Being Pregnant during the Kivu Ebola Virus Outbreak in DR Congo: The rVSV-ZEBOV Vaccine and Its Accessibility by Mothers and Infants during Humanitarian Crises and in Conflict Areas
Abstract
1. Introduction
2. Pregnancy and the Ebola Virus in Democratic Republic of the Congo
“Present data suggests that maternal mortality remains high (approximately 95%) and peri-natal mortality virtually 100% for infected pregnant women.”
3. Conflict in Kivu
“Violence is being fueled by a multi-million dollar illicit mining industry of minerals such as the 3 Ts (tin, tantalum, and tungsten) that can be found in all of our electronic devices including: smartphones, gaming systems, computers, and military equipment”(“conflict minerals”).
“Whenever there is fighting there is systematic rape—in villages, at checkpoints on roads, wherever.”
“The violence has to stop, we have enough areas where we cannot go due to violence, military violence or rebel violence going on. So this is very bad for the Ebola response.”
“This could prolong the outbreak. I’m concerned about the well-being of responders & communities. I call on all parties to halt the violence.”
“…the important message remains: that rape and sexual slavery have become amazingly commonplace in this region of the DRC and have defined this conflict as a war against women.”
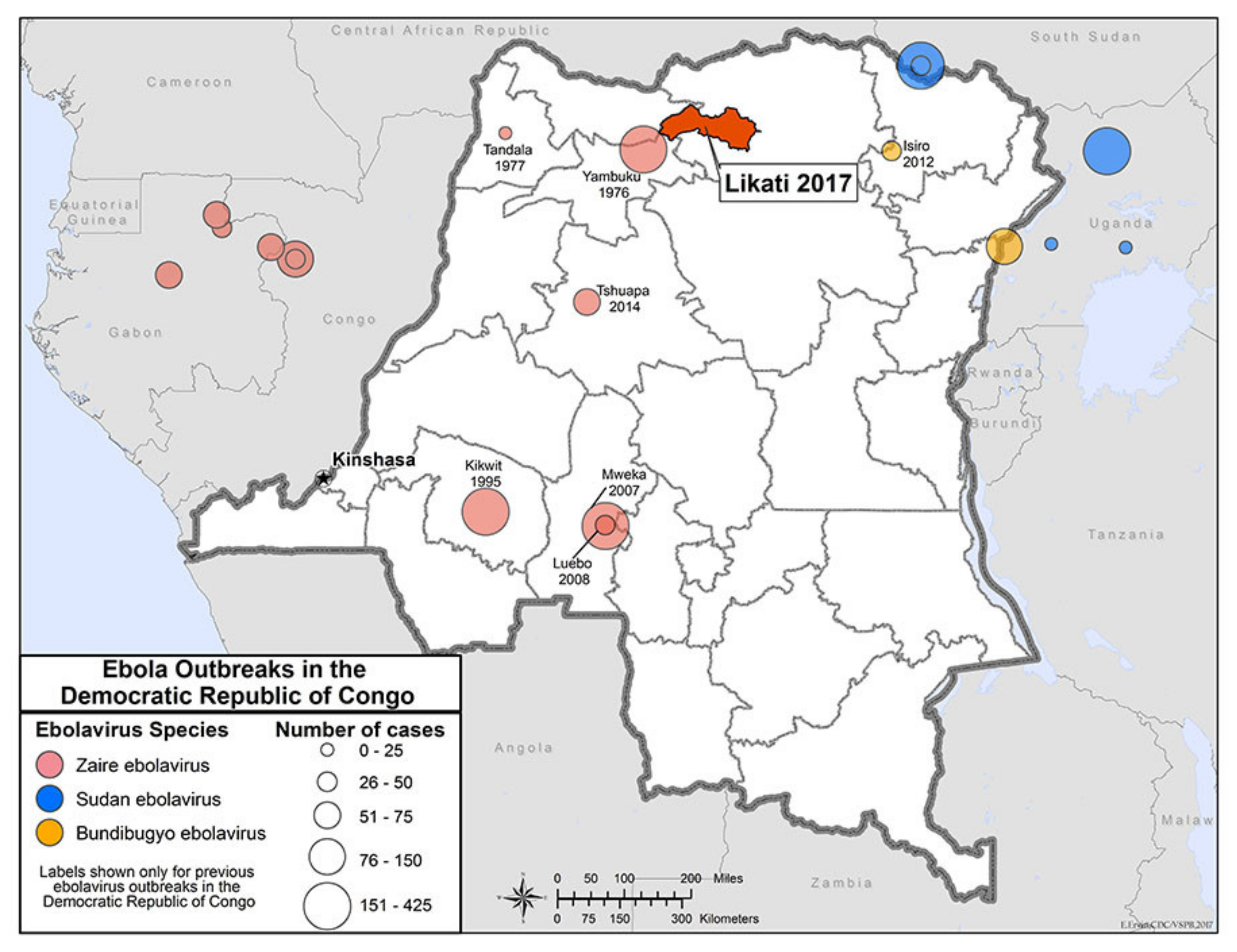
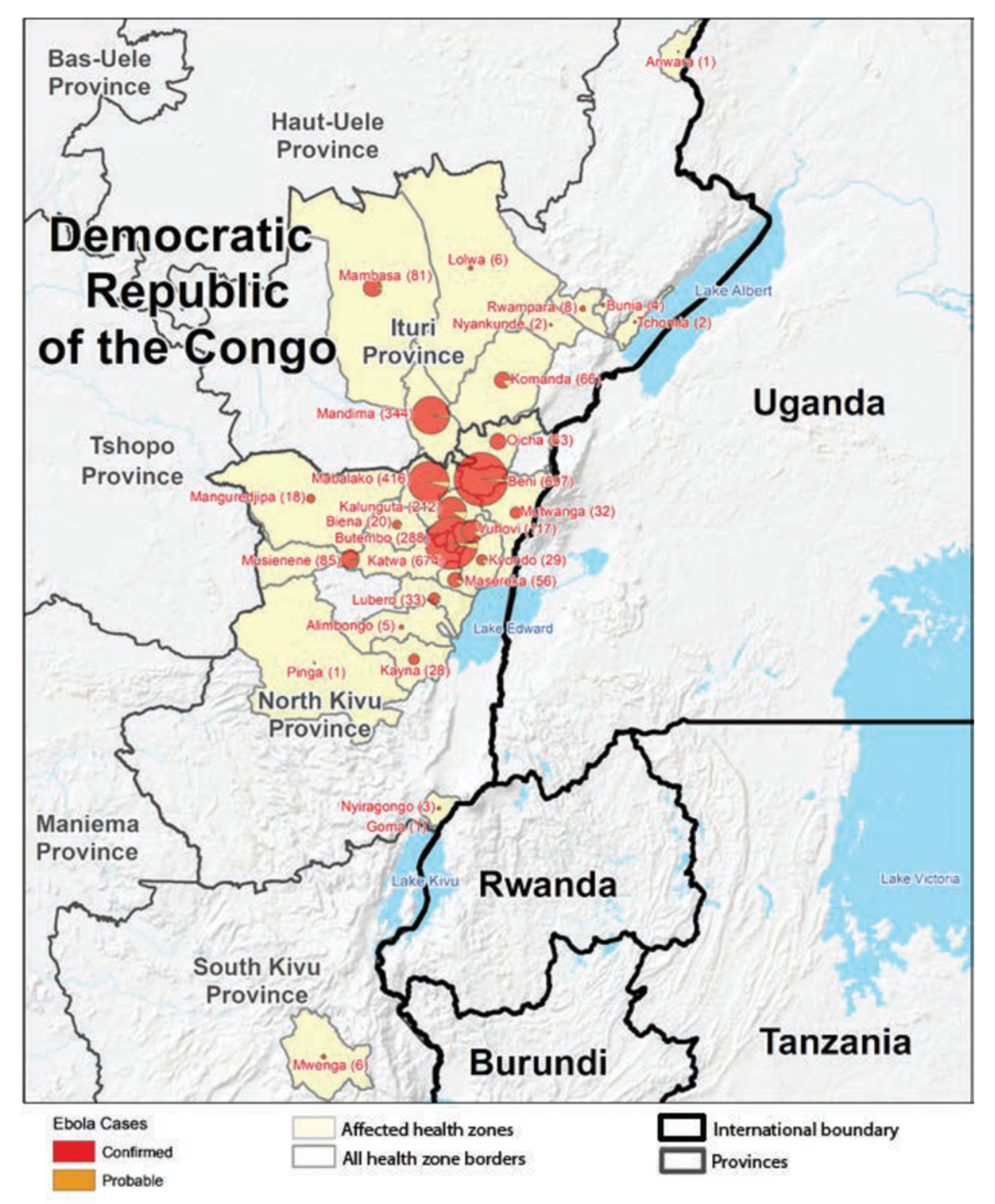
4. The Kivu Ebola Outbreak
“The rVSV-ZEBOV vaccine will give pregnant women, and the children they are carrying, a chance to live. Without it, most of the pregnant women infected with Ebola, and almost all of their infants, will die.”
“Additional safety data among other target populations such as children, HIV-positive individuals and pregnant women is required.”
“Now there is no option, you just send us to death.”
“You tell us to protect yourself with the vaccine, and then you tell us we cannot get the vaccine. So we have nothing left.”
“You told us to accept [the vaccine] and now we do, but now you don’t give it to save us.”
“In view of the severity of the outbreak and aligned with SAGE’s recommendation from October 2018 [1], SAGE welcomes and supports the recent recommendation of the ethics committee of DRC to also authorize the vaccination of pregnant women in outbreak affected areas, using the currently recommended vaccination strategies, with the live-replicating rVSV-ZEBOV-GP vaccine with informed consent and in compliance with GCP. As recommended by the ethics committee, every effort must be made to collect data on the safety of the vaccine in these populations, including a documentation of the pregnancy outcomes. SAGE advises that the use of rVSV-ZEBOV-GP vaccine in pregnant women currently remains limited to the EVD outbreak affected areas in DRC and should be continuously evaluated based on the emerging data on the safety and efficacy of the vaccine in this target population.”
“It was painful to separate from my family and my children but I had to do it.”
“The vaccination of pregnant women is usually a very complex decision to make, especially when they are using a new vaccine which is still under a trial.”
“Pregnant and lactating women in the DRC finally have access to … one of the best prevention tools we have against this deadly virus … Hopefully this will set a new precedent for ongoing and future Ebola vaccination efforts, avoiding costly delays in protocol approvals while women face the very real threats of Ebola infection.”
5. The Ad26.ZEBOV/MVA-BN Vaccine Becomes Available Including Pregnant Women and Children
6. Conclusions
Funding
Conflicts of Interest
References
- Soucheray, S. DRC’s 426-Case Ebola Outbreak Now 2nd Largest Ever. Available online: http://www.cidrap.umn.edu/news-perspective/2018/11/drcs-426-case-ebola-outbreak-now-2nd-largest-ever (accessed on 2 November 2019).
- European Centre for Disease Control and Prevention. Ebola Virus Disease Outbreak in Equateur Province, Democratic Republic of the Congo. Available online: https://ecdc.europa.eu/sites/portal/files/documents/17-05-2018-RRA-first-update-Ebola%20haemorrhagic%20fever-Democratic%20Republic%20of%20the%20Congo.pdf (accessed on 8 September 2019).
- Breman, J.G.; Heymann, D.L.; Lloyd, G.; McCormick, J.B.; Miatudila, M.; Murphy, F.A.; Muyembé-Tamfun, J.J.; Piot, P.; Ruppol, J.F.; Sureau, P.; et al. Discovery and description of Ebola Zaire virus in 1976 and relevance to the West African epidemic during 2013-2016. J. Infect. Dis. 2016, 214, S93–S101. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Anoko, J.A.; Abramowitz, S. (Eds.) Pregnant in the Time of Ebola: Women and Their Children in the 2013–2015 West African Epidemic; Springer Nature: New York, NY, USA; Berlin, Germany, 2019; ISBN 978-3-319-97636-5. [Google Scholar]
- Schwartz, D.A. Clinical trials and administration of Zika virus vaccine in pregnant women: Lessons (that should have been) learned from excluding immunization with the Ebola Vaccine during pregnancy and lactation. Vaccines 2018, 6, 81. Available online: https://www.mdpi.com/2076-393X/6/4/81/htm (accessed on 18 November 2019). [CrossRef] [PubMed]
- Gomes, M.F.; de la Fuente-Núñez, V.; Saxena, A.; Kuesel, A.C. Protected to death: Systematic exclusion of pregnant women from Ebola virus disease trials. Reprod. Health 2017, 14, 172. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5751665/ (accessed on 15 December 2019). [CrossRef] [PubMed]
- Bebell, L.M. Ebola virus disease and pregnancy: Perinatal transmission and epidemiology. In Pregnant in the Time of Ebola: Women and Their Children in the 2013–2015 West African Epidemic; Schwartz, D.A., Anoko, J.A., Abramowitz, S., Eds.; Springer Nature: New York, NY, USA, 2019; pp. 53–65. ISBN 978-3-319-97636-5. [Google Scholar]
- Schwartz, D.A. Maternal filovirus infection and death from Marburg and Ravn viruses: Highly lethal to pregnant women and their fetuses similar to Ebola Virus. In Re-Emerging Filovirus Diseases; Okware, S.I., Ed.; IntechOpen: New York, NY, USA, 2019; ISBN 978-1-78985-550-0. Available online: https://www.intechopen.com/online-first/maternal-filovirus-infection-and-death-from-marburg-and-ravn-viruses-highly-lethal-to-pregnant-women (accessed on 18 November 2019). [CrossRef]
- Bebell, L.M.; Oduyebo, T.; Riley, L.E. Ebola virus disease and pregnancy: A review of the current knowledge of Ebola virus pathogenesis, maternal, and neonatal outcomes. Birth Defects Res. 2017, 109, 353–362. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/bdra.23558#bdr223558-bib-0022 (accessed on 30 October 2019). [CrossRef] [PubMed]
- Faden, R.; Karron, R.; Krubiner, C. An ‘Indefensible’ Decision: Not Vaccinating Pregnant and Lactating Women in an Ebola Outbreak. Available online: https://www.statnews.com/2018/08/27/ebola-vaccine-pregnant-lactating-women/ (accessed on 15 October 2019).
- Schwartz, D.A. Maternal and infant death and the rVSV-ZEBOV vaccine through three recent Ebola virus epidemics-West Africa, DRC Équateur and DRC Kivu: 4 years of excluding pregnant and lactating women and their infants from immunization. Curr. Trop. Med. Rep. 2019, 6, 213–222. Available online: https://link.springer.com/article/10.1007/s40475-019-00195-w (accessed on 31 December 2019). [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J. Ebola Vaccine for Pregnant Women: One Step Closer but Still More to Go. Available online: https://www.statnews.com/2019/06/13/ebola-vaccine-pregnant-lactating-women-2/ (accessed on 25 October 2019).
- Schwartz, D.A. Maternal and infant survival following Ebola infection—Their exclusion from treatment and vaccine trials and “Primum non nocere”. In Pregnant in the Time of Ebola: Women and Their Children in the 2013–2015 West African Epidemic; Schwartz, D.A., Anoko, J.A., Abramowitz, S., Eds.; Springer Nature: New York, NY, USA, 2019; pp. 147–155. ISBN 978-3-319-97636-5. [Google Scholar]
- Higgins, A. A Landmark Policy Reversal in Congo will Now Allow Pregnant Women to Receive the Ebola Vaccine. Available online: https://www.thelily.com/a-landmark-policy-reversal-in-congo-will-now-allow-pregnant-women-to-receive-the-ebola-vaccine/ (accessed on 3 December 2019).
- Higgins, A. Pregnant Women in DRC Finally Receive Ebola Vaccine. Available online: https://www.devex.com/news/pregnant-women-in-drc-finally-receive-ebola-vaccine-95204 (accessed on 4 December 2019).
- Smith, J. Exclusion of Pregnant Women from Vaccine Research, Deployment is ‘Unacceptable,’ Health and Bioethics Experts Say. Available online: https://hub.jhu.edu/2018/12/10/pregnant-women-vaccine-exclusion-bioethics/ (accessed on 1 November 2019).
- Furneaux, R. Vaccine Studies Still Exclude Pregnant Women. That’s a Big Mistake. Just Ask the Women Living in Fear of Ebola. Available online: https://www.motherjones.com/politics/2018/12/vaccine-studies-still-exclude-pregnant-women-thats-a-big-mistake/ (accessed on 26 November 2019).
- Krubiner, C.B.; Faden, R.R.; Karron, R.A.; Little, M.O.; Lyerly, A.D.; Abramson, J.S.; Beigi, R.H.; Cravioto, A.R.; Durbin, A.P.; Gellin, B.G.; et al. Pregnant women & vaccines against emerging epidemic threats: Ethics guidance for preparedness, research, and response. Vaccine 2019. Available online: https://www.sciencedirect.com/science/article/pii/S0264410X19300453?via%3Dihub (accessed on 13 December 2019). [CrossRef]
- Lovell, D. In the Fight Against Ebola in the Congo, Pregnant Women Must not be Forgotten. Available online: https://news.syr.edu/blog/2018/08/30/in-the-fight-against-ebola-in-the-congo-pregnant-women-must-not-be-forgotten/ (accessed on 30 December 2019).
- Caluwaerts, S.; Bayliss, F. An Ebola Survivor Orphaned by Vaccine Policy. Available online: https://impactethics.ca/2016/11/25/nubia-an-ebola-survivor-orphaned-byvaccine-policy/ (accessed on 1 December 2019).
- Branswell, H. Experts Call for Reversing the Decision to Deny the Ebola Vaccine to Pregnant Women. Available online: https://www.statnews.com/2018/08/27/experts-call-for-reversing-denial-of-ebola-vaccine-to-pregnant-women/ (accessed on 1 September 2019).
- The PREVENT Working Group. Pregnant Women & Vaccines Against Emerging Epidemic Threats: Ethics Guidance for Preparedness, Research, and Response. Available online: https://static1.squarespace.com/static/574503059f72665be88193e9/t/5c082429c2241ba2553ee1f5/1544037418944/PREVENT-Web.pdf (accessed on 15 October 2019).
- Kratz, T.; Roddy, P.; Oloma, A.T.; Jeffs, B.; Ciruelo, D.P.; De La Rosa, O.; Borchert, M. Ebola virus disease outbreak in Isiro, Democratic Republic of the Congo, 2012: Signs and symptoms, management and outcomes. PLoS ONE 2015, 10, 1–18. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4479598/ (accessed on 18 December 2019). [CrossRef]
- CDC. 2017 Democratic Republic of the Congo, Bas Uélé District. Available online: https://www.cdc.gov/vhf/ebola/outbreaks/drc/2017-may.html (accessed on 15 October 2019).
- Beigi, R.H. Emerging infectious diseases in pregnancy. Obstet. Gynecol. 2017, 129, 896–906. [Google Scholar] [CrossRef]
- Jamieson, D.J.; Theiler, R.N.; Rasmussen, S.A. Emerging infections and pregnancy. Emerg. Infect. Dis. 2006, 12, 1638–1643. [Google Scholar] [CrossRef]
- Kourtis, A.P.; Read, J.S.; Jamieson, D.J. Pregnancy and infection. N. Engl. J. Med. 2014, 370, 2211–2218. [Google Scholar] [CrossRef]
- Silasi, M.; Cardenas, I.; Kwon, J.Y.; Racicot, K.; Aldo, P.; Mor, G. Viral infections during pregnancy. Am. J. Reprod. Immunol. 2015, 73, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Soma-Pillay, P.; Catherine, N.P.; Tolppanen, H.; Mebazaa, A.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Theiler, R.N.; Rasmussen, S.A.; Treadwell, T.A.; Jamieson, D.J. Emerging and zoonotic infections in women. Infect. Dis. Clin. N. Am. 2008, 22, 755–772. [Google Scholar] [CrossRef]
- Shapiro, D.; Tambashe, B.O. Recent Fertility Changes in the Democratic Republic of the Congo. Available online: http://www.niussp.org/article/recent-fertility-changes-in-the-democratic-republic-of-the-congo/ (accessed on 14 January 2020).
- Democratic Republic of Congo. Demographic and Health Survey 2013–2014. Key Findings. Available online: https://dhsprogram.com/pubs/pdf/SR218/SR218.e.pdf (accessed on 12 January 2020).
- Assaf, S.; Wang, W. Regional Disparities in Fertility Preferences and Demand Satisfied for Family Planning by Modern Methods Across Levels of Poverty. DHS Analytical Studies No. 71. Rockville, Maryland, USA. Available online: https://dhsprogram.com/pubs/pdf/AS71/AS71.pdf (accessed on 20 November 2019).
- World Health Organization. Addressing Sex and Gender in Epidemic-Prone Infectious Diseases. Available online: https://apps.who.int/iris/bitstream/handle/10665/43644/9789241595346_eng.pdf (accessed on 12 January 2020).
- Ravi, S.J.; Gauldin, E.M. Sociocultural dimensions of the Ebola virus disease outbreak in Liberia. Biosecur. Bioterror. 2014, 12, 301–305. [Google Scholar] [CrossRef]
- WHO Africa. Mothers Survive Ebola while Pregnant Against the Odds in the Democratic Republic of the Congo. Available online: https://www.afro.who.int/news/mothers-surviveebola-while-pregnant-against-odds-democratic-republic-congo (accessed on 12 January 2020).
- WHO. Report of an International Commission. Ebola haemorrhagic fever in Zaire, 1976. Bull. World Health Organ. 1978, 56, 271–293. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2395567/ (accessed on 29 November 2019).
- Mupapa, K.; Mukundu, W.; Bwaka, M.A.; Kipasa, M.; De Roo, A.; Kuvula, K.; Kibadi, K.; Massamba, M.; Ndaberey, D.; Colebunders, R.; et al. Ebola hemorrhagic fever and pregnancy. J. Infect. Dis. 1999, 179, S11–S12. [Google Scholar] [CrossRef] [PubMed]
- Krubiner, C.B.; Schwartz, D.A. Viral hemorrhagic fevers in pregnant women and the vaccine landscape: Comparisons between yellow fever, Ebola, and Lassa fever. Curr. Trop. Med. Rep. 2019, 6, 186–196. [Google Scholar] [CrossRef]
- Jamieson, D.J.; Uyeki, T.M.; Callaghan, W.M.; Meaney-Delman, D.; Rasmussen, S.A. What obstetrician–gynecologists should know about Ebola. Obstet. Gynecol. 2014, 124, 1005–1010. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Black, B.O.; Caluwaerts, S.; Achar, J. Ebola viral disease and pregnancy. Obstet. Med. 2015, 8, 108–113. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4582839/ (accessed on 29 September 2019). [CrossRef]
- Reuters. Ebola Clinic for Pregnant Women Opens. Pregnant Women Survival Rate from the Virus is Virtually Zero. Available online: https://www.phillyvoice.com/ebola-clinic-pregnant-women-opens/ (accessed on 7 September 2019).
- RCOG. Principles of Management for Pregnant Women with Ebola: A Western Context. Available online: https://www.rcog.org.uk/globalassets/documents/news/ebola-and-pregnancy-western.pdf (accessed on 20 October 2019).
- Alirol, E.; Kuesel, A.C.; Guraiib, M.M.; de la Fuente-Núñez, V.; Saxena, A.; Gomes, M.F. Ethics review of studies during public health emergencies - the experience of the WHO ethics review committee during the Ebola virus disease epidemic. BMC Med. Ethics 2017, 18, 43. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5485606/ (accessed on 30 December 2019). [CrossRef]
- Venkatraman, N.; Silman, D.; Folegatti, P.M.; Hill, A.V. Vaccines against Ebola virus. Vaccine 2018, 36, 5454–5459. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, J.; Jarvis, C. Benefits Risk Analysis of Vaccination of Pregnant Women with rVSV-ZEBOV as Part of an Expanded Access Programme. Available online: https://www.who.int/immunization/sage/meetings/2018/october/SAGE_october_2018_ebola_Edmunds_Jarvis.pdf (accessed on 11 January 2020).
- Samai, M.; Seward, J.F.; Goldstein, S.T.; Mahon, B.E.; Lisk, D.R.; Widdowson, M.A.; Jalloh, M.I.; Schrag, S.S.; Idriss, A.; Carter, R.J.; et al. The Sierra Leone trial to introduce a vaccine against Ebola: An evaluation of rVSVΔG-ZEBOV-GP vaccine tolerability and safety during theWest Africa Ebola outbreak. J. Infect. Dis. 2018, 217, S6–S15. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5961340/ (accessed on 14 January 2020). [CrossRef] [PubMed]
- Maganga, G.D.; Kapetshi, J.; Berthet, N.; Kebela Ilunga, B.; Kabange, F.; Mbala Kingebeni, P.; Mondonge, V.; Muyembe, J.-J.T.; Bertherat, E.; Briand, S.; et al. Ebola virus disease in the Democratic Republic of Congo. N. Engl. J. Med. 2014, 371, 2083–2091. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa1411099 (accessed on 1 January 2020). [CrossRef] [PubMed]
- WHO. Classical Ebola Virus Disease in the Democratic Republic of Congo. Available online: https://www.who.int/csr/disease/ebola/one-year-report/drc/en/ (accessed on 3 November 2019).
- Henao-Restrepo, A.M.; Camacho, A.; Longini, I.M.; Watson, C.H.; Edmunds, W.J.; Egger, M.; Carroll, M.W.; Dean, N.E.; Diatta, I.; Doumbia, M.; et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: Final results from the Guinea ring vaccination, open-label, cluster randomized trial (Ebola Ca Suffit!). Lancet 2017, 389, 857–866. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5364328/ (accessed on 1 January 2020). [CrossRef]
- INRB; WHO. Preliminary Results on the Efficacy of rVSV-ZEBOV-GP Ebola Vaccine Using the Ring Vaccination Strategy in the Control of an Ebola Outbreak in the Democratic Republic of the Congo: An Example of Integration of Research into Epidemic Response. Available online: https://www.who.int/csr/resources/publications/ebola/ebola-ring-vaccination-results-12-april-2019.pdf (accessed on 30 December 2019).
- Leonard, K. Health Officials Contain Ebola’s Spread in the Congo. Washington Examiner. Available online: https://www.washingtonexaminer.com/policy/healthcare/health-officials-contain-ebolas-spread-in-the-congo (accessed on 1 January 2020).
- Katunga, J. Minerals, Forests, and Violent Conflict in the Democratic Republic of Congo. Available online: https://www.wilsoncenter.org/sites/default/files/Katunga12.pdf (accessed on 1 January 2020).
- Jewish World Watch. Conflict Minerals. Available online: https://www.jww.org/conflict-areas/drc/conflict-minerals/ (accessed on 19 December 2019).
- Lyall, G. Rebellion and Conflict Minerals in North Kivu. Available online: https://www.accord.org.za/conflict-trends/rebellion-conflict-minerals-north-kivu/ (accessed on 21 December 2019).
- Bosuandole, M. Kivu: The Forgotten War. Available online: https://mg.co.za/article/2018-09-26-kivu-the-forgotten-war (accessed on 20 December 2019).
- Congo Research Group. Congo, Forgotten. The Numbers Behind Africa’s Longest Humanitarian Crisis. Available online: https://kivusecurity.nyc3.digitaloceanspaces.com/reports/28/KST%20biannual%20report%20August%2012%20%281%29.pdf (accessed on 5 December 2019).
- United Nations. UN Honours 14 Peacekeepers Killed in Eastern DR Congo. Available online: https://news.un.org/en/story/2017/12/639022-un-honours-14-peacekeepers-killed-eastern-dr-congo (accessed on 19 November 2019).
- Adetunji, J. Forty-eight Women Raped Every Hour in Congo, Study Finds. Available online: https://www.theguardian.com/world/2011/may/12/48-women-raped-hour-congo (accessed on 5 December 2019).
- Jewish World Watch. Current Situation in the DRC. Available online: https://www.jww.org/conflict-areas/drc/current-situation/#rape (accessed on 5 December 2019).
- Cunningham, H.K. DRC: The Boy Soldiers and Girl Brides of War-Torn North Kivu. Available online: https://www.genocidewatch.com/single-post/2018/10/16/DRC-The-boy-soldiers-and-girl-brides-of-war-torn-North-Kivu (accessed on 27 November 2019).
- Burke, J. ‘The Wars will Never Stop’–Millions Flee Bloodshed as Congo Falls Apart. Available online: https://www.theguardian.com/world/2018/apr/03/millions-flee-bloodshed-as-congos-army-steps-up-fight-with-rebels-in-east (accessed on 28 November 2019).
- Mednick, S. Congo’s Instability is Fueling Grave Violations Against Children, and Driving them to Armed Groups. Available online: https://www.vice.com/en_us/article/a3mj4z/congos-instability-is-fueling-grave-violations-against-children-and-driving-them-to-armed-groups (accessed on 17 November 2019).
- Paquette, D.; Sun, L.H. With more than 1100 dead, Congo’s Ebola Outbreak is only Getting Worse. Now Doctors Are Forced to Go Undercover. Available online: https://www.washingtonpost.com/world/africa/with-more-than-1100-dead-congos-ebola-outbreak-is-only-getting-worse-now-doctors-are-forced-to-go-undercover/2019/05/16/b7e15d80-7712-11e9-a7bf-c8a43b84ee31_story.html (accessed on 1 January 2020).
- DW News. DR Congo: Rebels Attack Ebola Hospital in North Kivu. Available online: https://www.dw.com/en/dr-congo-rebels-attack-ebola-hospital-in-north-kivu/a-48417432 (accessed on 21 December 2019).
- Médecins Sans Frontières. Medical Activities Suspended after Ebola Treatment Centre Attack. Available online: https://www.msf.org/medical-activities-suspended-after-ebola-treatment-centre-attack (accessed on 1 January 2020).
- Belluz, J. Assailants Attacked an Ebola Treatment Center in Congo—Again. It’s a Major Setback. Available online: https://www.vox.com/2019/3/1/18245905/ebola-outbreak-congo (accessed on 30 December 2019).
- Mallet, R.; Bomani, J.S. Attacks on MSF Ebola treatment centers in DRC fuel fears. Available online: https://www.dw.com/en/attacks-on-msf-ebola-treatment-centers-in-drc-fuel-fears/a-47752972 (accessed on 28 December 2019).
- Nebehay, S. WHO, UNICEF Evacuate 76 Staff from Ebola Teams in Congo due to Insecurity. Available online: https://uk.mobile.reuters.com/article/amp/idUKKBN1Y015J?__twitter_impression=true (accessed on 20 January 2020).
- Schniring, L. More Violence Continues to Stall Ebola Response in DRC Hot Spots. Available online: http://www.cidrap.umn.edu/news-perspective/2019/11/more-violence-continues-stall-ebola-response-drc-hot-spots (accessed on 28 December 2019).
- Larson, K.; Dodds, P. UN Peacekeepers in Congo Hold Record for Rape, Sex Abuse. Available online: https://apnews.com/69e56ab46cab400f9f4b3753bd79c930/UN-peacekeepers-in-Congo-hold-record-for-rape,-sex-abuse (accessed on 27 December 2019).
- WHO. Ebola Virus Disease Democratic Republic of the Congo. Available online: http://apps.who.int/iris/bitstream/handle/10665/275658/SITREP_EVD_DRC_20181030-eng.pdf?ua=1 (accessed on 30 September 2019).
- WHO. Cluster of Presumptive Ebola Cases in North Kivu in the Democratic Republic of the Congo. Available online: https://www.who.int/news-room/detail/01-08-2018-cluster-of-presumptive-ebola-cases-in-north-kivu-in-the-democratic-republic-of-the-congo (accessed on 15 November 2019).
- Médecins Sans Frontières. New Ebola Outbreak Declared in North Kivu. Available online: https://www.msf.org/new-ebola-outbreak-declared-north-kivu (accessed on 1 January 2020).
- WHO. SAGE meeting of October 2018. Available online: https://www.who.int/immunization/sage/meetings/2018/october/YB_SAGE_October_2018_final.pdf?ua=1 (accessed on 15 December 2019).
- Edmunds, J.; Jarvis, C. Benefits Risk Analysis of Vaccination of Pregnant Women with rVSV-ZEBOV as Part of Expanded Access Programme. Presentation to WHO SAGE. October 2018. Available online: https://www.who.int/immunization/sage/meetings/2018/october/presentations_background_docs/en/index1.html (accessed on 23 December 2019).
- UNICEF. Children Account for More than one Third of Ebola Cases in Eastern Democratic Republic of the Congo–UNICEF. Available online: https://www.unicef.org/wca/press-releases/children-account-more-one-third-ebola-cases-eastern-democratic-republic-congo-unicef (accessed on 1 January 2020).
- WHO. Weekly Bulletin on Outbreaks and Other Emergencies. 11 January 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/279455/OEW02-0511012019.pdf (accessed on 8 November 2019).
- Bardosh, K.; Gercama, I.; Bedford, J. Social Science and Behavioural Data Compilation, DRC Ebola outbreak, November 2018–February 2019. Available online: https://reliefweb.int/report/democratic-republic-congo/social-science-and-behavioural-data-compilation-drc-ebola-outbreak (accessed on 1 January 2020).
- Bedford, J.; Gercama, I.; Bardosh, K. Social Science and Behavioural Data Compilation–November 2018. Available online: https://reliefweb.int/sites/reliefweb.int/files/resources/SSHAP%20data%20compilation%20brief%20November%202018.pdf (accessed on 23 December 2019).
- UN News. Pregnant, Nursing Women Can Now be Given Ebola Vaccine, UN Health Agency Announces. Available online: https://news.un.org/en/story/2019/02/1033281 (accessed on 27 October 2019).
- WHO. SAGE Interim Recommendations on Vaccination against Ebola Virus Disease (EVD). Available online: https://www.who.int/immunization/interim_ebola_recommendations_feb_2019.pdf (accessed on 10 December 2019).
- Hackett, D.W. Ebola Vaccination Policy Reversed, Pregnant and Lactating Women Now Included. Available online: https://www.precisionvaccinations.com/merck-v920-ebola-virus-vaccine-candidate-now-offered-pregnant-and-lactating-women-democratic (accessed on 29 December 2019).
- Branswell, H. Ebola Vaccine will be Provided to Women who are Pregnant, Marking Reversal in Policy. Available online: https://www.statnews.com/2019/02/20/ebola-pregnancy-reversal/ (accessed on 15 November 2019).
- Karron, R.; Krubiner, C.; Faden, F. As the World Prepares to Fight Lassa Fever, the Interests of Pregnant Women Must be Part of the Planning. Available online: https://www.statnews.com/2019/01/15/lassa-fever-vaccine-pregnant-women/ (accessed on 13 December 2019).
- UNFPA. Eight Months Pregnant and Infected with Ebola in Conflict-Affected DRC—A Mother’s Story. Available online: https://reliefweb.int/report/democratic-republic-congo/eight-months-pregnant-and-infected-ebola-conflict-affected-drc (accessed on 30 December 2019).
- Burke, J. DRC Ebola Cases Pass 2000, Prompting Call for ‘Total Reset’. Available online: https://www.theguardian.com/world/2019/jun/03/drc-set-to-exceed-2000-ebola-cases-in-second-largest-outbreak-ever (accessed on 29 November 2019).
- WHO. Ebola Virus Disease—Democratic Republic of the Congo. Available online: https://www.who.int/csr/don/25-april-2019-ebola-drc/en/ (accessed on 14 January 2020).
- UNHCR. Attacks in Congo’s North Kivu Province Push Tens of Thousands to Flee—UNHCR. Available online: https://www.unhcr.org/en-us/news/briefing/2019/5/5ccbf72f4/attacks-congos-north-kivu-province-push-tens-thousands-flee-unhcr.html (accessed on 14 January 2020).
- WHO. WHO Adapts Ebola Vaccination Strategy in the Democratic Republic of the Congo to Account for Insecurity and Community Feedback. Available online: https://www.who.int/news-room/detail/07-05-2019-who-adapts-ebola-vaccination-strategy-in-the-democratic-republic-of-the-congo-to-account-for-insecurity-and-community-feedback (accessed on 21 December 2019).
- Anywaine, Z.; Whitworth, H.; Kaleebu, P.; Praygod, G.; Shukarev, G.; Manno, D.; Kapiga, S.; Grosskurth, H.; Kalluvya, S.; Bockstal, V.; et al. Safety and Immunogenicity of a 2-dose Heterologous Vaccination Regimen with Ad26.ZEBOV and MVA-BN-Filo Ebola vaccines: 12-month data from a Phase 1 Randomized Clinical Trial in Uganda and Tanzania. J. Infect. Dis. 2019, 220, 46–56. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6548900/ (accessed on 1 January 2020). [CrossRef]
- CMRE. Evolution de L’épidémie Dans les Provinces du Nord-Kivu et de L’ITURI au 11 Novembre 2019. Available online: https://mailchi.mp/2e48cbb0e962/situation-pidmiologique-du-02-novembre-3698289?e=6a8e4f1f4d (accessed on 15 December 2019).
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwartz, D.A. Being Pregnant during the Kivu Ebola Virus Outbreak in DR Congo: The rVSV-ZEBOV Vaccine and Its Accessibility by Mothers and Infants during Humanitarian Crises and in Conflict Areas. Vaccines 2020, 8, 38. https://doi.org/10.3390/vaccines8010038
Schwartz DA. Being Pregnant during the Kivu Ebola Virus Outbreak in DR Congo: The rVSV-ZEBOV Vaccine and Its Accessibility by Mothers and Infants during Humanitarian Crises and in Conflict Areas. Vaccines. 2020; 8(1):38. https://doi.org/10.3390/vaccines8010038
Chicago/Turabian StyleSchwartz, David A. 2020. "Being Pregnant during the Kivu Ebola Virus Outbreak in DR Congo: The rVSV-ZEBOV Vaccine and Its Accessibility by Mothers and Infants during Humanitarian Crises and in Conflict Areas" Vaccines 8, no. 1: 38. https://doi.org/10.3390/vaccines8010038
APA StyleSchwartz, D. A. (2020). Being Pregnant during the Kivu Ebola Virus Outbreak in DR Congo: The rVSV-ZEBOV Vaccine and Its Accessibility by Mothers and Infants during Humanitarian Crises and in Conflict Areas. Vaccines, 8(1), 38. https://doi.org/10.3390/vaccines8010038




