Impact of Key Nicotinic AChR Subunits on Post-Stroke Pneumococcal Pneumonia
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Experimental Model of Stroke
2.3. Antibiotic Treatment
2.4. Bronchoscopy-Guided Application of S. Pneumoniae Three Days after MCAo
2.5. Microbiological Investigation
2.6. Flow Cytometry
2.7. Analysis of Cytokines in BAL and Albumin in BAL and Plasma
2.8. Quantitative Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR)
2.9. Statistics
3. Results
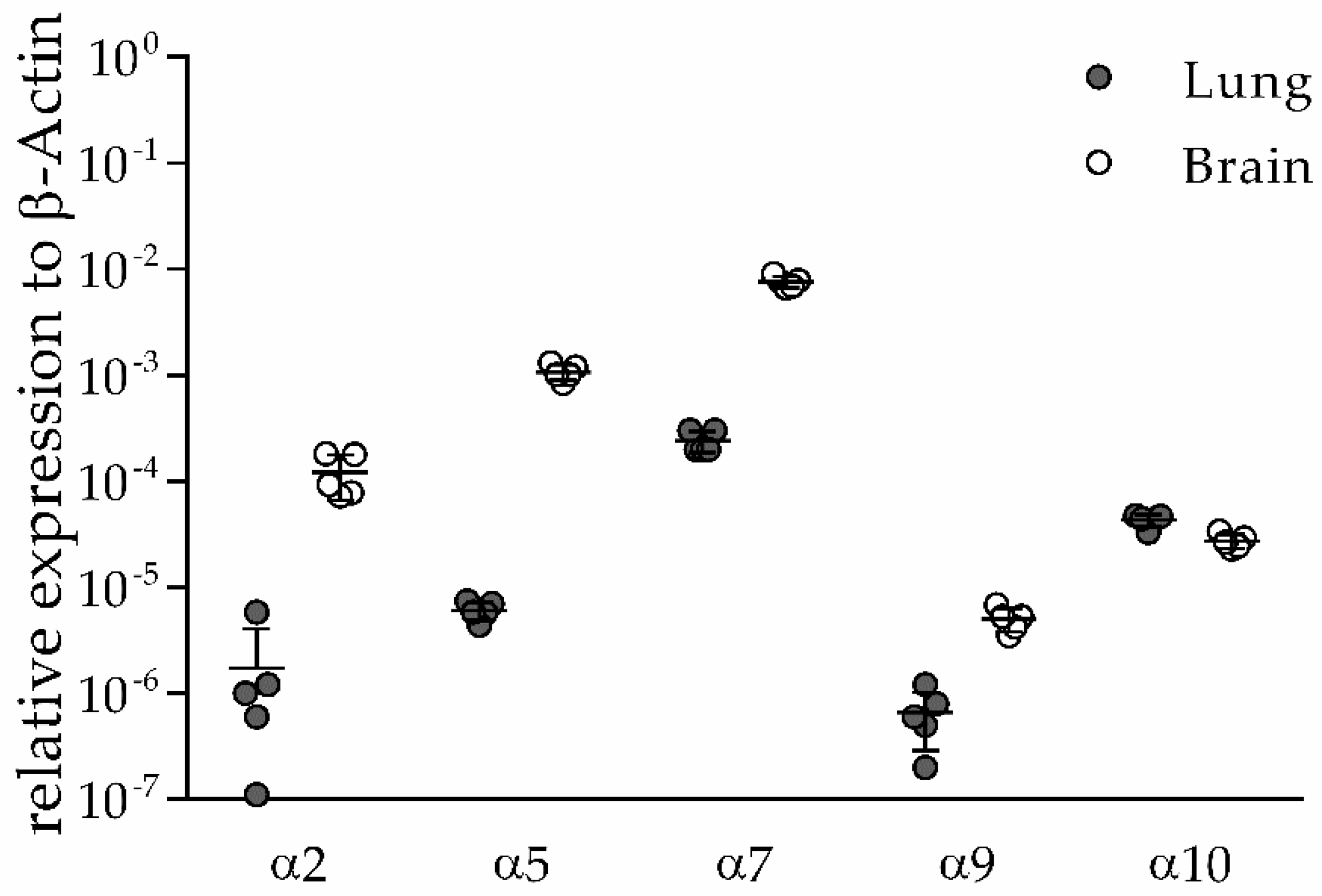
3.1. α2, α5, α9 and α10 nAChR Subunits are Expressed in Lung and Brain of Naïve Mice
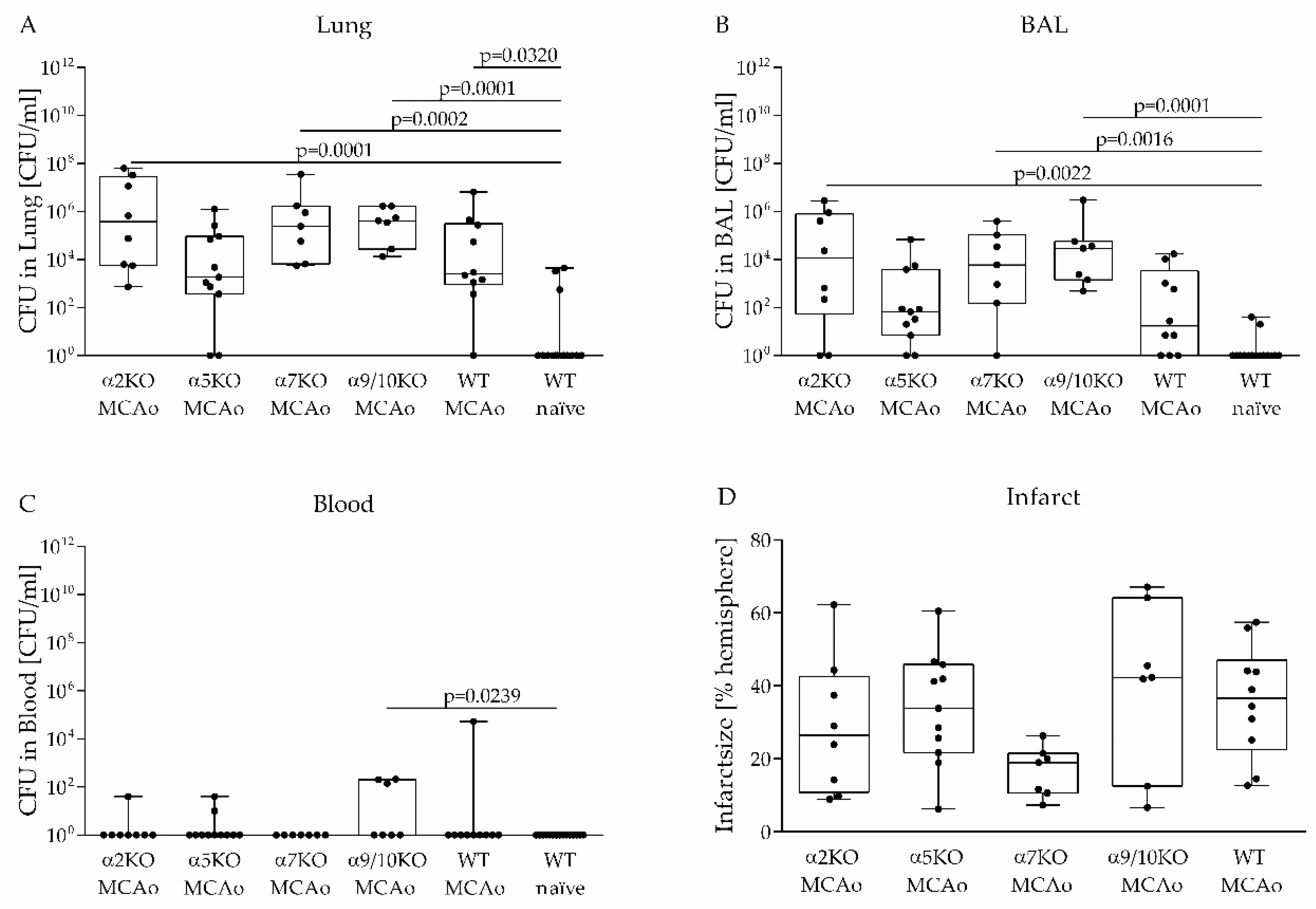
3.2. Role of Various nAChRs in an Aspiration-Induced Post-Stroke Pneumococcal Pneumonia
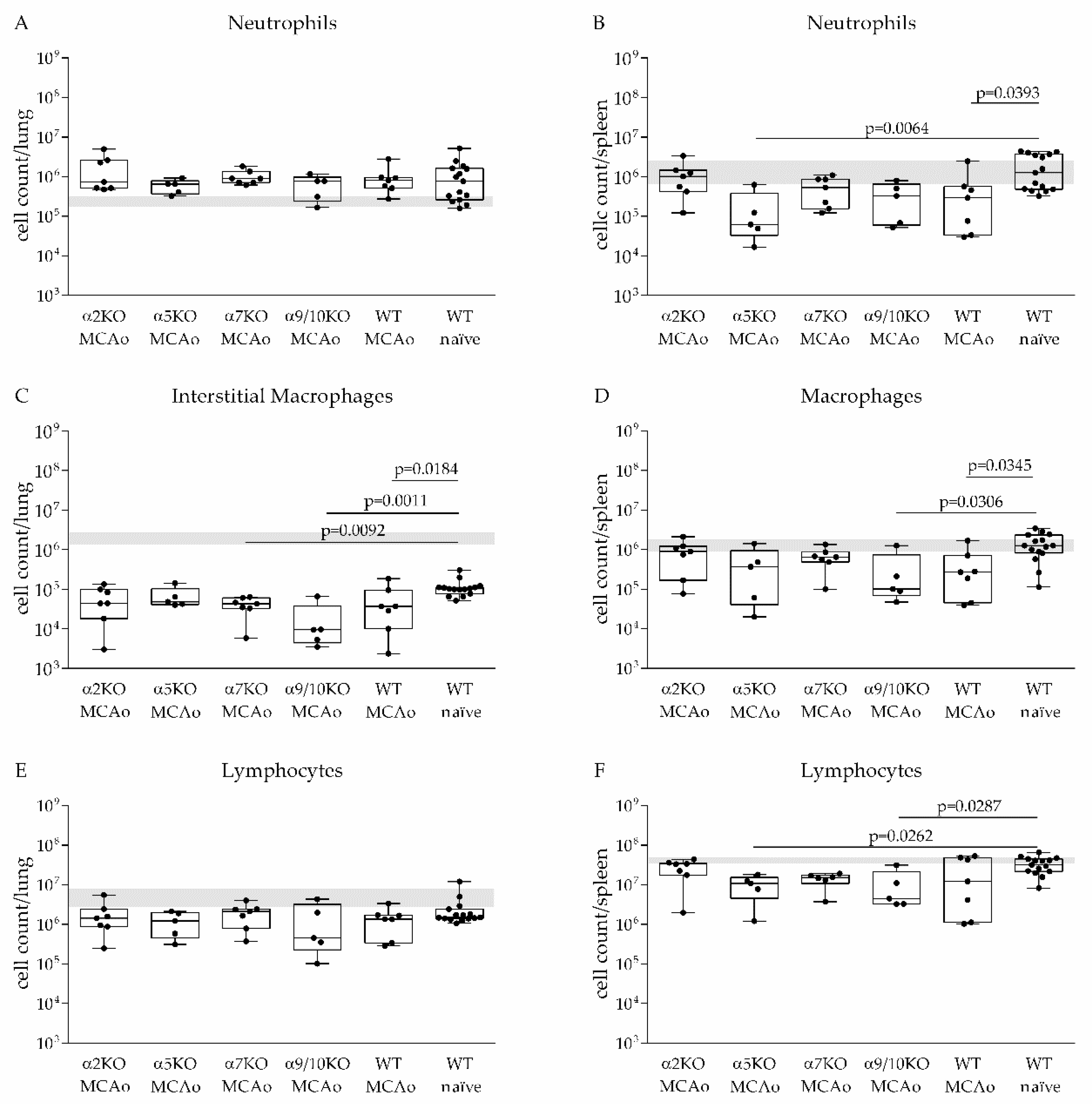
3.3. α2, α5, α7, α9/10 nAChRs Have No Effect on Immune Cell Recruitment after Stroke
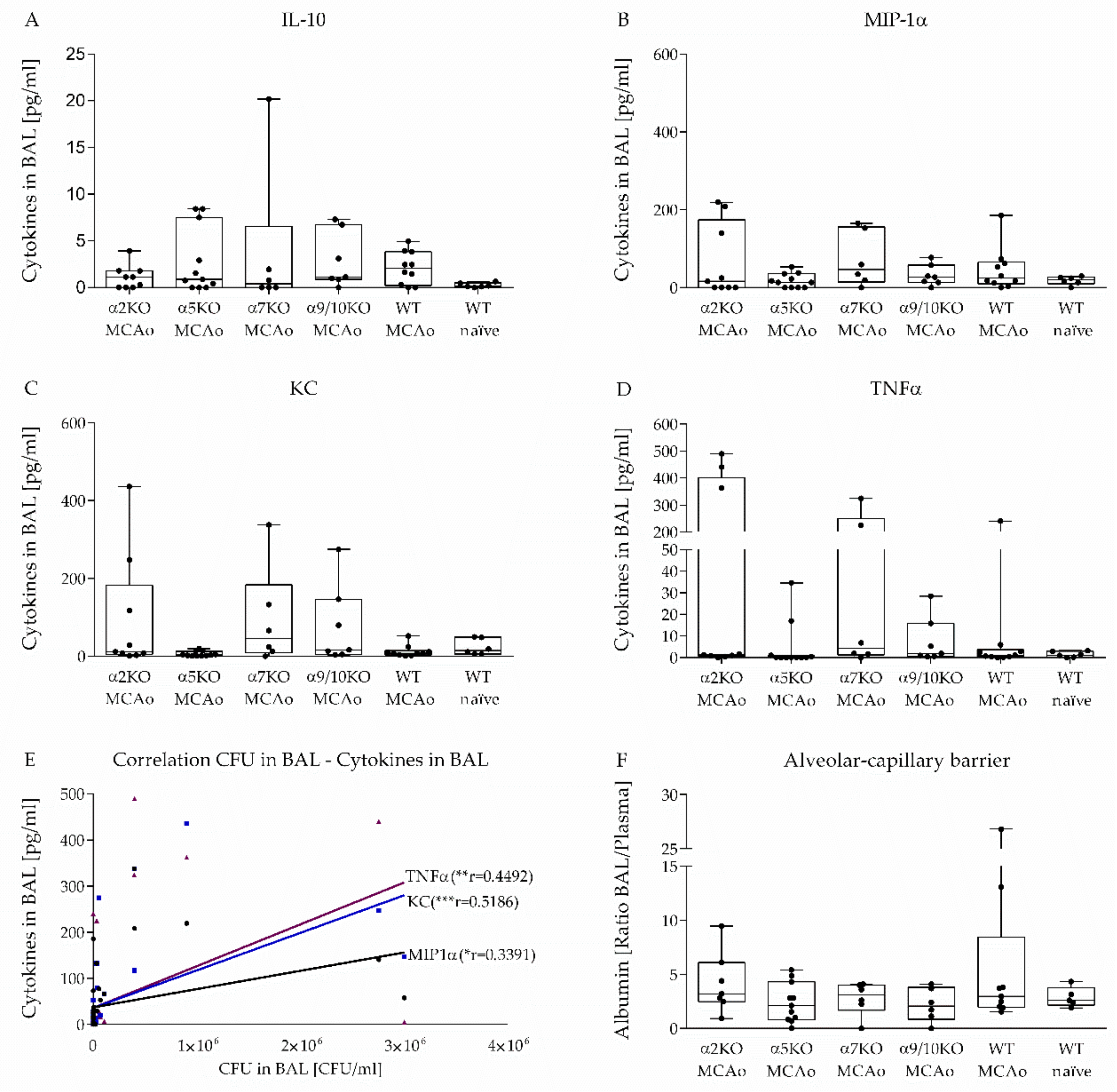
3.4. Effect of α2, α5, α7, α9/10 nAChRs on Alveolar-Capillary Barrier and Cytokine Secretion in BAL after Stroke
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davenport, R.J.; Dennis, M.S.; Wellwood, I.; Warlow, C.P. Complications after acute stroke. Stroke 1996, 27, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.C.; Li, J.Y.; Lyden, P.D.; Hanson, S.K.; Feasby, T.E.; Adams, R.J.; Faught, R.E., Jr.; Haley, E.C., Jr. Medical and neurological complications of ischemic stroke: Experience from the RANTTAS trial. RANTTAS Investigators. Stroke 1998, 29, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Langhorne, P.; Stott, D.J.; Robertson, L.; MacDonald, J.; Jones, L.; McAlpine, C.; Dick, F.; Taylor, G.S.; Murray, G. Medical complications after stroke: A multicenter study. Stroke 2000, 31, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Weimar, C.; Roth, M.P.; Zillessen, G.; Glahn, J.; Wimmer, M.L.; Busse, O.; Haberl, R.L.; Diener, H.C.; German Stroke Data Bank Collaborators O. Complications following acute ischemic stroke. Eur. Neurol. 2002, 48, 133–140. [Google Scholar] [CrossRef]
- Meisel, C.; Schwab, J.M.; Prass, K.; Meisel, A.; Dirnagl, U. Central nervous system injury-induced immune deficiency syndrome. Nat. Rev. Neurosci. 2005, 6, 775–786. [Google Scholar] [CrossRef]
- Sellars, C.; Bowie, L.; Bagg, J.; Sweeney, M.P.; Miller, H.; Tilston, J.; Langhorne, P.; Stott David, J. Risk Factors for Chest Infection in Acute Stroke. Stroke 2007, 38, 2284–2291. [Google Scholar] [CrossRef]
- Hoffmann, S.; Harms, H.; Ulm, L.; Nabavi, D.G.; Mackert, B.-M.; Schmehl, I.; Jungehulsing, G.J.; Montaner, J.; Bustamante, A.; Hermans, M.; et al. Stroke-induced immunodepression and dysphagia independently predict stroke-associated pneumonia—The PREDICT study. J. Cereb. Blood Flow Metab. 2017, 37, 3671–3682. [Google Scholar] [CrossRef]
- Engel, O.; Akyuz, L.; da Costa Goncalves, A.C.; Winek, K.; Dames, C.; Thielke, M.; Herold, S.; Bottcher, C.; Priller, J.; Volk, H.D.; et al. Cholinergic Pathway Suppresses Pulmonary Innate Immunity Facilitating Pneumonia after Stroke. Stroke 2015, 46, 3232–3240. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Wang, H.; Czura, C.J.; Friedman, S.G.; Tracey, K.J. The cholinergic anti-inflammatory pathway: A missing link in neuroimmunomodulation. Mol. Med. 2003, 9, 125–134. [Google Scholar] [CrossRef]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Physiological functions of the cholinergic system in immune cells. J. Pharmacol. Sci. 2017, 134, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Galitovskiy, V.; Chernyavsky, A.I.; Marchenko, S.; Grando, S.A. Plasticity of the murine spleen T-cell cholinergic receptors and their role in in vitro differentiation of naïve CD4 T cells toward the Th1, Th2 and Th17 lineages. Genes Immun. 2011, 12, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Papke, R.L.; Lindstrom, J.M. Nicotinic acetylcholine receptors: Conventional and unconventional ligands and signaling. Neuropharmacology 2020, 168, 108021. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.; Love, C.P. Screening for dysphagia and aspiration in acute stroke: A systematic review. Dysphagia 2001, 16, 7–18. [Google Scholar] [CrossRef]
- Kollef, M.H.; Shorr, A.; Tabak, Y.P.; Gupta, V.; Liu, L.Z.; Johannes, R.S. Epidemiology and outcomes of health-care-associated pneumonia: Results from a large US database of culture-positive pneumonia. Chest 2005, 128, 3854–3862. [Google Scholar] [CrossRef]
- Trouillet, J.L.; Chastre, J.; Vuagnat, A.; Joly-Guillou, M.L.; Combaux, D.; Dombret, M.C.; Gibert, C. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am. J. Respir. Crit. Care Med. 1998, 157, 531–539. [Google Scholar] [CrossRef]
- Kishore Amit, K.; Vail, A.; Jeans Adam, R.; Chamorro, A.; Di Napoli, M.; Kalra, L.; Langhorne, P.; Roffe, C.; Westendorp, W.; Nederkoorn Paul, J.; et al. Microbiological Etiologies of Pneumonia Complicating Stroke. Stroke 2018, 49, 1602–1609. [Google Scholar] [CrossRef]
- Prass, K.; Braun Johann, S.; Dirnagl, U.; Meisel, C.; Meisel, A. Stroke Propagates Bacterial Aspiration to Pneumonia in a Model of Cerebral Ischemia. Stroke 2006, 37, 2607–2612. [Google Scholar] [CrossRef]
- McCulloch, L.; Smith, C.J.; McColl, B.W. Adrenergic-mediated loss of splenic marginal zone B cells contributes to infection susceptibility after stroke. Nat. Commun. 2017, 8, 15051. [Google Scholar] [CrossRef]
- Wong, C.H.; Jenne, C.N.; Lee, W.Y.; Léger, C.; Kubes, P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science 2011, 334, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Lotfipour, S.; Byun, J.S.; Leach, P.; Fowler, C.D.; Murphy, N.P.; Kenny, P.J.; Gould, T.J.; Boulter, J. Targeted deletion of the mouse α2 nicotinic acetylcholine receptor subunit gene (Chrna2) potentiates nicotine-modulated behaviors. J. Neurosci. 2013, 33, 7728–7741. [Google Scholar] [CrossRef]
- Salas, R.; Orr-Urtreger, A.; Broide, R.S.; Beaudet, A.; Paylor, R.; De Biasi, M. The Nicotinic Acetylcholine Receptor Subunit α5 Mediates Short-Term Effects of Nicotine in Vivo. Mol. Pharmacol. 2003, 63, 1059. [Google Scholar] [CrossRef]
- Orr-Urtreger, A.; Göldner, F.M.; Saeki, M.; Lorenzo, I.; Goldberg, L.; De Biasi, M.; Dani, J.A.; Patrick, J.W.; Beaudet, A.L. Mice Deficient in the α7 Neuronal Nicotinic Acetylcholine Receptor Lack α-Bungarotoxin Binding Sites and Hippocampal Fast Nicotinic Currents. J. Neurosci. 1997, 17, 9165. [Google Scholar] [CrossRef] [PubMed]
- Vetter, D.E.; Katz, E.; Maison, S.F.; Taranda, J.; Turcan, S.; Ballestero, J.; Liberman, M.C.; Elgoyhen, A.B.; Boulter, J. The α10 nicotinic acetylcholine receptor subunit is required for normal synaptic function and integrity of the olivocochlear system. Proc. Natl. Acad. Sci. USA 2007, 104, 20594. [Google Scholar] [CrossRef] [PubMed]
- Vetter, D.E.; Liberman, M.C.; Mann, J.; Barhanin, J.; Boulter, J.; Brown, M.C.; Saffiote-Kolman, J.; Heinemann, S.F.; Elgoyhen, A.B. Role of α9 Nicotinic ACh Receptor Subunits in the Development and Function of Cochlear Efferent Innervation. Neuron 1999, 23, 93–103. [Google Scholar] [CrossRef]
- Engel, O.; Kolodziej, S.; Dirnagl, U.; Prinz, V. Modeling stroke in mice-middle cerebral artery occlusion with the filament model. J. Vis. Exp. 2011. [Google Scholar] [CrossRef]
- Jeong, D.-G.; Jeong, E.-S.; Seo, J.-H.; Heo, S.-H.; Choi, Y.-K. Difference in Resistance to Streptococcus pneumoniae Infection in Mice. Lab. Anim. Res. 2011, 27, 91–98. [Google Scholar] [CrossRef]
- Schulte-Herbrüggen, O.; Klehmet, J.; Quarcoo, D.; Meisel, C.; Meisel, A. Mouse strains differ in their susceptibility to poststroke infections. Neuroimmunomodulation 2006, 13, 13–18. [Google Scholar] [CrossRef]
- Dames, C.; Akyüz, L.; Reppe, K.; Tabeling, C.; Dietert, K.; Kershaw, O.; Gruber, A.D.; Meisel, C.; Meisel, A.; Witzenrath, M.; et al. Miniaturized bronchoscopy enables unilateral investigation, application, and sampling in mice. Am. J. Respir. Cell Mol. Biol. 2014, 51, 730–737. [Google Scholar] [CrossRef]
- Sun, F.; Xiao, G.; Qu, Z. Murine Bronchoalveolar Lavage. Bio-protocol 2017, 7, e2287. [Google Scholar] [CrossRef] [PubMed]
- Müller-Redetzky, H.C.; Felten, M.; Hellwig, K.; Wienhold, S.-M.; Naujoks, J.; Opitz, B.; Kershaw, O.; Gruber, A.D.; Suttorp, N.; Witzenrath, M. Increasing the inspiratory time and I:E ratio during mechanical ventilation aggravates ventilator-induced lung injury in mice. Crit. Care 2015, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Li, X.; Li, X.; Sheng, Y.-Y.; Zhuang, P.-W.; Zhang, Y.-J. Neuroimmune crosstalk in central nervous system injury-induced infection and pharmacological intervention. Brain Res. Bull. 2019, 153, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Wood, K.; Shi, F.-D.; Wang, X.; Liu, Q. Stroke-induced immunosuppression and poststroke infection. Stroke Vasc. Neurol. 2018, 3, 34. [Google Scholar] [CrossRef]
- El Husseini, N.; Laskowitz, D.T. The role of neuroendocrine pathways in prognosis after stroke. Expert Rev. Neur. 2014, 14, 217–232. [Google Scholar] [CrossRef]
- Dirnagl, U.; Klehmet, J.; Braun Johann, S.; Harms, H.; Meisel, C.; Ziemssen, T.; Prass, K.; Meisel, A. Stroke-Induced Immunodepression. Stroke 2007, 38, 770–773. [Google Scholar] [CrossRef]
- Chamorro, Á.; Meisel, A.; Planas, A.M.; Urra, X.; van de Beek, D.; Veltkamp, R. The immunology of acute stroke. Nat. Rev. Neurol. 2012, 8, 401. [Google Scholar] [CrossRef]
- Kummer, W.; Lips, K.S.; Pfeil, U. The epithelial cholinergic system of the airways. Histochem. Cell Biol. 2008, 130, 219. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Koarai, A.; Ichinose, M. Possible involvement of acetylcholine-mediated inflammation in airway diseases. Allergol. Int. 2018, 67, 460–466. [Google Scholar] [CrossRef]
- Frinchi, M.; Nuzzo, D.; Scaduto, P.; Di Carlo, M.; Massenti, M.F.; Belluardo, N.; Mudò, G. Anti-inflammatory and antioxidant effects of muscarinic acetylcholine receptor (mAChR) activation in the rat hippocampus. Sci. Rep. 2019, 9, 14233. [Google Scholar] [CrossRef] [PubMed]
- Guarini, S.; Cainazzo, M.M.; Giuliani, D.; Mioni, C.; Altavilla, D.; Marini, H.; Bigiani, A.; Ghiaroni, V.; Passaniti, M.; Leone, S.; et al. Adrenocorticotropin reverses hemorrhagic shock in anesthetized rats through the rapid activation of a vagal anti-inflammatory pathway. Cardiovasc. Res. 2004, 63, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.A.; Ochani, M.; Gallowitsch-Puerta, M.; Ochani, K.; Huston, J.M.; Czura, C.J.; Al-Abed, Y.; Tracey, K.J. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc. Natl. Acad. Sci. USA 2006, 103, 5219–5223. [Google Scholar] [CrossRef] [PubMed]
- Zoli, M.; Pucci, S.; Vilella, A.; Gotti, C. Neuronal and Extraneuronal Nicotinic Acetylcholine Receptors. Curr. Neuropharmacol. 2018, 16, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Giebelen, I.A.J.; Le Moine, A.; van den Pangaart, P.S.; Sadis, C.; Goldman, M.; Florquin, S.; van der Poll, T. Deficiency of α7 Cholinergic Receptors Facilitates Bacterial Clearance in Escherichia coli Peritonitis. J. Infect. Dis. 2008, 198, 750–757. [Google Scholar] [CrossRef]
- Speer, P.; Zhang, Y.; Gu, Y.; Lucas, M.J.; Wang, Y. Effects of nicotine on intercellular adhesion molecule expression in endothelial cells and integrin expression in neutrophils in vitro. Am. J. Obstet. Gynecol. 2002, 186, 551–556. [Google Scholar] [CrossRef]
- Kawashima, K.; Yoshikawa, K.; Fujii, Y.X.; Moriwaki, Y.; Misawa, H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 2007, 80, 2314–2319. [Google Scholar] [CrossRef]
- Nouri-Shirazi, M.; Guinet, E. Evidence for the immunosuppressive role of nicotine on human dendritic cell functions. Immunology 2003, 109, 365–373. [Google Scholar] [CrossRef]
- Wang, F.; Gerzanich, V.; Wells, G.B.; Anand, R.; Peng, X.; Keyser, K.; Lindstrom, J. Assembly of human neuronal nicotinic receptor alpha5 subunits with alpha3, beta2, and beta4 subunits. J. Biol. Chem. 1996, 271, 17656–17665. [Google Scholar] [CrossRef]
- Kedmi, M.; Beaudet, A.L.; Orr-Urtreger, A. Mice lacking neuronal nicotinic acetylcholine receptor beta4-subunit and mice lacking both alpha5- and beta4-subunits are highly resistant to nicotine-induced seizures. Physiol. Genom. 2004, 17, 221–229. [Google Scholar] [CrossRef]
- Bagdas, D.; AlSharari, S.D.; Freitas, K.; Tracy, M.; Damaj, M.I. The role of alpha5 nicotinic acetylcholine receptors in mouse models of chronic inflammatory and neuropathic pain. Biochem. Pharmacol. 2015, 97, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Orr-Urtreger, A.; Chapman, J.; Rabinowitz, R.; Nachman, R.; Korczyn, A.D. Autonomic function in mice lacking alpha5 neuronal nicotinic acetylcholine receptor subunit. J. Physiol. 2002, 542, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.; Yu, H.; Gong, J.; Jiang, J.; Qiao, X.; Perkey, E.; Kim, D.-i.; Emont, M.P.; Zestos, A.G.; Cho, J.-S.; et al. An immune-beige adipocyte communication via nicotinic acetylcholine receptor signaling. Nat. Med. 2018, 24, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Prass, K.; Meisel, C.; Höflich, C.; Braun, J.; Halle, E.; Wolf, T.; Ruscher, K.; Victorov, I.V.; Priller, J.; Dirnagl, U.; et al. Stroke-induced Immunodeficiency Promotes Spontaneous Bacterial Infections and Is Mediated by Sympathetic Activation Reversal by Poststroke T Helper Cell Type 1–like Immunostimulation. J. Exp. Med. 2003, 198, 725. [Google Scholar] [CrossRef] [PubMed]
- Kishore, A.K.; Jeans, A.R.; Garau, J.; Bustamante, A.; Kalra, L.; Langhorne, P.; Chamorro, A.; Urra, X.; Katan, M.; Napoli, M.D.; et al. Antibiotic treatment for pneumonia complicating stroke: Recommendations from the pneumonia in stroke consensus (PISCES) group. Eur. Stroke J. 2019, 4, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Mracsko, E.; Stegemann-Koniszewski, S.; Na, S.Y.; Dalpke, A.; Bruder, D.; Lasitschka, F.; Veltkamp, R. A Mouse Model of Post-Stroke Pneumonia Induced by Intra-Tracheal Inoculation with Streptococcus pneumoniae. Cerebrovasc. Dis. 2017, 43, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Fillion, I.; Ouellet, N.; Simard, M.; Bergeron, Y.; Sato, S.; Bergeron, M.G. Role of chemokines and formyl peptides in pneumococcal pneumonia-induced monocyte/macrophage recruitment. J. Immunol. 2001, 166, 7353–7361. [Google Scholar] [CrossRef]
- Haste, L.; Hulland, K.; Bolton, S.; Yesilkaya, H.; McKechnie, K.; Andrew, P.W. Development and Characterization of a Long-Term Murine Model of Streptococcus pneumoniae Infection of the Lower Airways. Infect. Immun. 2014, 82, 3289. [Google Scholar] [CrossRef]
- Peñaloza, H.F.; Nieto, P.A.; Muñoz-Durango, N.; Salazar-Echegarai, F.J.; Torres, J.; Parga, M.J.; Alvarez-Lobos, M.; Riedel, C.A.; Kalergis, A.M.; Bueno, S.M. Interleukin-10 plays a key role in the modulation of neutrophils recruitment and lung inflammation during infection by Streptococcus pneumoniae. Immunology 2015, 146, 100–112. [Google Scholar] [CrossRef]
- Farris, B.Y.; Monaghan, K.L.; Zheng, W.; Amend, C.D.; Hu, H.; Ammer, A.G.; Coad, J.E.; Ren, X.; Wan, E.C.K. Ischemic stroke alters immune cell niche and chemokine profile in mice independent of spontaneous bacterial infection. Immun. Inflamm. Dis. 2019, 7, 326–341. [Google Scholar] [CrossRef]
- Kemp, K.; Bruunsgaard, H.; Skinhøj, P.; Klarlund Pedersen, B. Pneumococcal infections in humans are associated with increased apoptosis and trafficking of type 1 cytokine-producing T cells. Infect. Immun. 2002, 70, 5019–5025. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schreiber, T.; Swanson, P.E.; Chang, K.C.; Davis, C.C.; Dunne, W.M.; Karl, I.E.; Reinhart, K.; Hotchkiss, R.S. Both gram-negative and gram-positive experimental pneumonia induce profound lymphocyte but not respiratory epithelial cell apoptosis. Shock 2006, 26. [Google Scholar] [CrossRef] [PubMed]
- Grayson, K.M.; Blevins, L.K.; Oliver, M.B.; Ornelles, D.A.; Swords, W.E.; Alexander-Miller, M.A. Activation-dependent modulation of Streptococcus pneumoniae-mediated death in human lymphocytes. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, C.W.; Klonoski, J.M.; Maier, T.; Trujillo, G.; Vitiello, P.F.; Huber, V.C.; Lee, L. Enhanced response to pulmonary Streptococcus pneumoniae infection is associated with primary ciliary dyskinesia in mice lacking Pcdp1 and Spef2. Cilia 2013, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Nazarova, E.V.; Tan, S.; Liu, Y.; Russell, D.G. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J. Exp. Med. 2018, 215, 1135–1152. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, A.; Weiser, J.N.; Paton, J.C.; Andrew, P.W. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 2008, 6, 288. [Google Scholar] [CrossRef]
- Chignard, M.; Balloy, V. Neutrophil recruitment and increased permeability during acute lung injury induced by lipopolysaccharide. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L1083–L1090. [Google Scholar] [CrossRef]
- O’Grady, N.P.; Preas, H.L.; Pugin, J.; Fiuza, C.; Tropea, M.; Reda, D.; Banks, S.M.; Suffredini, A.F. Local Inflammatory Responses following Bronchial Endotoxin Instillation in Humans. Am. J. Respir. Crit. Care Med. 2001, 163, 1591–1598. [Google Scholar] [CrossRef]
- Speyer, C.L.; Neff, T.A.; Warner, R.L.; Guo, R.-F.; Sarma, J.V.; Riedemann, N.C.; Murphy, M.E.; Murphy, H.S.; Ward, P.A. Regulatory effects of iNOS on acute lung inflammatory responses in mice. Am. J. Pathol. 2003, 163, 2319–2328. [Google Scholar] [CrossRef]
- Williams, A.E.; José, R.J.; Brown, J.S.; Chambers, R.C. Enhanced inflammation in aged mice following infection with Streptococcus pneumoniae is associated with decreased IL-10 and augmented chemokine production. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2015, 308, L539–L549. [Google Scholar] [CrossRef]
- José, R.J.; Williams, A.E.; Mercer, P.F.; Sulikowski, M.G.; Brown, J.S.; Chambers, R.C. Regulation of neutrophilic inflammation by proteinase-activated receptor 1 during bacterial pulmonary infection. J. Immunol. 2015, 194, 6024–6034. [Google Scholar] [CrossRef] [PubMed]
- Kantrow, S.P.; Shen, Z.; Jagneaux, T.; Zhang, P.; Nelson, S. Neutrophil-mediated lung permeability and host defense proteins. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L738–L745. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, S.; Zhou, B.; Liu, B.; Zhou, Y.; Zhang, H.; Li, T.; Zuo, X. Activation of the cholinergic anti-inflammatory system by nicotine attenuates arthritis via suppression of macrophage migration. Mol. Med. Rep. 2016, 14, 5057–5064. [Google Scholar] [CrossRef] [PubMed]
- Sadis, C.; Teske, G.; Stokman, G.; Kubjak, C.; Claessen, N.; Moore, F.; Loi, P.; Diallo, B.; Barvais, L.; Goldman, M.; et al. Nicotine protects kidney from renal ischemia/reperfusion injury through the cholinergic anti-inflammatory pathway. PLoS ONE 2007, 2, e469. [Google Scholar] [CrossRef] [PubMed]
- Lafargue, M.; Xu, L.; Carlès, M.; Serve, E.; Anjum, N.; Iles, K.E.; Xiong, X.; Giffard, R.; Pittet, J.-F. Stroke-induced activation of the α7 nicotinic receptor increases Pseudomonas aeruginosa lung injury. FASEB J. 2012, 26, 2919–2929. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jin, W.-N.; Liu, Y.; Shi, K.; Sun, H.; Zhang, F.; Zhang, C.; Gonzales, R.J.; Sheth, K.N.; La Cava, A.; et al. Brain Ischemia Suppresses Immunity in the Periphery and Brain via Different Neurogenic Innervations. Immunity 2017, 46, 474–487. [Google Scholar] [CrossRef]
- Fernandez-Cabezudo, M.J.; Lorke, D.E.; Azimullah, S.; Mechkarska, M.; Hasan, M.Y.; Petroianu, G.A.; al-Ramadi, B.K. Cholinergic stimulation of the immune system protects against lethal infection by Salmonella enterica serovar Typhimurium. Immunology 2010, 130, 388–398. [Google Scholar] [CrossRef]
- Su, X.; Matthay, M.A.; Malik, A.B. Requisite role of the cholinergic alpha7 nicotinic acetylcholine receptor pathway in suppressing Gram-negative sepsis-induced acute lung inflammatory injury. J. Immunol. 2010, 184, 401–410. [Google Scholar] [CrossRef]
- Giebelen, I.A.J.; Leendertse, M.; Florquin, S.; van der Poll, T. Stimulation of acetylcholine receptors impairs host defence during pneumococcal pneumonia. Eur. Respir. J. 2009, 33, 375. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagdmann, S.; Dames, C.; Berchtold, D.; Winek, K.; Weitbrecht, L.; Meisel, A.; Meisel, C. Impact of Key Nicotinic AChR Subunits on Post-Stroke Pneumococcal Pneumonia. Vaccines 2020, 8, 253. https://doi.org/10.3390/vaccines8020253
Jagdmann S, Dames C, Berchtold D, Winek K, Weitbrecht L, Meisel A, Meisel C. Impact of Key Nicotinic AChR Subunits on Post-Stroke Pneumococcal Pneumonia. Vaccines. 2020; 8(2):253. https://doi.org/10.3390/vaccines8020253
Chicago/Turabian StyleJagdmann, Sandra, Claudia Dames, Daniel Berchtold, Katarzyna Winek, Luis Weitbrecht, Andreas Meisel, and Christian Meisel. 2020. "Impact of Key Nicotinic AChR Subunits on Post-Stroke Pneumococcal Pneumonia" Vaccines 8, no. 2: 253. https://doi.org/10.3390/vaccines8020253
APA StyleJagdmann, S., Dames, C., Berchtold, D., Winek, K., Weitbrecht, L., Meisel, A., & Meisel, C. (2020). Impact of Key Nicotinic AChR Subunits on Post-Stroke Pneumococcal Pneumonia. Vaccines, 8(2), 253. https://doi.org/10.3390/vaccines8020253




