Evidence for Anti-Viral Effects of Complete Freund’s Adjuvant in the Mouse Model of Enterovirus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Virus Propagation and Infection
2.3. Challenge Studies in Animals Immunized with CFA
2.4. Histology
2.5. Statistics
3. Results and Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BCG | Bacillus Calmette–Guérin |
| CFA | Complete Freund’s adjuvant |
| CVB | Coxsackievirus B |
| EMEM | Eagle’s minimum essential medium |
| FBS | Fetal bovine serum |
| IFN | Interferon |
| I.p. | Intraperitoneal |
| M.tb | Mycobacterium tuberculosis |
| NK | Natural killer |
| PBS | Phosphate-buffered saline |
| RNA | Ribonucleic acid |
| T1D | Type 1 diabetes |
| Th | T helper |
| VP | Viral protein |
References
- Baggen, J.; Thibaut, H.J.; Strating, J.R.P.M.; Van Kuppeveld, F.J.M. The life cycle of non-polio enteroviruses and how to target it. Nat. Rev. Microbiol. 2018, 16, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Krilov, L.R. Enterovirus Infections. Pediatr. Rev. 2016, 37, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Pons-Salort, M.; Parker, E.P.K.; Grassly, N.C. The epidemiology of non-polio enteroviruses: Recent advances and outstanding questions. Curr. Opin. Infect. Dis. 2015, 28, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Lugo, D.; Krogstad, P. Enteroviruses in the early 21st century: New manifestations and challenges. Curr. Opin. Pediatr. 2016, 28, 107–113. [Google Scholar] [CrossRef]
- Midgley, C.M.; Watson, J.T.; Nix, W.A.; Curns, A.T.; Rogers, S.L.; Brown, B.A.; Conover, C.; Dominguez, S.R.; Feikin, D.R.; Gray, S.; et al. Severe respiratory illness associated with a nationwide outbreak of enterovirus D68 in the USA (2014): A descriptive epidemiological investigation. Lancet Respir. Med. 2015, 3, 879–887. [Google Scholar] [CrossRef]
- Cihakova, D.; Rose, N.R. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv. Immunol. 2008, 99, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Jaidane, H.; Hober, D. Role of coxsackievirus B4 in the pathogenesis of type 1 diabetes. Diabetes Metab. 2008, 34, 537–548. [Google Scholar] [CrossRef]
- Lasrado, N.; Gangaplara, A.; Arumugam, R.; Massilamany, C.; Pokal, S.; Zhou, Y.; Xiang, S.H.; Steffen, D.; Reddy, J. Identification of Immunogenic Epitopes That Permit the Detection of Antigen-Specific T Cell Responses in Multiple Serotypes of Group B Coxsackievirus Infections. Viruses 2020, 12, 347. [Google Scholar] [CrossRef]
- O’Hagan, D.T. Vaccine Adjuvants—Preparation Methods and Research Protocols; Springer: Berlin, Germany, 2000; Volume 42. [Google Scholar]
- Traub, S.; Von Aulock, S.; Hartung, T.; Hermann, C. MDP and other muropeptides--direct and synergistic effects on the immune system. J. Endotoxin Res. 2006, 12, 69–85. [Google Scholar] [CrossRef]
- Su, S.B.; Silver, P.B.; Grajewski, R.S.; Agarwal, R.K.; Tang, J.; Chan, C.C.; Caspi, R.R. Essential role of the MyD88 pathway, but nonessential roles of TLRs 2, 4, and 9, in the adjuvant effect promoting Th1-mediated autoimmunity. J. Immunol. 2005, 175, 6303–6310. [Google Scholar] [CrossRef]
- Gangaplara, A.; Massilamany, C.; Brown, D.M.; Delhon, G.; Pattnaik, A.K.; Chapman, N.; Rose, N.; Steffen, D.; Reddy, J. Coxsackievirus B3 infection leads to the generation of cardiac myosin heavy chain-α-reactive CD4 T cells in A/J mice. Clin. immunol. 2012, 144, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Gebhard, J.R.; Perry, C.M.; Harkins, S.; Lane, T.; Mena, I.; Asensio, V.C.; Campbell, I.L.; Whitton, J.L. Coxsackievirus B3-induced myocarditis: Perforin exacerbates disease, but plays no detectable role in virus clearance. Am. J. Pathol. 1998, 153, 417–428. [Google Scholar] [CrossRef]
- Crocker, S.J.; Frausto, R.F.; Whitmire, J.K.; Benning, N.; Milner, R.; Whitton, J.L. Amelioration of coxsackievirus B3-mediated myocarditis by inhibition of tissue inhibitors of matrix metalloproteinase-1. Am. J. Pathol. 2007, 171, 1762–1773. [Google Scholar] [CrossRef] [PubMed]
- Opie, E.L.; Freund, J. An Experimental Study of Protective Inoculation with Heat Killed Tubercle Bacilli. J. Exp. Med. 1937, 66, 761–788. [Google Scholar] [CrossRef]
- Massilamany, C.; Gangaplara, A.; Steffen, D.; Reddy, J. Identification of novel mimicry epitopes for cardiac myosin heavy chain-α that induce autoimmune myocarditis in A/J mice. Cell. Immunol. 2011, 271, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Massilamany, C.; Gangaplara, A.; Basavalingappa, R.H.; Rajasekaran, R.A.; Vu, H.; Riethoven, J.J.; Steffen, D.; Pattnaik, A.K.; Reddy, J. Mutations in the 5’ NTR and the Non-Structural Protein 3A of the Coxsackievirus B3 Selectively Attenuate Myocarditogenicity. PLoS ONE 2015, 10, e0131052. [Google Scholar] [CrossRef]
- Barnard, G.A. A New Test for 2 × 2 Tables. Nature 1945, 156, 177. [Google Scholar] [CrossRef]
- Fairweather, D.; Rose, N.R. Coxsackievirus-induced myocarditis in mice: A model of autoimmune disease for studying immunotoxicity. Methods 2007, 41, 118–122. [Google Scholar] [CrossRef]
- Comoy, E.E.; Capron, A.; Thyphronitis, G. Adjuvant is the major parameter influencing the isotype profiles generated during immunization with a protein antigen, the Schistosoma mansoni Sm28-GST. Scand. J. Immunol. 1998, 47, 444–452. [Google Scholar] [CrossRef]
- Leentjens, J.; Kox, M.; Stokman, R.; Gerretsen, J.; Diavatopoulos, D.A.; van Crevel, R.; Rimmelzwaan, G.F.; Pickkers, P.; Netea, M.G. BCG Vaccination Enhances the Immunogenicity of Subsequent Influenza Vaccination in Healthy Volunteers: A Randomized, Placebo-Controlled Pilot Study. J. Infect. Dis. 2015, 212, 1930–1938. [Google Scholar] [CrossRef]
- Scheid, A.; Borriello, F.; Pietrasanta, C.; Christou, H.; Diray-Arce, J.; Pettengill, M.A.; Joshi, S.; Li, N.; Bergelson, I.; Kollmann, T.; et al. Adjuvant Effect of Bacille Calmette-Guerin on Hepatitis B Vaccine Immunogenicity in the Preterm and Term Newborn. Front. Immunol. 2018, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Lodmell, D.L.; Ewalt, L.C. Enhanced resistance against encephalomyocarditis virus infection in mice, induced by a nonviable Mycobacterium tuberculosis oil-droplet vaccine. Infect. Immun. 1978, 19, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Starr, S.E.; Visintine, A.M.; Tomeh, M.O.; Nahmias, A.J. Effects of immunostimulants on resistance of newborn mice to herpes simplex type 2 infection. Proc. Soc. Exp. Biol. Med. 1976, 152, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Floch, F.; Werner, G.H. Increased resistance to virus infections of mice inoculated with BCG (Bacillus calmette-guerin). Ann. Immunol. (Paris) 1976, 127, 173–186. [Google Scholar]
- Moorlag, S.; Arts, R.J.W.; van Crevel, R.; Netea, M.G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019, 25, 1473–1478. [Google Scholar] [CrossRef]
- Mathurin, K.S.; Martens, G.W.; Kornfeld, H.; Welsh, R.M. CD4 T-Cell-Mediated Heterologous Immunity between Mycobacteria and Poxviruses. J. Virol. 2009, 83, 3528–3539. [Google Scholar] [CrossRef]
- Suenaga, T.; Okuyama, T.; Yoshida, I.; Azuma, M. Effect of Mycobacterium tuberculosis BCG infection on the resistance of mice to ectromelia virus infection: Participation of interferon in enhanced resistance. Infect. Immun. 1978, 20, 312–314. [Google Scholar] [CrossRef]
- Chu, R.S.; Targoni, O.S.; Krieg, A.M.; Lehmann, P.V.; Harding, C.V. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 1997, 186, 1623–1631. [Google Scholar] [CrossRef]
- Suzuki, Y.; Conley, F.K.; Remington, J.S. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J. Immunol. 1989, 143, 2045–2050. [Google Scholar]
- Shrestha, B.; Wang, T.; Samuel, M.A.; Whitby, K.; Craft, J.; Fikrig, E.; Diamond, M.S. Gamma interferon plays a crucial early antiviral role in protection against West Nile virus infection. J. Virol. 2006, 80, 5338–5348. [Google Scholar] [CrossRef]
- Yamagami, H.; Matsumoto, T.; Fujiwara, N.; Arakawa, T.; Kaneda, K.; Yano, I.; Kobayashi, K. Trehalose 6,6’-dimycolate (cord factor) of Mycobacterium tuberculosis induces foreign-body- and hypersensitivity-type granulomas in mice. Infect. Immun. 2001, 69, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Kuhn, C.; Feillet, H.; Bach, J.F. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: An update. Clin. Exp. Immunol. 2010, 160, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Dominguez-Andres, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; van der Meer, J.W. Trained Immunity: An Ancient Way of Remembering. Cell Host Microbe 2017, 21, 297–300. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef]
- van’t Wout, J.W.; Poell, R.; van Furth, R. The role of BCG/PPD-activated macrophages in resistance against systemic candidiasis in mice. Scand. J. Immunol. 1992, 36, 713–719. [Google Scholar] [CrossRef]
- Sher, N.A.; Chaparas, S.D.; Greenberg, L.E.; Bernard, S. Effects of BCG, Corynebacterium parvum, and methanol-extration residue in the reduction of mortality from Staphylococcus aureus and Candida albicans infections in immunosuppressed mice. Infect. Immun. 1975, 12, 1325–1330. [Google Scholar] [CrossRef]
- Tribouley, J.; Tribouley-Duret, J.; Appriou, M. Effect of Bacillus Callmette Guerin (BCG) on the receptivity of nude mice to Schistosoma mansoni. C. R. Seances Soc. Biol. Fil. 1978, 172, 902–904. [Google Scholar]
- Kuhtreiber, W.M.; Faustman, D.L. BCG Therapy for Type 1 Diabetes: Restoration of Balanced Immunity and Metabolism. Trends Endocrinol. Metab. 2019, 30, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Faustman, D.L. TNF, TNF inducers, and TNFR2 agonists: A new path to type 1 diabetes treatment. Diabetes Metab. Res. Rev. 2018, 34. [Google Scholar] [CrossRef] [PubMed]
- Ristori, G.; Romano, S.; Cannoni, S.; Visconti, A.; Tinelli, E.; Mendozzi, L.; Cecconi, P.; Lanzillo, R.; Quarantelli, M.; Buttinelli, C.; et al. Effects of Bacille Calmette-Guerin after the first demyelinating event in the CNS. Neurology 2014, 82, 41–48. [Google Scholar] [CrossRef]
- Ristori, G.; Faustman, D.; Matarese, G.; Romano, S.; Salvetti, M. Bridging the gap between vaccination with Bacille Calmette-Guerin (BCG) and immunological tolerance: The cases of type 1 diabetes and multiple sclerosis. Curr. Opin. Immunol. 2018, 55, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Karaci, M. The Protective Effect of the BCG Vaccine on the Development of Type 1 Diabetes in Humans. Value BCG TNF Autoimmun. 2014. [Google Scholar] [CrossRef]
- Shet, A.; Ray, D.; Malavige, N.; Santosham, M.; Bar-Zeev, N. Differential COVID-19-attributable mortality and BCG vaccine use in countries. medRxiv 2020. [Google Scholar] [CrossRef]
- Miller, A.; Reandelar, M.J.; Fasciglione, K.; Roumenova, V.; Li, Y.; Otazu, G.H. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: An epidemiological study. medRxiv 2020. [Google Scholar] [CrossRef]
- Jensen, K.J.; Karkov, H.S.; Lund, N.; Andersen, A.; Eriksen, H.B.; Barbosa, A.G.; Kantso, B.; Aaby, P.; Benn, C.S. The immunological effects of oral polio vaccine provided with BCG vaccine at birth: A randomised trial. Vaccine 2014, 32, 5949–5956. [Google Scholar] [CrossRef]
- Malone, K.M.; Rue-Albrecht, K.; Magee, D.A.; Conlon, K.; Schubert, O.T.; Nalpas, N.C.; Browne, J.A.; Smyth, A.; Gormley, E.; Aebersold, R.; et al. Comparative ’omics analyses differentiate Mycobacterium tuberculosis and Mycobacterium bovis and reveal distinct macrophage responses to infection with the human and bovine tubercle bacilli. Microb. Genom. 2018, 4. [Google Scholar] [CrossRef]
- Garnier, T.; Eiglmeier, K.; Camus, J.C.; Medina, N.; Mansoor, H.; Pryor, M.; Duthoy, S.; Grondin, S.; Lacroix, C.; Monsempe, C.; et al. The complete genome sequence of Mycobacterium bovis. Proc. Natl. Acad. Sci. USA 2003, 100, 7877–7882. [Google Scholar] [CrossRef]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonca, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.C.; et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 2018, 172, 176–190. [Google Scholar] [CrossRef]
- Fairweather, D.; Yusung, S.; Frisancho, S.; Barrett, M.; Gatewood, S.; Steele, R.; Rose, N.R. IL-12 receptor beta 1 and Toll-like receptor 4 increase IL-1 beta- and IL-18-associated myocarditis and coxsackievirus replication. J. Immunol. 2003, 170, 4731–4737. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E.; Nuwer, M.R.; Merigan, T.C. Potentiating Effect of Freund’s Adjuvant on Interferon Production by Endotoxin or Poly rI*Poly rC. Infect. Immun. 1970, 2, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Rowe, T.; Leon, A.J.; Banner, D.; Danesh, A.; Xu, L.; Ran, L.; Bosinger, S.E.; Guan, Y.; Chen, H.; et al. Molecular characterization of in vivo adjuvant activity in ferrets vaccinated against influenza virus. J. Virol. 2010, 84, 8369–8388. [Google Scholar] [CrossRef] [PubMed]
- Van Kaer, L. Innate and virtual memory T cells in man. Eur. J. Immunol. 2015, 45, 1916–1920. [Google Scholar] [CrossRef] [PubMed]
- White, J.T.; Cross, E.W.; Burchill, M.A.; Danhorn, T.; McCarter, M.D.; Rosen, H.R.; O’Connor, B.; Kedl, R.M. Virtual memory T cells develop and mediate bystander protective immunity in an IL-15-dependent manner. Nat. Commun. 2016, 7, 11291. [Google Scholar] [CrossRef] [PubMed]
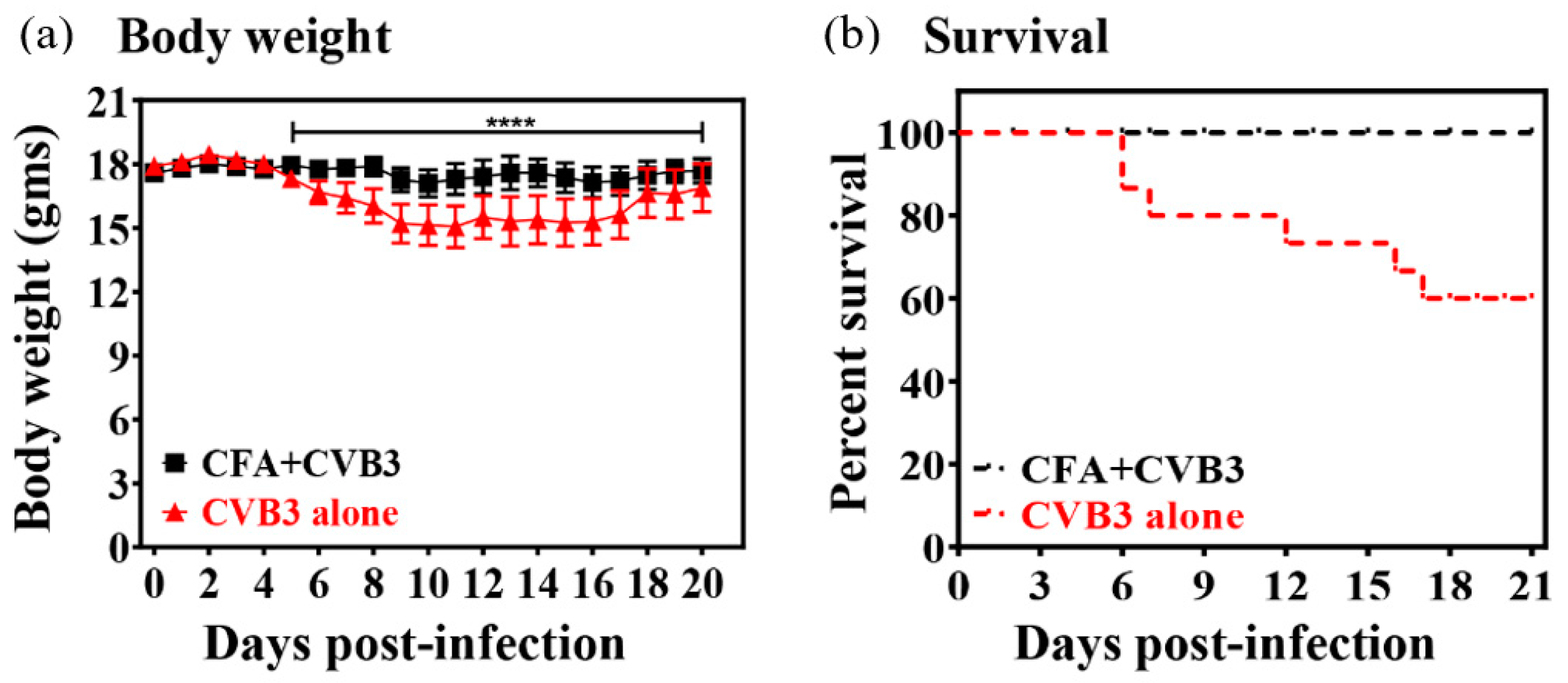
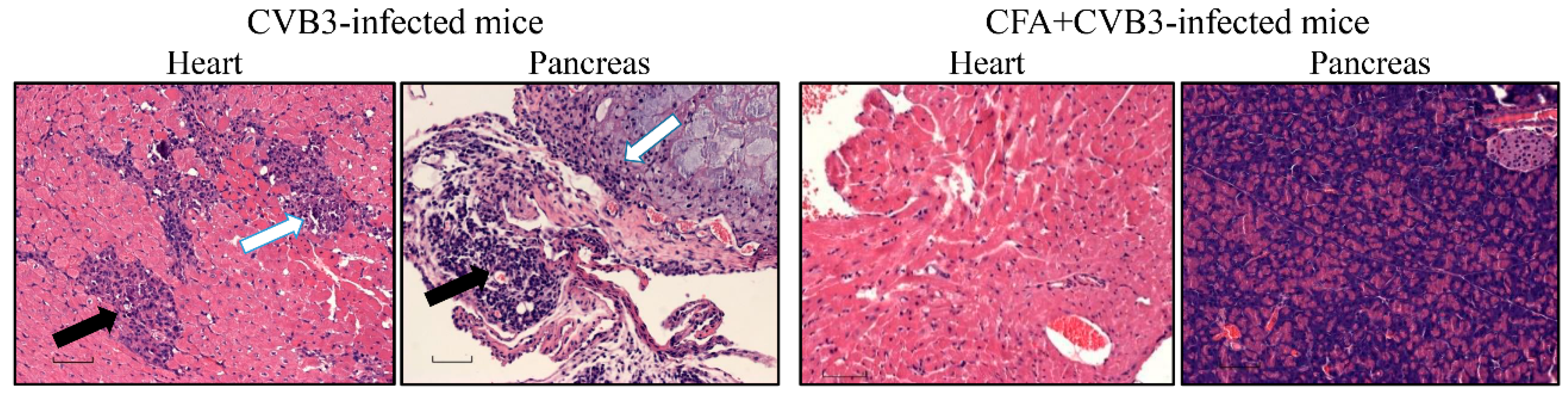
| Parameters | CVB3 Group | CFA + CVB3-Challenged Group |
|---|---|---|
| Myocarditis | ||
| Incidence | 6/15 (40.0) | 0/10 (0.0) |
| Inflammatory foci | 54.4 ± 17.7 | 0.0 |
| Pancreatitis | ||
| Incidence | 11/15 (73.3) | 1/10 (10.0) |
| Atrophy | 7/15 (46.7) | 1/10 (10.0) |
| Inflammation | 11/15 (73.3) | 1/10 (10.0) |
| Necrosis | 8/15 (53.3) | 0/10 (0.0) |
| Mineralization | 4/15 (26.7) | 1/10 (10.0) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangaplara, A.; Massilamany, C.; Lasrado, N.; Steffen, D.; Reddy, J. Evidence for Anti-Viral Effects of Complete Freund’s Adjuvant in the Mouse Model of Enterovirus Infection. Vaccines 2020, 8, 364. https://doi.org/10.3390/vaccines8030364
Gangaplara A, Massilamany C, Lasrado N, Steffen D, Reddy J. Evidence for Anti-Viral Effects of Complete Freund’s Adjuvant in the Mouse Model of Enterovirus Infection. Vaccines. 2020; 8(3):364. https://doi.org/10.3390/vaccines8030364
Chicago/Turabian StyleGangaplara, Arunakumar, Chandirasegaran Massilamany, Ninaad Lasrado, David Steffen, and Jay Reddy. 2020. "Evidence for Anti-Viral Effects of Complete Freund’s Adjuvant in the Mouse Model of Enterovirus Infection" Vaccines 8, no. 3: 364. https://doi.org/10.3390/vaccines8030364
APA StyleGangaplara, A., Massilamany, C., Lasrado, N., Steffen, D., & Reddy, J. (2020). Evidence for Anti-Viral Effects of Complete Freund’s Adjuvant in the Mouse Model of Enterovirus Infection. Vaccines, 8(3), 364. https://doi.org/10.3390/vaccines8030364





