influenza A N2 | Increased survival | [76] |
| New Zealand rabbits | Chromatography-purified influenza A N2 | Increased NAI titers | [77,78] |
| Ferrets | Human embryonic kidney cell expression system influenza A N1 | Increased NAI titers, decreased pulmonary virus titers, decreased lung pathology | [65] |
| Humans | Chromatography-purified influenza A N2 | Increased NAI titers, increased ELISA antibody titers | [79] |
| Vaccine Type | Vaccine Year | NA Activity (mU/mL) | NA Concentration (μg/mL) | Reference |
|---|---|---|---|---|
| Monovalent whole virus (H3N2) | 1968/1969 | 112,000 a | 43 | [118] |
| Monovalent split virus (B) | 1973/1974 | 21,000 a | 165 | [118] |
| Bivalent whole virus (H3N2 + B) | 1973/1974 | 78,000 a | 284 | [118] |
| Bivalent whole virus (H3N2 + B) | 1974/1975 | 164,000–184,000 a | 372–692 | [118] |
| Trivalent whole virus | 1975/1976 | 206,000 a | 596 | [118] |
| Trivalent split virus | 1975/1976 | 50,000 a | 135 | [118] |
| Bivalent whole virus (H3N2 + H1N1) | 1976/1977 | 10,400-60,000 a | 81–242 | [118] |
| Monovalent whole virus (H1N1) | 1976/1977 | <500 a | 45–98 | [118] |
| Monovalent (pH1N1) c | 2009 | - | 0.73–5.28 | [119] |
| Monovalent (pH1N1) c | 2009 | 2–56 b | 9 | [16] |
| Trivalent split virus | 2008/2009 | 194–3293 b | - | [16] |
| Trivalent c | 2011/2012 | 2–3105 b | 22 | [16] |
| Trivalent c | 2012/2013 | 4521 b | - | [16] |
| Trivalent subunit (egg derived) | 2013/2014 | - | 5 | [120] |
| Trivalent subunit (cell derived) | 2013/2014 | - | 0.02 | [120] |
| Trivalent split virus (egg derived) | 2013/2014 | - | 10.5 | [120] |
| Trivalent split virus (egg derived) | 2013/2014 | - | 4.4 | [120] |
| Quadrivalent split virus (egg derived) | 2015/2016 | - | 2.7 | [127] |
| Quadrivalent split virus (egg derived) | 2015/2016 | - | 3.9 | [127] |
| Quadrivalent split virus high-dose (egg derived) | 2015/2016 | - | 12.9 | [127] |
| Trivalent split virus (egg derived) | 2015/2016 | - | 2.4 | [127] |
| Trivalent subunit (egg derived) | 2015/2016 | - | 3.4 | [127] |
| Quadrivalent live-attenuated virus | 2015/2016 | - | 1.1 | [127] |
| Quadrivalent split virus (egg derived) | 2016/2017 | - | 2.8 | [127] |
| Quadrivalent split virus (egg derived) | 2016/2017 | - | 3.5 | [127] |
| Quadrivalent split virus high-dose (egg derived) | 2016/2017 | - | 9.5 | [127] |
| Trivalent split virus (egg derived) | 2016/2017 | - | 1.6 | [127] |
| Trivalent subunit (egg derived) | 2016/2017 | - | 2.8 | [127] |
| Quadrivalent live-attenuated virus | 2016/2017 | - | 0.4 | [127] |
| Quadrivalent split virus (egg derived) | 2017/2018 | - | 2.0 | [127] |
| Quadrivalent split virus (egg derived) | 2017/2018 | - | 3.2 | [127] |
| Quadrivalent split virus high-dose (egg derived) | 2017/2018 | - | 7.9 | [127] |
| Trivalent split virus (egg derived) | 2017/2018 | - | 1.5 | [127] |
| Trivalent subunit (egg derived) | 2017/2018 | - | 3.1 | [127] |
| Quadrivalent live-attenuated virus | 2017/2018 | - | 0.7 | [127] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giurgea, L.T.; Morens, D.M.; Taubenberger, J.K.; Memoli, M.J. Influenza Neuraminidase: A Neglected Protein and Its Potential for a Better Influenza Vaccine. Vaccines 2020, 8, 409. https://doi.org/10.3390/vaccines8030409
Giurgea LT, Morens DM, Taubenberger JK, Memoli MJ. Influenza Neuraminidase: A Neglected Protein and Its Potential for a Better Influenza Vaccine. Vaccines. 2020; 8(3):409. https://doi.org/10.3390/vaccines8030409
Chicago/Turabian StyleGiurgea, Luca T., David M. Morens, Jeffery K. Taubenberger, and Matthew J. Memoli. 2020. "Influenza Neuraminidase: A Neglected Protein and Its Potential for a Better Influenza Vaccine" Vaccines 8, no. 3: 409. https://doi.org/10.3390/vaccines8030409
APA StyleGiurgea, L. T., Morens, D. M., Taubenberger, J. K., & Memoli, M. J. (2020). Influenza Neuraminidase: A Neglected Protein and Its Potential for a Better Influenza Vaccine. Vaccines, 8(3), 409. https://doi.org/10.3390/vaccines8030409





