Vaccination with Ectoparasite Proteins Involved in Midgut Function and Blood Digestion Reduces Salmon Louse Infestations
Abstract
1. Introduction
2. Materials and Methods
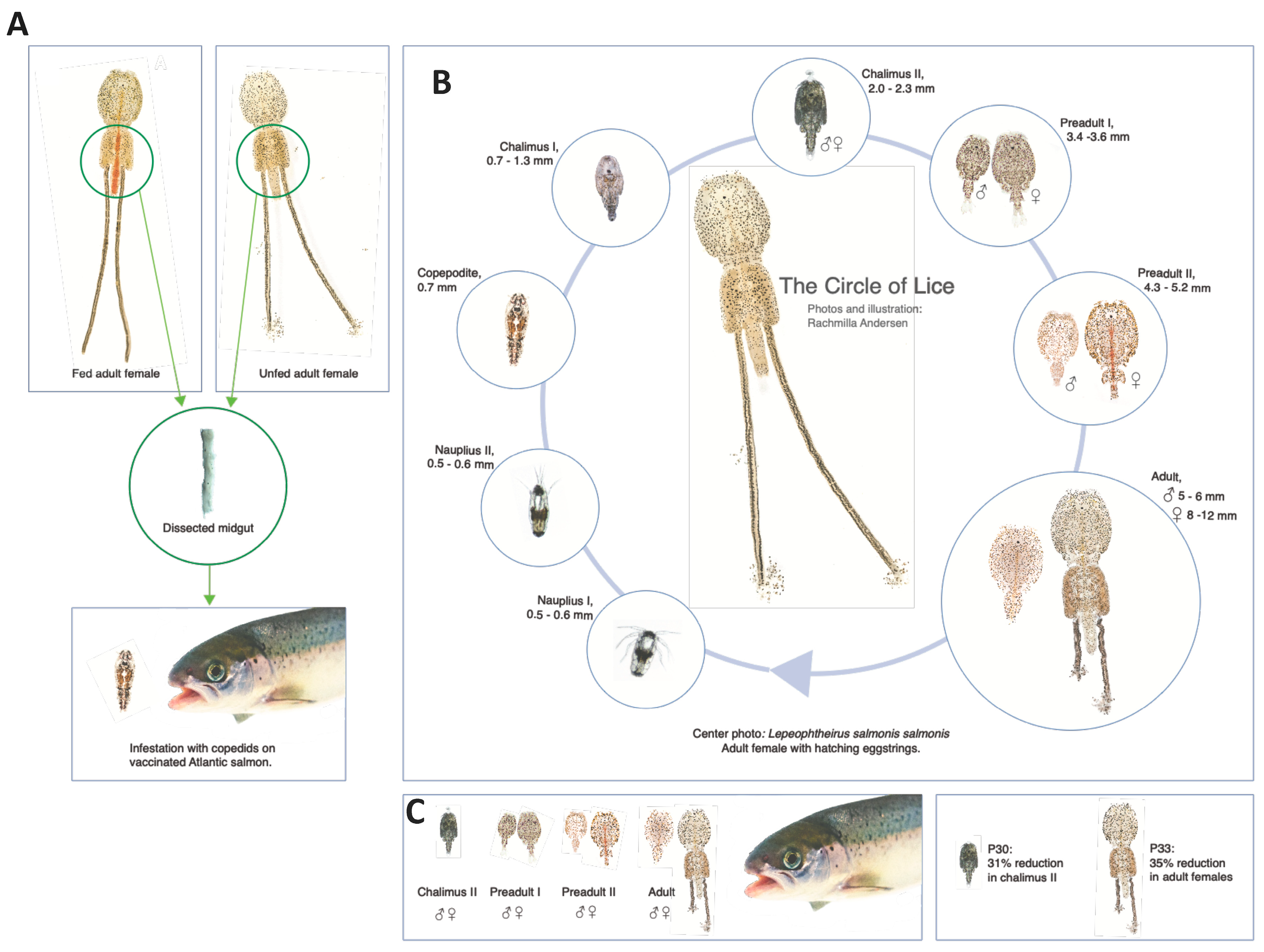
2.1. Salmon Lice and Tissues
2.2. Salmon Louse RNA and Protein Extraction
2.3. Fish, Husbandry, and Ethics Approval
2.4. Production of Recombinant Proteins and Vaccine Formulation
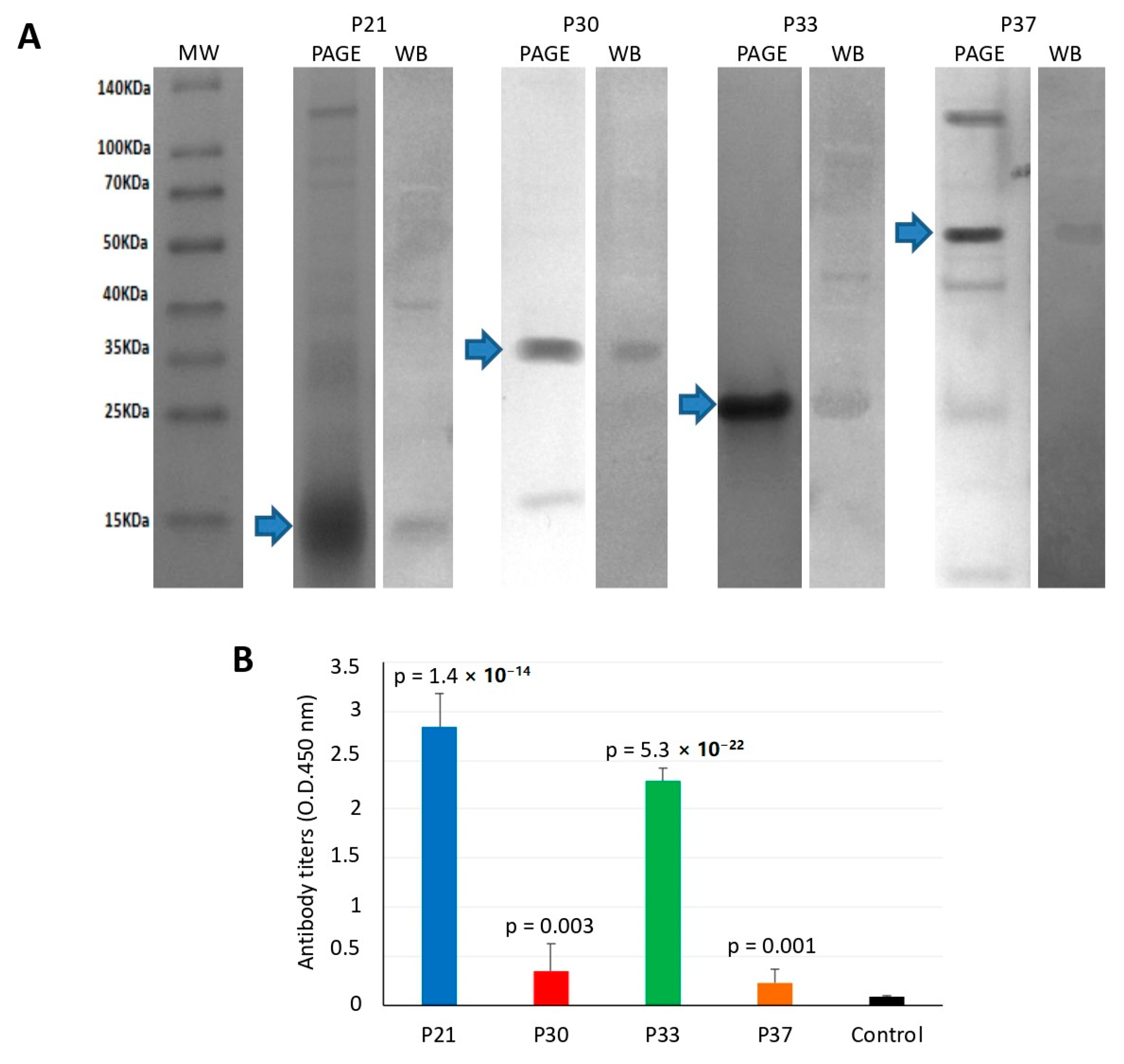
2.5. Polyacrylamide Gel Electrophoresis and Western Blot for the Characterization of Recombinant Proteins
2.6. Fish Vaccination and Challenge Infestation with Salmon Lice
2.7. Salmon Lice Counts and Eggstring Sampling and Hatching
2.8. ELISA for the Quantitation of Fish Antibody Titers
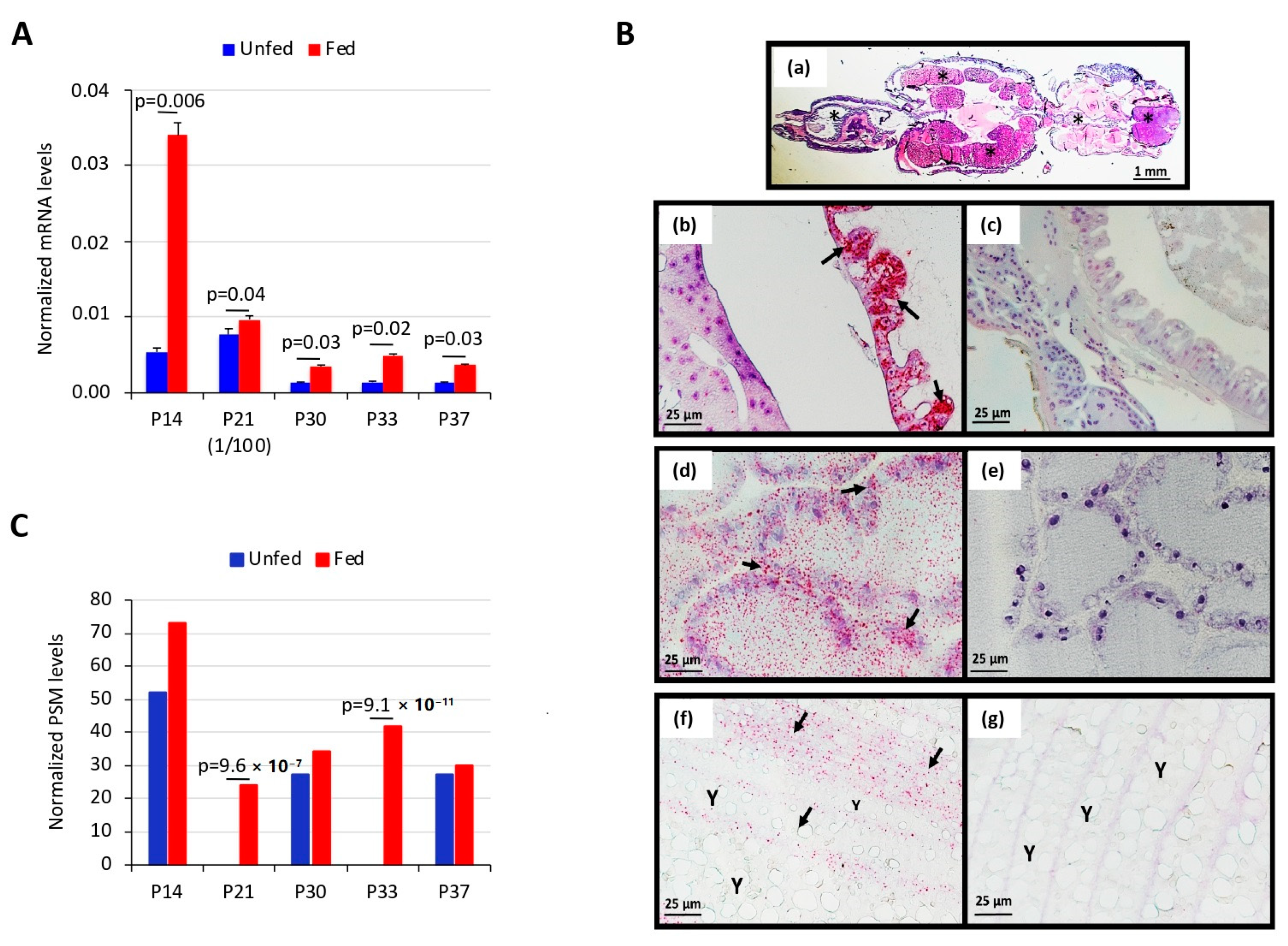
2.9. Analysis of Gene Expression by qRT-PCR
2.10. In Situ Hybridization
2.11. Targeted Protein Identification
2.12. Immunofluorescence Assay
3. Results
3.1. Salmon Louse Gut Membrane Proteins Overrepresented in Response to Feeding may Constitute Candidate Vaccine Protective Antigens
3.2. Vaccination of Atlantic Salmon with Selected Salmon Louse Midgut Proteins Affects Ectoparasite Infestations
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Torrissen, O.; Jones, S.; Asche, F.; Guttormsen, A.; Skilbrei, O.T.; Nilsen, F.; Horsberg, T.E.; Jackson, D. Salmon lice--impact on wild salmonids and salmon aquaculture. J. Fish Dis. 2013, 36, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Lhorente, J.P.; Gallardo, J.A.; Villanueva, B.; Carabaño, M.J.; Neira, R. Disease resistance in Atlantic salmon (Salmo salar): Coinfection of the intracellular bacterial pathogen Piscirickettsia salmonis and the sea louse Caligus rogercresseyi. PLoS ONE 2014, 9, e95397. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.E.; Bricknell, I.R.; Covello, J.; Purcell, S.; Fast, M.D.; Wolters, W.; Bouchard, D.A. Sea lice, Lepeophtheirus salmonis (Krøyer 1837), infected Atlantic salmon (Salmo salar L.) are more susceptible to infectious salmon anemia virus. PLoS ONE 2019, 14, e0209178, Erratum in PLoS One 2019, 14, e0213232. [Google Scholar] [CrossRef]
- Nylund, S.; Nylund, A.; Watanabe, K.; Arnesen, C.E.; Karlsbakk, E. Paranucleospora theridion n. gen., n. sp. (Microsporidia, Enterocytozoonidae) with a life cycle in the salmon louse (Lepeophtheirus salmonis, Copepoda) and Atlantic salmon (Salmo salar). J. Eukaryot. Microbiol. 2010, 57, 95–114. [Google Scholar] [CrossRef]
- Jakob, E.; Barker, D.E.; Garver, K.A. Vector potential of the salmon louse Lepeophtheirus salmonis in the transmission of infectious haematopoietic necrosis virus (IHNV). Dis. Aquat. Organ. 2011, 97, 155–165. [Google Scholar] [CrossRef]
- Johnson, S.C.; Albright, L.J. The developmental stages of Lepeophtheirus salmonis (Krøyer, 1837) (Copepoda: Caligidae). Can. J. Zool. 1991, 69, 929–950. [Google Scholar] [CrossRef]
- Hamre, L.A.; Eichner, C.; Caipang, C.M.; Dalvin, S.T.; Bron, J.E.; Nilsen, F.; Boxshall, G.; Skern-Mauritzen, R. The Salmon Louse Lepeophtheirus salmonis (Copepoda: Caligidae) life cycle has only two Chalimus stages. PLoS ONE 2013, 8, e73539. [Google Scholar] [CrossRef]
- Vollset, K.W.; Qviller, L.; Skår, B.; Barlaup, B.T.; Dohoo, I. Parasitic sea louse infestations on wild sea trout: Separating the roles of fish farms and temperature. Parasit. Vectors 2018, 11, 609. [Google Scholar] [CrossRef]
- Aaen, S.M.; Helgesen, K.O.; Bakke, M.J.; Kaur, K.; Horsberg, T.E. Drug resistance in sea lice: A threat to salmonid aquaculture. Trends Parasitol. 2015, 31, 72–81. [Google Scholar] [CrossRef]
- Overton, K.; Dempster, T.; Oppedal, F.; Kristiansen, T.S.; Gismervik, K.; Stien, L.H. Salmon lice treatments and salmon mortality in Norwegian aquaculture: A review. Rev. Aquacult. 2019, 11, 1398–1417. [Google Scholar] [CrossRef]
- Global Salmon Initiative. Non-medical Approaches to Sea Lice Management. Available online: https://globalsalmoninitiative.org/en/what-is-the-gsi-working-on/biosecurity/non-medicinal-approaches-to-sea-lice-management/ (accessed on 3 November 2019).
- Leclercq, E.; Davie, A.; Migaud, H. Delousing efficiency of farmed ballan wrasse (Labrus bergylta) against Lepeophtheirus salmonis infecting Atlantic salmon (Salmo salar) post-smolts. Pest Manag. Sci. 2014, 70, 1274–1282. [Google Scholar] [CrossRef]
- Imsland, A.K.D.; Hanssen, A.; Nytrø, A.V.; Reynolds, P.; Jonassen, T.M.; Hangstad, T.A.; Elvegård, T.A.; Urskog, T.C.; Mikalsen, B. It works! Lumpfish can significantly lower sea lice infestation in large-scale salmon farming. Biol. Open 2018, 7, bio036301. [Google Scholar] [CrossRef]
- Woo, P.T. Immunization against parasitic diseases of fish. Dev. Biol. Stand 1997, 90, 233–241. [Google Scholar]
- Raynard, R.S.; Bricknell, I.R.; Billingsley, P.F.; Nisbet, A.J.; Vigneau, A.; Sommerville, C. Development of vaccines against sea lice. Pest Manag. Sci. 2002, 58, 569–575. [Google Scholar] [CrossRef]
- Carpio, Y.; Basabem, L.; Acostam, J.; Rodríguez, A.; Mendoza, A.; Lisperger, A.; Zamorano, E.; González, M.; Rivas, M.; Contreras, S.; et al. Novel gene isolated from Caligus rogercresseyi: A promising target for vaccine development against sea lice. Vaccine 2011, 29, 2810–2820. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Moreno-Cid, J.A.; Galindo, R.C.; Almazan, C.; Kocan, K.M.; Merino, O.; Perez de la Lastra, J.M.; Estrada-Peña, A.; Blouin, E.F. Subolesin/Akirin vaccines for the control of arthropod vectors and vector-borne pathogens. Transbound. Emerg. Dis. 2013, 60 (Suppl. 2), 172–178. [Google Scholar] [CrossRef]
- McNair, C.M. Ectoparasites of medical and veterinary importance: Drug resistance and the need for alternative control methods. J. Pharm. Pharmacol. 2015, 67, 351–363. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Estrada-Peña, A. Why new vaccines for the control of ectoparasite vectors have not been registered and commercialized? Vaccines 2019, 7, 75. [Google Scholar] [CrossRef]
- De la Fuente, J.; Antunes, S.; Bonnet, S.; Cabezas-Cruz, A.; Domingos, A.; Estrada-Peña, A.; Johnson, N.; Kocan, K.M.; Mansfield, K.L.; Nijhof, A.M.; et al. Tick-pathogen interactions and vector competence: Identification of molecular drivers for tick-borne diseases. Front. Cell. Infect. Microbiol. 2017, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; de la Fuente, J. Vaccinomics approach to the development of vaccines for the control of multiple ectoparasite infestations. Nova Acta Leopold. 2016, 411, 185–200. [Google Scholar]
- De la Fuente, J.; Contreras, M. Tick vaccines: Current status and future directions. Expert Rev. Vaccines 2015, 14, 1367–1376. [Google Scholar] [CrossRef]
- De la Fuente, J.; Almazán, C.; Canales, M.; Pérez de la Lastra, J.M.; Kocan, K.M.; Willadsen, P. A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Anim. Health Res. Rev. 2007, 8, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Villar, M.; Artigas-Jerónimo, S.; Kornieieva, L.; Mytrofanov, S.; de la Fuente, J. A reverse vaccinology approach to the identification and characterization of Ctenocephalides felis candidate protective antigens for the control of cat flea infestations. Parasit. Vectors 2018, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Kopáček, P.; Lew-Tabor, A.; Maritz-Olivier, C. Strategies for new and improved vaccines against ticks and tick-borne diseases. Parasite Immunol. 2016, 38, 754–769. [Google Scholar] [CrossRef] [PubMed]
- Hamre, L.A.; Glover, K.A.; Nilsen, F. Establishment and characterisation of salmon louse (Lepeophtheirus salmonis (Krøyer 1837)) laboratory strains. Parasitol. Int. 2009, 58, 451–460. [Google Scholar] [CrossRef]
- Hamre, L.A.; Nilsen, F. Individual fish tank arrays in studies of Lepeophtheirus salmonis and lice loss variability. Dis. Aquat. Organ. 2011, 97, 47–56. [Google Scholar] [CrossRef]
- Frost, P.; Nilsen, F. Validation of reference genes for transcription profiling in the salmon louse, Lepeophtheirus salmonis, by quantitative real-time PCR. Vet. Parasitol. 2003, 118, 169–174. [Google Scholar] [CrossRef]
- Ayllón, N.; Villar, M.; Busby, A.T.; Kocan, K.M.; Blouin, E.F.; Bonzón-Kulichenko, E.; Galindo, R.C.; Mangold, A.J.; Alberdi, P.; Pérez de la Lastra, J.M.; et al. Anaplasma phagocytophilum inhibits apoptosis and promotes cytoskeleton rearrangement for infection of tick cells. Infect. Immun. 2013, 81, 2415–2425. [Google Scholar] [CrossRef]
- Wang, F.; Flanagan, J.; Su, N.; Wang, L.-C.; Bui, S.; Nielson, A.; Wu, X.; Vo, H.-T.; Ma, X.-J.; Luo, Y. RNAscope: A novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diagn. 2012, 14, 22–29. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef] [PubMed]
- Ohlstein, B.; Spradling, A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science 2007, 315, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Fiúza, U.M.; Arias, A.M. Cell and molecular biology of Notch. J. Endocrinol. 2007, 194, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Melmed, G.; Thomas, L.S.; Lee, N.; Tesfay, S.Y.; Lukasek, K.; Michelsen, K.S.; Zhou, Y.; Hu, B.; Arditi, M.; Abreu, M.T. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: Implications for host-microbial interactions in the gut. J. Immunol. 2003, 170, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Couto, J.; Antunes, S.; Pinheiro-Silva, R.; do Rosário, V.; de la Fuente, J.; Domingos, A. Solute carriers affect Anopheles stephensi survival and Plasmodium berghei infection in the salivary glands. Sci. Rep. 2017, 7, 6141. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, R.; Manzano-Román, R.; Obolo-Mvoulouga, P.; Oleaga, A. Function-guided selection of midgut antigens from Ornithodoros erraticus ticks and an evaluation of their protective efficacy in rabbits. Vet. Parasitol. 2019, 272, 1–12. [Google Scholar] [CrossRef]
- Guerrero, F.D.; Andreotti, R.; Bendele, K.G.; Cunha, R.C.; Miller, R.J.; Yeater, K.; Pérez de León, A.A. Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations. Parasit. Vectors 2014, 7, 475. [Google Scholar]
- Contreras, M.; de la Fuente, J. Control of infestations by Ixodes ricinus tick larvae in rabbits vaccinated with aquaporin recombinant antigens. Vaccine 2017, 35, 1323–1328. [Google Scholar] [CrossRef]
- Évora, P.M.; Sanches, G.S.; Guerrero, F.D.; León, A.P.; Bechara, G.H. Immunogenic potential of Rhipicephalus (Boophilus) microplus aquaporin 1 against Rhipicephalus sanguineus in domestic dogs. Rev. Bras. Parasitol. Vet. 2017, 26, 60–66. [Google Scholar] [CrossRef]
- Hajdusek, O.; Almazán, C.; Loosova, G.; Villar, M.; Canales, M.; Grubhoffer, L.; Kopacek, P.; de la Fuente, J. Characterization of ferritin 2 for the control of tick infestations. Vaccine 2010, 28, 2993–2998. [Google Scholar] [CrossRef]
- Galay, R.L.; Miyata, T.; Umemiya-Shirafuji, R.; Maeda, H.; Kusakisako, K.; Tsuji, N.; Mochizuki, M.; Fujisaki, K.; Tanaka, T. Evaluation and comparison of the potential of two ferritins as anti-tick vaccines against Haemaphysalis longicornis. Parasit. Vectors 2014, 7, 482. [Google Scholar] [CrossRef] [PubMed]
- Knorr, S.; Anguita, J.; Cortazar, J.T.; Hajdusek, O.; Kopáček, P.; Trentelman, J.J.; Kershaw, O.; Hovius, J.W.; Nijhof, A.M. Preliminary evaluation of tick protein extracts and recombinant ferritin 2 as anti-tick vaccines targeting Ixodes ricinus in cattle. Front. Physiol. 2018, 9, 1696. [Google Scholar] [CrossRef]
- Manjunathachar, H.V.; Kumar, B.; Saravanan, B.C.; Choudhary, S.; Mohanty, A.K.; Nagar, G.; Chigure, G.; Ravi Kumar, G.V.P.P.S.; de la Fuente, J.; Ghosh, S. Identification and characterization of vaccine candidates against Hyalomma anatolicum-vector of Crimean-Congo haemorrhagic fever virus. Transbound. Emerg. Dis. 2019, 66, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Trimnell, A.R.; Davies, G.M.; Lissina, O.; Hails, R.S.; Nuttall, P.A. A cross-reactive tick cement antigen is a candidate broad-spectrum tick vaccine. Vaccine 2005, 23, 4329–4341. [Google Scholar] [CrossRef]
- Labuda, M.; Trimnell, A.R.; Licková, M.; Kazimírová, M.; Davies, G.M.; Lissina, O.; Hails, R.S.; Nuttall, P.A. An antivector vaccine protects against a lethal vector-borne pathogen. PLoS Pathog. 2006, 2, e27. [Google Scholar] [CrossRef]
- Tort, L.; Balasch, J.C.; Mackenzie, S. Fish immune system. A crossroads between innate and adaptive responses. Inmunología 2003, 22, 277–286. [Google Scholar]
- Halliday, A.; Turner, J.D.; Guimarães, A.; Bates, P.A.; Taylor, M.J. The TLR2/6 ligand PAM2CSK4 is a Th2 polarizing adjuvant in Leishmania major and Brugia malayi murine vaccine models. Parasit. Vectors 2016, 9, 96. [Google Scholar] [CrossRef]
- Contreras, M.; Villar, M.; de la Fuente, J. A vaccinomics approach to the identification of tick protective antigens for the control of Ixodes ricinus and Dermacentor reticulatus infestations in companion animals. Front. Physiol. 2019, 10, 977. [Google Scholar] [CrossRef]
- Awomoyi, A.A. The human solute carrier family 11 member 1 protein (SLC11A1): Linking infections, autoimmunity and cancer? FEMS Immunol. Med. Microbiol. 2007, 49, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, G. The host transfer ability of Lepeophtheirus salmonis (Copepoda: Caligidae) from farmed Atlantic salmon, Salmo salar L. J. Fish Dis. 1997, 20, 153–157. [Google Scholar] [CrossRef]
- De la Fuente, J.; Rodríguez, M.; Redondo, M.; Montero, C.; García-García, J.C.; Méndez, L.; Serrano, E.; Valdés, M.; Enríquez, A.; Canales, M.; et al. Field studies and cost-effectiveness analysis of vaccination with GavacTM against the cattle tick Boophilus microplus. Vaccine 1998, 16, 366–373. [Google Scholar] [CrossRef]
- Merino, M.; Antunes, S.; Mosqueda, J.; Moreno-Cid, J.A.; Pérez de la Lastra, J.M.; Rosario-Cruz, R.; Rodríguez, S.; Domingos, A.; de la Fuente, J. Vaccination with proteins involved in tick-pathogen interactions reduces vector infestations and pathogen infection. Vaccine 2013, 31, 5889–5896. [Google Scholar] [CrossRef] [PubMed]

| Protein: Accession No. | Description | GO | References |
|---|---|---|---|
| P21: A0A0K2VDM5 | Delta-like protein | BP: Metabolism, multicellular organism development, Notch signaling pathway MF: Calcium ion binding CC: Membrane | [33,34] |
| P30: A0A0K2T2M9 | Putative Toll-like receptor 6 | BP: Metabolism, signal transduction, innate immune response MF: Receptor CC: Membrane | [35] |
| P33: A0A0K2TQ92 | Potassium chloride and amino acid transporter | BP: Metabolism, amino acid transmembrane transport, Cellular hypotonic salinity response MF: Transmembrane transporter activity, Potassium:chloride symporter activity CC: Membrane | [36] |
| P37: A0A0K2UYH4 | Bifunctional heparan sulfate N-deacetylase/N-sulfotransferase | BP: Metabolism, cell surface receptor signaling pathway, Synaptic vesicle endocytosis, Wnt signaling pathway MF: [heparan sulfate]-glucosamine N-sulfotransferase activity CC: Membrane | [34] |
| Antigen | Fish (n) | Chalimus II | Adult Male | Adult Female | Reduction of Chalimus II | Reduction of Adult Females |
|---|---|---|---|---|---|---|
| P33 | 36 | 13.64 (12.05–15.32) | 4.17 (3.36–4.98) | 3.81 (3.12–4.49) | 14% p = 0.12 | 35% p = 0.004 |
| Control P33 | 40 | 15.85 (13.58–18.12) | 4.95 (4.14–5.76) | 5.83 (4.66–6.99) | ||
| P30 | 35 | 12.14 (9.85–14.44) | 2.66 (1.80–3.52) | 1.17 (0.58–1.76) | 31% p = 0.02 | 25% p = 0.30 |
| Control P30 | 40 | 17.56 (13.94–21.21) | 2.67 (2.05–3.28) | 1.56 (1.07–2.06) | ||
| P37 | 40 | 27.98 (24.78–31.17) | 8.46 (7.10–9.56) | 8.00 (6.53–9.47) | 7% p = 0.28 | 4% p = 0.69 |
| Control P37 | 40 | 30.20 (27.51-32.88) | 8.02 (6.87–9.17) | 8.37 (7.17–9.56) | ||
| P21 | 39 | 19.72 (16.48-22.96) | 1.31 (0.96–1.66) | 0.13 (0.04–0.30) | 20% p = 0.09 | 0% |
| Control P21 | 40 | 24.50 (19.81–29.19) | 1.18 (0.81–1.54) | 0.13 (0.02–0.23) | ||
| P14 | 40 | 5.05 (3.99–6.11) | 2.42 (1.73–3.22) | 2.39 (1.79–2.99) | 12% p = 0.40 | 0% |
| Control P14 | 40 | 5.71 (4.55–6.87) | 2.47 (1.85–2.99) | 2.39 (1.71–3.07) |
| Antigen | Fish (n) | Eggstring Length (mm) | Copepodids/mm Eggstring | Reduction of Eggstring Length | Reduction of Hatching Success |
|---|---|---|---|---|---|
| P33 | 10 | 9.23 (7.57–10.89) | 10.59 (7.66–13.52) | 15% p = 0.09 | 1% p = 0.74 |
| Control P33 | 10 | 10.82 (9.63–12.00) | 10.74 (8.09–13.38) | ||
| P30 | 6 | 12.63 (10.48–14.78) | 8.28 (5.47–11.08) | 16% p = 0.04 | −13% p = 0.49 |
| Control P30 | 8 | 15.08 (14.25–15.91) | 7.36 (4.50–10.21) | ||
| P37 | 10 | 17.15 (16.24–18.05) | 10.10 (8.38–11.83) | -5% p = 0.40 | −44% p = 0.17 |
| Control P37 | 10 | 16.40 (15.00–17.80) | 7.01 (3.81–10.21) | ||
| P21 | 8 | 6.69 (2.69–10.69) | 5.05 (-0.87–10.97) | 24% p = 0.42 | −44% p = 0.70 |
| Control P21 | 7 | 8.79 (4.58–12.99) | 3.50 (-2.20–9.19) | ||
| P14 | 9 | 13.94 (9.86–18.03) | 7.99 (4.60–11.39) | 7% p = 0.54 | −9% p = 0.52 |
| Control P14 | 8 | 15.00 (11.32–18.67) | 7.35 (4.17–10.54) |
| Antigen | Number of Pre-Adults at Challenge | Number of Adults at Termination | Reduction | |||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Adult Males | Adult Females | |
| P33 | 7.30 (±0.62) | 6.90 (±0.19) | 0.90 (±0.43) | 2.80 (±0.61) | 50% p = 0.07 | 13% p = 0.45 |
| Control P33 | 7.40 (±0.50) | 7.00 (±0.00) | 1.80 (±0.82) | 3.30 (±0.88) | ||
| P30 | 6.70 (±0.30) | 8.00 (±0.00) | 0.80 (±0.46) | 1.20 (±0.67) | 27 % p = 0.43 | 29% p = 0.38 |
| Control P30 | 6.80 (±0.25) | 8.00 (±0.00) | 1.10 (±0.51) | 1.70 (±0.80) | ||
| P37 | 10.00 (±0.00) | 10.00 (±0.00) | 1.70 (±0.68) | 4.30 (±1.08) | 32% p = 0.16 | -10% p = 0.60 |
| Control P37 | 10.00 (±0.00) | 10.00 (±0.00) | 2.50 (±0.75) | 3.90 (±0.90) | ||
| P21 | 7.00 (±0.00) | 8.00 (±0.00) | 0.20 (±0.25) | 1.30 (±0.56) | −100% p = 0.56 | −44% p = 0.31 |
| Control P21 | 7.00 (±0.00) | 8.00 (±0.00) | 0.10 (±0.19) | 0.90 (±0.43) | ||
| P14 | 10.00 (±0.00) | 10.00 (±0.00) | 1.20 (±0.67) | 2.80 (±1.03) | −33% p = 0.52 | −87% p = 0.07 |
| Control P14 | 10.00 (±0.00) | 10.00 (±0.00) | 0.90 (±0.51) | 1.50 (±0.69) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras, M.; Karlsen, M.; Villar, M.; Olsen, R.H.; Leknes, L.M.; Furevik, A.; Yttredal, K.L.; Tartor, H.; Grove, S.; Alberdi, P.; et al. Vaccination with Ectoparasite Proteins Involved in Midgut Function and Blood Digestion Reduces Salmon Louse Infestations. Vaccines 2020, 8, 32. https://doi.org/10.3390/vaccines8010032
Contreras M, Karlsen M, Villar M, Olsen RH, Leknes LM, Furevik A, Yttredal KL, Tartor H, Grove S, Alberdi P, et al. Vaccination with Ectoparasite Proteins Involved in Midgut Function and Blood Digestion Reduces Salmon Louse Infestations. Vaccines. 2020; 8(1):32. https://doi.org/10.3390/vaccines8010032
Chicago/Turabian StyleContreras, Marinela, Marius Karlsen, Margarita Villar, Rolf Hetlelid Olsen, Lisa Marie Leknes, Anette Furevik, Karine Lindmo Yttredal, Haitham Tartor, Soren Grove, Pilar Alberdi, and et al. 2020. "Vaccination with Ectoparasite Proteins Involved in Midgut Function and Blood Digestion Reduces Salmon Louse Infestations" Vaccines 8, no. 1: 32. https://doi.org/10.3390/vaccines8010032
APA StyleContreras, M., Karlsen, M., Villar, M., Olsen, R. H., Leknes, L. M., Furevik, A., Yttredal, K. L., Tartor, H., Grove, S., Alberdi, P., Brudeseth, B., & de la Fuente, J. (2020). Vaccination with Ectoparasite Proteins Involved in Midgut Function and Blood Digestion Reduces Salmon Louse Infestations. Vaccines, 8(1), 32. https://doi.org/10.3390/vaccines8010032






