Co-Administration of Injected and Oral Vaccine Candidates Elicits Improved Immune Responses over Either Route Alone
Abstract
1. Introduction
2. Materials and Methods
2.1. Maize Material
2.2. Seed Processing
2.3. Vaccine Preparation
2.4. HBsAg Mouse Vaccine Trial
2.5. HBsAg and Ag2 Quantification
2.6. Hepatitis B Vaccine Mouse Study
2.7. Anti-HBsAg Antibody Detection in Mice
2.8. Coccidioides Vaccine Mouse Study
2.9. Analysis of CFUs and Weight Change
3. Results
3.1. HBsAg Vaccine Production
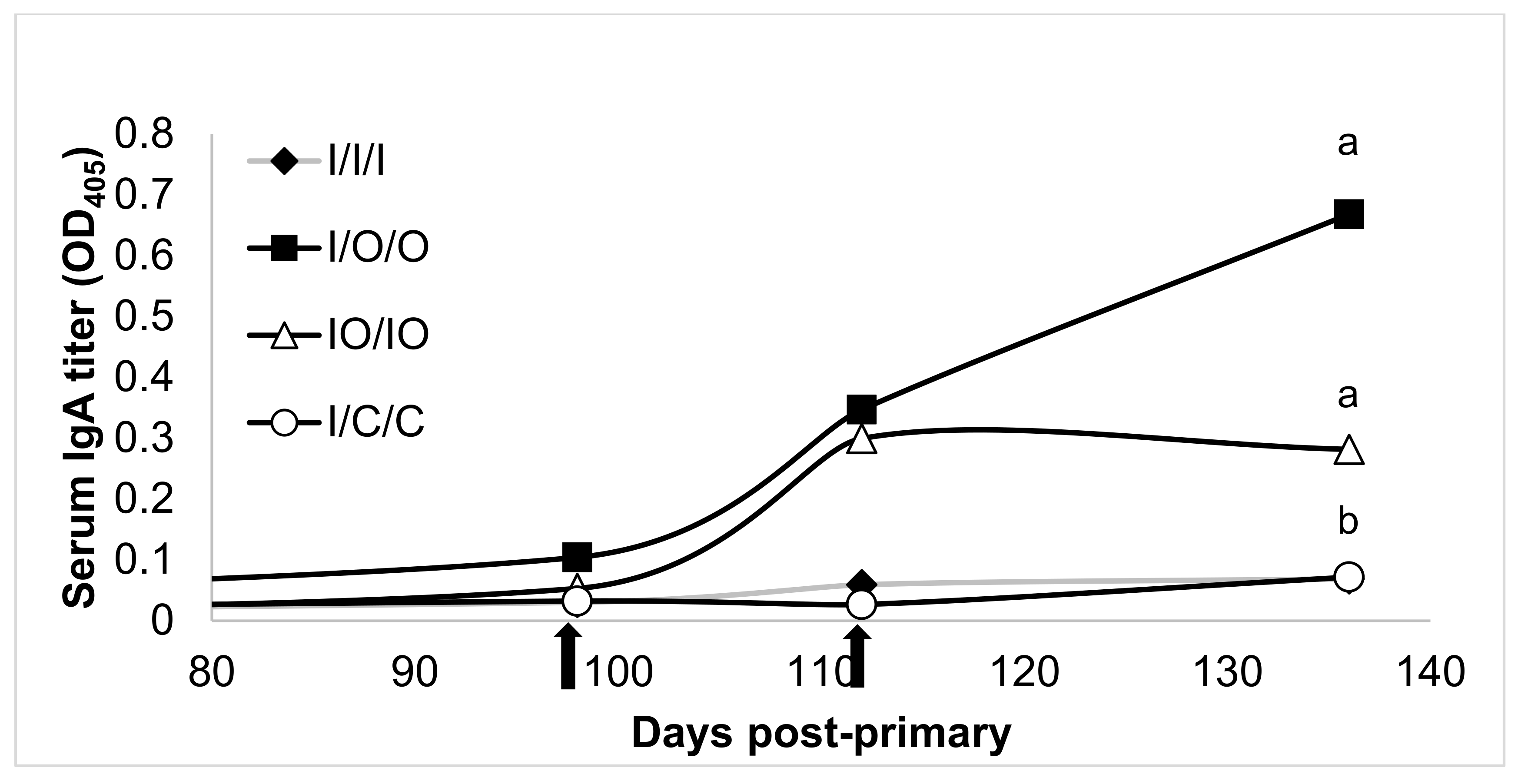
3.2. Mucosal Response
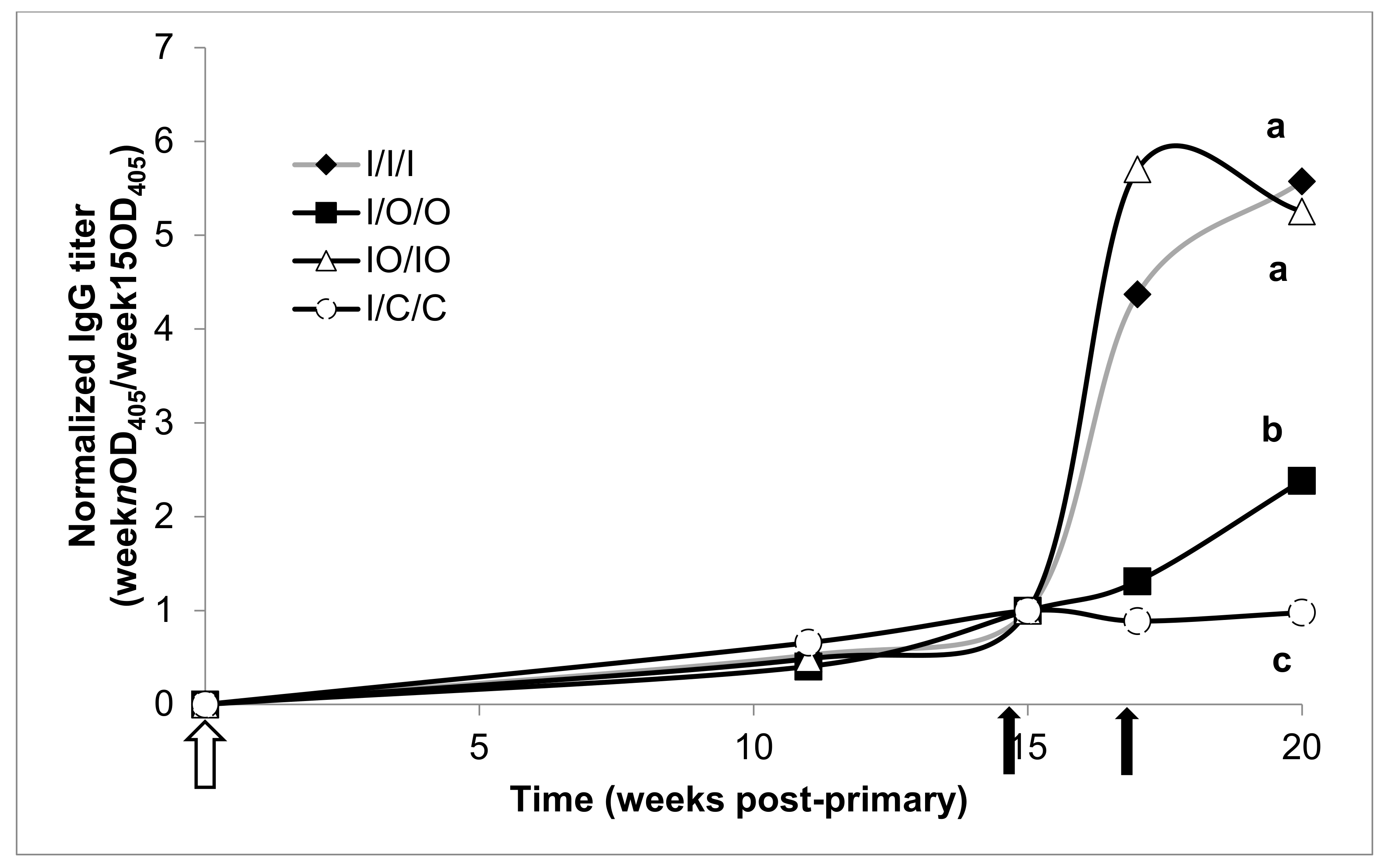
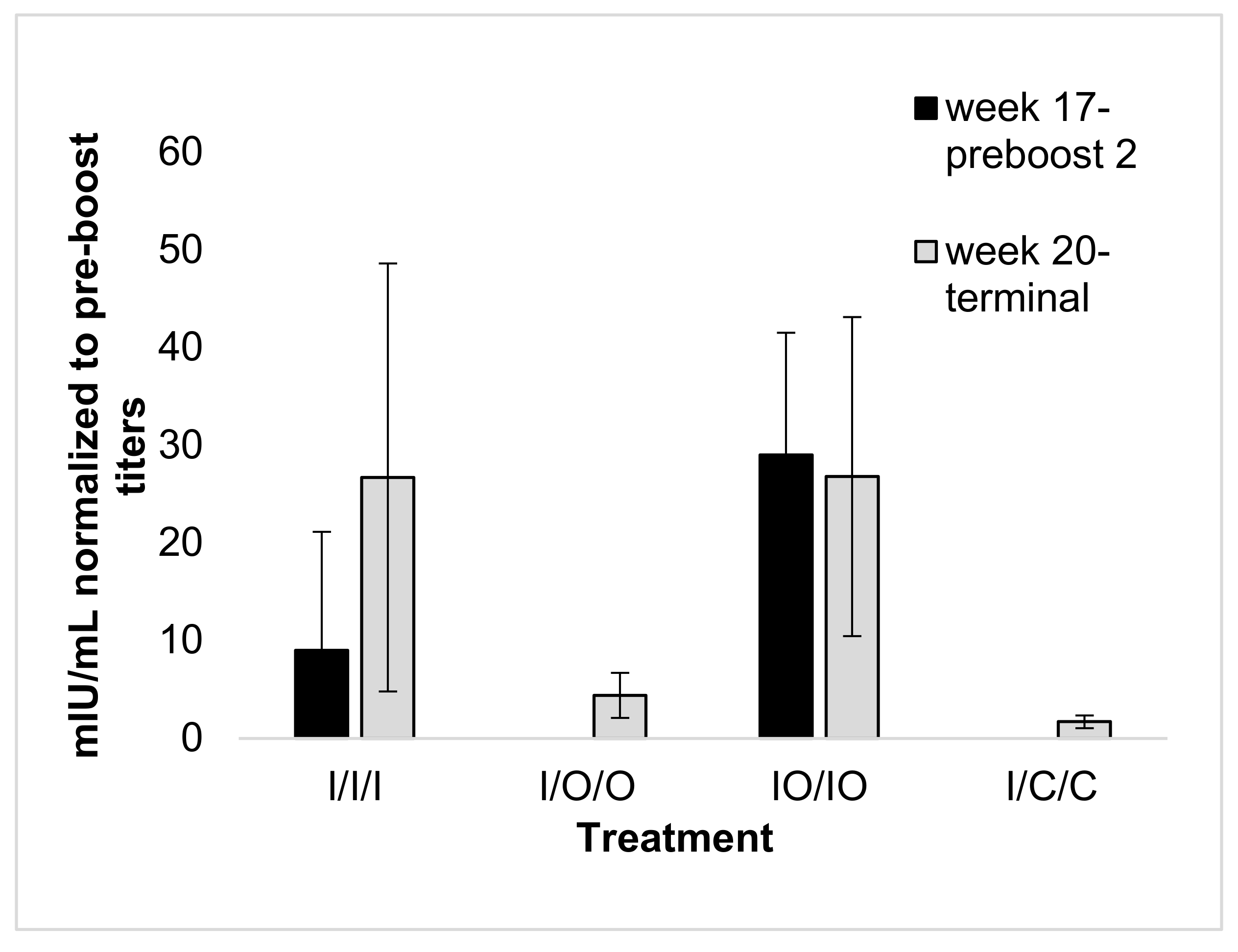
3.3. Serum Response
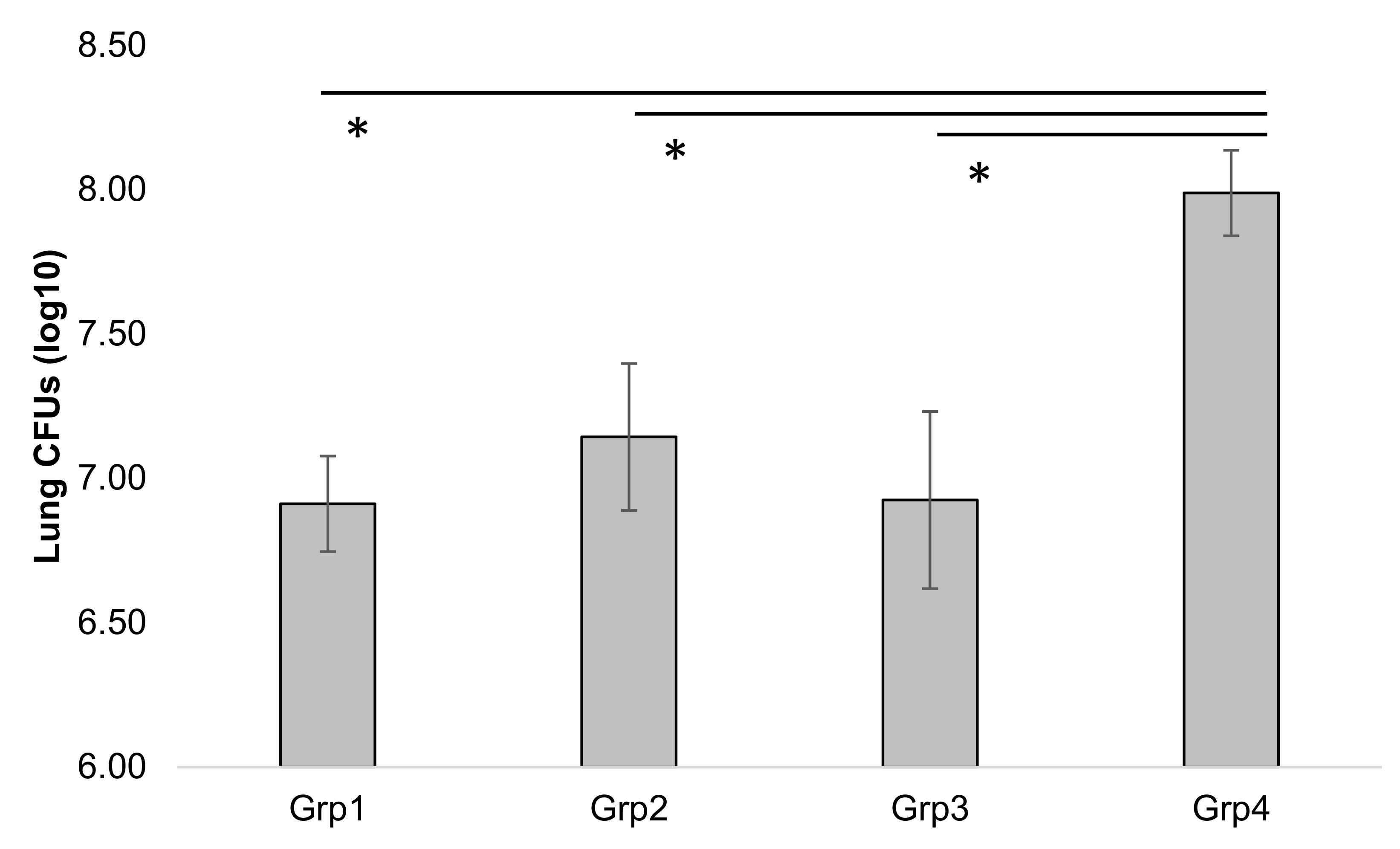
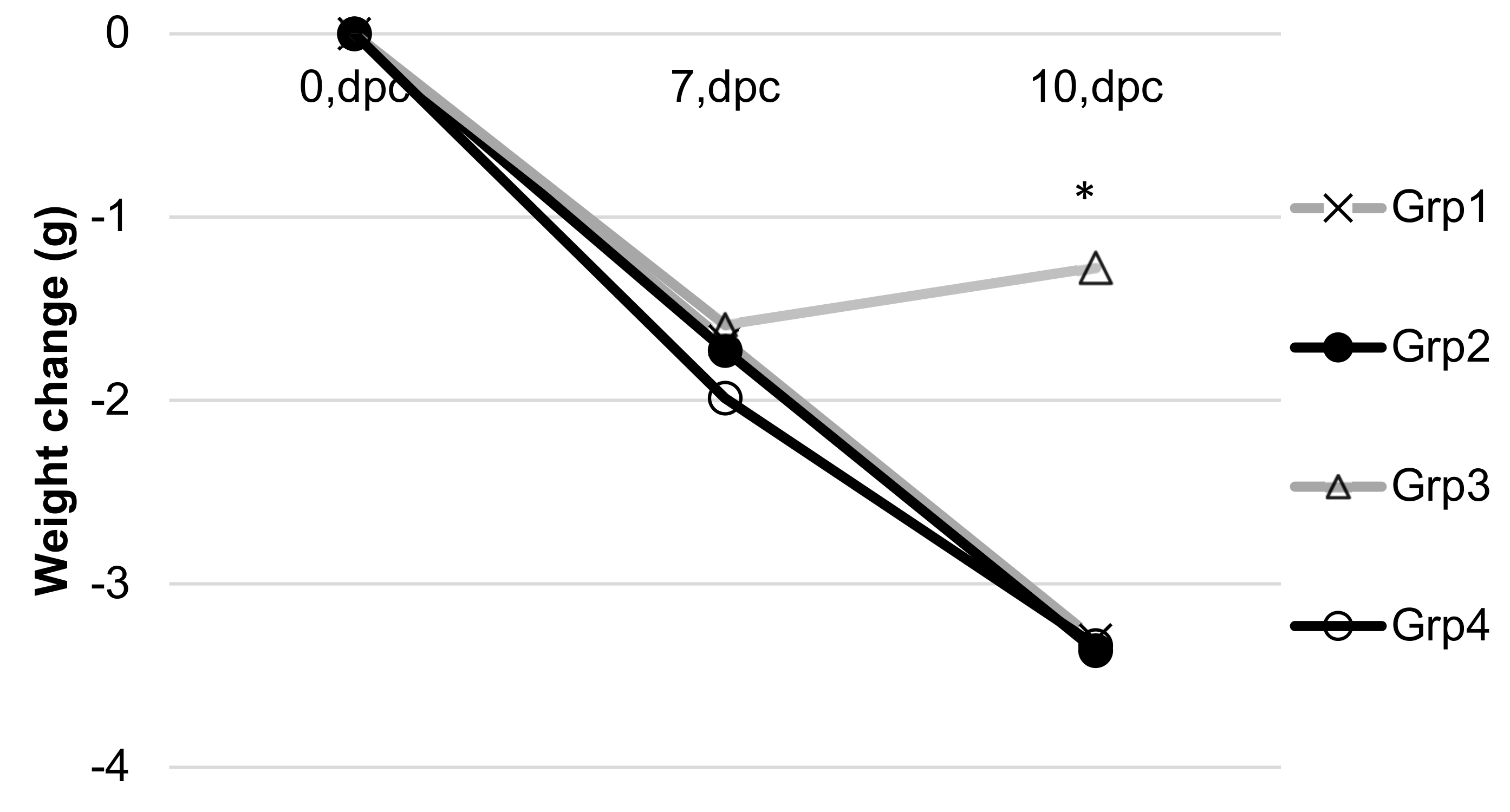
3.4. Coccidioides Mouse Study
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ott, J.J.; Stevens, G.A.; Groeger, J.; Wiersma, S.T. Global epidemiology of hepatitis B virus infection: New estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 2012, 30, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Hayden, C.A. An Oral Vaccine for Hepatitis B: Challenges, Setbacks, and Breakthroughs. In Commercial Plant-Produced Recombinant Protein Products, Biotechnology in Agriculture and Forestry; Howard, J., Hood, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 68. [Google Scholar]
- Mahoney, F.J.; Stewart, K.; Hu, H.; Coleman, P.; Alter, M.J. Progress toward the elimination of hepatitis B virus transmission among health care workers in the United States. Arch. Intern. Med. 1997, 157, 2601. [Google Scholar] [CrossRef] [PubMed]
- Simard, E.P.; Miller, J.T.; George, P.A.; Wasley, A.; Alter, M.J.; Bell, B.P.; Finelli, L. Hepatitis B Vaccination Coverage Levels Among Healthcare Workers in the United States, 2002–2003. Infect. Control Hosp. Epidemiol. 2007, 28, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Ahishali, E.; Boztas, G.; Akyuz, F.; Ibrisim, D.; Poturoglu, S.; Pinarbasi, B.; Ozdil, S.; Mungan, Z. Response to hepatitis B vaccination in patients with celiac disease. Dig. Dis. Sci. 2008, 53, 2156–2159. [Google Scholar] [CrossRef]
- Landrum, M.L.; Hullsiek, K.H.; O’Connell, R.J.; Chun, H.M.; Ganesan, A.; Okulicz, J.F.; Lalani, T.; Weintrob, A.C.; Crum-Cianflone, N.F.; Agan, B.K. Hepatitis B Vaccine Antibody Response and the Risk of Clinical AIDS or Death. PLoS ONE 2012, 7, e33488. [Google Scholar] [CrossRef]
- Laurence, J.C. Hepatitis A and B immunizations of individuals infected with human immunodeficiency virus. Am. J. Med. 2005, 118, 75–83. [Google Scholar] [CrossRef]
- Leonardi, S.; Spina, M.; Spicuzza, L.; Rotolo, N.; La Rosa, M. Hepatitis B vaccination failure in celiac disease: Is there a need to reassess current immunization strategies? Vaccine 2009, 27, 6030–6033. [Google Scholar] [CrossRef]
- Roome, A.J.; Walsh, S.J.; Cartter, M.L.; Hadler, J.L. Hepatitis B vaccine responsiveness in Connecticut public safety personnel. JAMA J. Am. Med Assoc. 1993, 270, 2931. [Google Scholar] [CrossRef]
- Tohme, R.A.; Awosika-Olumo, D.; Nielsen, C.; Khuwaja, S.; Scott, J.; Xing, J.; Drobeniuc, J.; Hu, D.J.; Turner, C.; Wafeeg, T. Evaluation of hepatitis B vaccine immunogenicity among older adults during an outbreak response in assisted living facilities. Vaccine 2011, 29, 9316–9320. [Google Scholar] [CrossRef]
- Vida Pérez, L.; Gómez Camacho, F.; García Sánchez, V.; Iglesias Flores, E.M.; Castillo Molina, L.; Cerezo Ruiz, A.; Casáis Juanena, L.; De Dios Vega, J.F. Eficacia de la vacuna contra el virus de la hepatitis B en pacientes con enfermedad inflamatoria intestinal. Med. Clín. 2009, 132, 331–335. [Google Scholar] [CrossRef]
- Williams, R.E.; Sena, A.C.; Moorman, A.C.; Moore, Z.S.; Sharapov, U.M.; Drobenuic, J.; Hu, D.J.; Wood, H.W.; Xing, J.; Spradling, P.R. Hepatitis B vaccination of susceptible elderly residents of long term care facilities during a hepatitis B outbreak. Vaccine 2012, 30, 3147–3150. [Google Scholar] [CrossRef]
- Zitt, E.; Sprenger-Mähr, H.; Knoll, F.; Neyer, U.; Lhotta, K. Vitamin D deficiency is associated with poor response to active hepatitis B immunisation in patients with chronic kidney disease. Vaccine 2011, 30, 931–935. [Google Scholar] [CrossRef]
- Nisihara, R.; De Bem, R.; Negreiros, P.; Utiyama, S.; Oliveira, N.; Amarante, H. Low hepatitis B vaccine response in children with Down syndrome from Brazil. Child. Care Health Dev. 2013, 40, 607–609. [Google Scholar] [CrossRef]
- Vermeiren, A.; Hoebe, C.J.; Dukers-Muijrers, N.H. High non-responsiveness of males and the elderly to standard hepatitis B vaccination among a large cohort of healthy employees. J. Clin. Virol. 2013, 58, 262–264. [Google Scholar] [CrossRef]
- Hayden, C.; Smith, E.; Turner, D.D.; Keener, T.K.; Wong, J.C.; Walker, J.H.; Tizard, I.R.; Jimenez-Flores, R.; Howard, J.A. Supercritical Fluid Extraction Provides an Enhancement to the Immune Response for Orally-Delivered Hepatitis B Surface Antigen. Vaccine 2014, 32, 1240–1246. [Google Scholar] [CrossRef]
- Boisen, S. Protease inhibitors in cereals. Occurrence, properties, physiological role, and nutritional influence. Acta Agric. Scand. 1983, 33, 369–381. [Google Scholar] [CrossRef]
- Arakawa, T.; Timasheff, S.N. Stabilization of protein structure by sugars. Biochemistry 1982, 21, 6536–6544. [Google Scholar] [CrossRef]
- Bailey, M.R. A model system for edible vaccination using recombinant avidin produced in corn seed. Master’s Thesis, Texas A&M University, College Station, TX, USA, 2000. [Google Scholar]
- Lu P-j Byrd, K.K.; Murphy, T.V.; Weinbaum, C. Hepatitis B vaccination coverage among high-risk adults 18–49 years, US, 2009. Vaccine 2011, 29, 7049–7057. [Google Scholar]
- Lum, P.J.; Ochoa, K.C.; Hahn, J.A.; Shafer, K.P.; Evans, J.L.; Moss, A.R. Hepatitis B virus immunization among young injection drug users in San Francisco, Calif: The UFO Study. Am. J. Public Health 2003, 93, 919–923. [Google Scholar] [CrossRef]
- Nyamathi, A.M.; Marlow, E.; Branson, C.; Marfisee, M.; Nandy, K. Hepatitis A/B vaccine completion among homeless adults with history of incarceration. J. Forensic Nurs. 2012, 13–22. [Google Scholar] [CrossRef]
- Linkins, R.W.; Chonwattana, W.; Holtz, T.H.; Wasinrapee, P.; Chaikummao, S.; Varangrat, A.; Tongtoyai, J.; Mock, P.A.; Curlin, M.E.; Sirivongrangson, P. Hepatitis A and hepatitis B infection prevalence and associated risk factors in men who have sex with men, Bangkok, 2006–2008. J. Med Virol. 2013, 85, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- MacKellar, D.A.; Valleroy, L.A.; Secura, G.M.; McFarland, W.; Shehan, D.; Ford, W.; LaLota, M.; Celentano, D.D.; Koblin, B.A.; Torian, L.V. Two decades after vaccine license: Hepatitis B immunization and infection among young men who have sex with men. Am. J. Public Health 2001, 91, 965. [Google Scholar] [PubMed]
- Hayden, C.A.; Egelkrout, E.M.; Moscoso, A.M.; Enrique, C.; Keener, T.K.; Jimenez-Flores, R.; Wong, J.C.; Howard, J.A. Production of highly concentrated, heat-stable hepatitis B surface antigen in maize. Plant Biotechnol. J. 2012, 10, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, S.; Pastor, A.R.; Malm, M.; López-Guerrero, V.; Esquivel-Guadarrama, F.; Palomares, L.A.; Vesikari, T.; Blazevic, V. Protection against live rotavirus challenge in mice induced by parenteral and mucosal delivery of VP6 subunit rotavirus vaccine. Arch. Virol. 2015, 160, 2075–2078. [Google Scholar] [CrossRef]
- Wu, H.; Singh, N.K.; Locy, R.D.; Scissum-Gunn, K.; Giambrone, J.J. Immunization of chickens with VP2 protein of infectious bursal disease virus expressed in Arabidopsis thaliana. Avian Dis. 2004, 48, 663–668. [Google Scholar] [CrossRef]
- Smith, C.E.; Beard, R.R. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am. J. Public Health Nations Health 1946, 36, 1394–1402. [Google Scholar] [CrossRef]
- Nguyen, C.; Barker, B.M.; Hoover, S.; Nix, D.E.; Ampel, N.M.; Frelinger, J.A.; Orbach, M.J.; Galgiani, J.N. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin. Microbiol. Rev. 2013, 26, 505–525. [Google Scholar] [CrossRef]
- Pappagianis, D. Seeking a Vaccine against Coccidioides immitis and Serologic Studies: Expectations and Realities. Fungal Genet. Biol. 2001, 32, 1–9. [Google Scholar] [CrossRef]
- Borchers, A.T.; Gershwin, M.E. The immune response in Coccidioidomycosis. Autoimmun. Rev. 2010, 10, 94–102. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Increase in reported coccidioidomycosis-United States, 1998–2011. Morb. Mortal. Wkly. Rep. 2013, 62, 217–221. [Google Scholar]
- Mirza, Z.; Soto, E.R.; Dikengil, F.; Levitz, S.M.; Ostroff, G.R. Beta-Glucan Particles as Vaccine Adjuvant Carriers. Vaccines Invasive Fungal Infect. Methods Protoc. 2017, 1625, 143–157. [Google Scholar]
- Huang, H.; Ostroff, G.R.; Lee, C.K.; Specht, C.A.; Levitz, S.M. Characterization and optimization of the glucan particle-based vaccine platform. Clin. Vaccine Immunol. 2013, 20, 1585–1591. [Google Scholar] [CrossRef]
- Hung, C.-Y.; Zhang, H.; Castro-Lopez, N.; Ostroff, G.R.; Khoshlenar, P.; Abraham, A.; Cole, G.T.; Negron, A.; Forsthuber, T.; Peng, T. Glucan-chitin particles enhance Th17 response and improve protective efficacy of a multivalent antigen (rCpa1) against pulmonary Coccidioides posadasii infection. Infect. Immun. 2018, 86, e00070-18. [Google Scholar] [CrossRef]
- Hayden, C.A.; Hung, C.-Y.; Zhang, H.; Negron, A.; Esquerra, R.; Ostroff, G.; Abraham, A.; Lopez, A.G.; Gonzales, J.E.; Howard, J.A. Maize-Produced Ag2 as a Subunit Vaccine for Valley Fever. J. Infect. Dis. 2019, 220, 615–623. [Google Scholar] [CrossRef]
- Wahren, B.; Hinkula, J.; Ståhle, E.L.; Borrebaeck, C.A.; Schwartz, S.; Wigzell, H. Nucleic acid vaccination with HIV regulatory genes. Ann. N. Y. Acad. Sci. 1995, 772, 278–281. [Google Scholar] [CrossRef]
- Iglesias, E.; García, D.; Márquez, G.; Prieto, Y.C.; Sánchez, J.; Trimiño, L.; Soria, Y.; García, D. Two mucosal–parenteral schedules to coadminister a multiantigenic formulation against HIV-1 in Balb/c mice. Int. Immunopharmacol. 2012, 12, 487–493. [Google Scholar] [CrossRef]
- Mäkitalo, B.; Lundholm, P.; Hinkula, J.; Nilsson, C.; Karlen, K.; Mörner, A.; Sutter, G.; Erfle, V.; Heeney, J.; Wahren, B. Enhanced cellular immunity and systemic control of SHIV infection by combined parenteral and mucosal administration of a DNA prime MVA boost vaccine regimen. J. Gen. Virol. 2004, 85, 2407–2419. [Google Scholar] [CrossRef]
- Resik, S.; Tejeda, A.; Mach, O.; Fonseca, M.; Diaz, M.; Alemany, N.; Heng Hung, L.; Aleman, Y.; Mesa, I.; Garcia, G. Does Simultaneous Administration of Bivalent (Types 1 and 3) Oral Poliovirus Vaccine and Inactivated Poliovirus Vaccine Induce Mucosal Cross-immunity to Poliovirus Type 2? Clin. Infect. Dis. 2018, 67, S51–S56. [Google Scholar] [CrossRef]
- Hurtgen, B.J.; Hung, C.-Y.; Ostroff, G.R.; Levitz, S.M.; Cole, G.T. Construction and evaluation of a novel recombinant T cell epitope-based vaccine against coccidioidomycosis. Infect. Immun 2012, 80, 3960–3974. [Google Scholar] [CrossRef]
- Hayden, C.A.; Streatfield, S.J.; Lamphear, B.J.; Fake, G.M.; Keener, T.K.; Walker, J.H.; Clements, J.D.; Turner, D.D.; Tizard, I.R.; Howard, J.A. Bioencapsulation of the hepatitis B surface antigen and its use as an effective oral immunogen. Vaccine 2012, 30, 2937–2942. [Google Scholar] [CrossRef]
- Hayden, C.A.; Fischer, M.E.; Andrews, B.L.; Chilton, H.C.; Turner, D.D.; Walker, J.H.; Tizard, I.R.; Howard, J.A. Oral delivery of wafers made from HBsAg-expressing maize germ induces long-term immunological systemic and mucosal responses. Vaccine 2015, 33, 2881–2886. [Google Scholar] [CrossRef]
- Delgado, N.; Xue, J.; Yu, J.-J.; Hung, C.-Y.; Cole, G.T. A recombinant β-1, 3-glucanosyltransferase homolog of Coccidioides posadasii protects mice against coccidioidomycosis. Infect. Immun. 2003, 71, 3010–3019. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayden, C.A.; Landrock, D.; Hung, C.Y.; Ostroff, G.; Fake, G.M.; Walker, J.H.; Kier, A.; Howard, J.A. Co-Administration of Injected and Oral Vaccine Candidates Elicits Improved Immune Responses over Either Route Alone. Vaccines 2020, 8, 37. https://doi.org/10.3390/vaccines8010037
Hayden CA, Landrock D, Hung CY, Ostroff G, Fake GM, Walker JH, Kier A, Howard JA. Co-Administration of Injected and Oral Vaccine Candidates Elicits Improved Immune Responses over Either Route Alone. Vaccines. 2020; 8(1):37. https://doi.org/10.3390/vaccines8010037
Chicago/Turabian StyleHayden, Celine A., Danilo Landrock, Chiung Yu Hung, Gary Ostroff, Gina M. Fake, John H. Walker, Ann Kier, and John A. Howard. 2020. "Co-Administration of Injected and Oral Vaccine Candidates Elicits Improved Immune Responses over Either Route Alone" Vaccines 8, no. 1: 37. https://doi.org/10.3390/vaccines8010037
APA StyleHayden, C. A., Landrock, D., Hung, C. Y., Ostroff, G., Fake, G. M., Walker, J. H., Kier, A., & Howard, J. A. (2020). Co-Administration of Injected and Oral Vaccine Candidates Elicits Improved Immune Responses over Either Route Alone. Vaccines, 8(1), 37. https://doi.org/10.3390/vaccines8010037



