HPV-Specific Systemic Antibody Responses and Memory B Cells are Independently Maintained up to 6 Years and in a Vaccine-Specific Manner Following Immunization with Cervarix and Gardasil in Adolescent and Young Adult Women in Vaccination Programs in Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Samples Purification
2.3. Plasmids
2.4. VLPs Preparation
2.5. Pseudovirion-Based Neutralization Assay (PBNA)
2.6. Determination of IgG Titers and Avidity
2.7. Polyclonal Stimulation of Memory B-Cells and B-Cell Elispot
2.8. Statistical Analysis
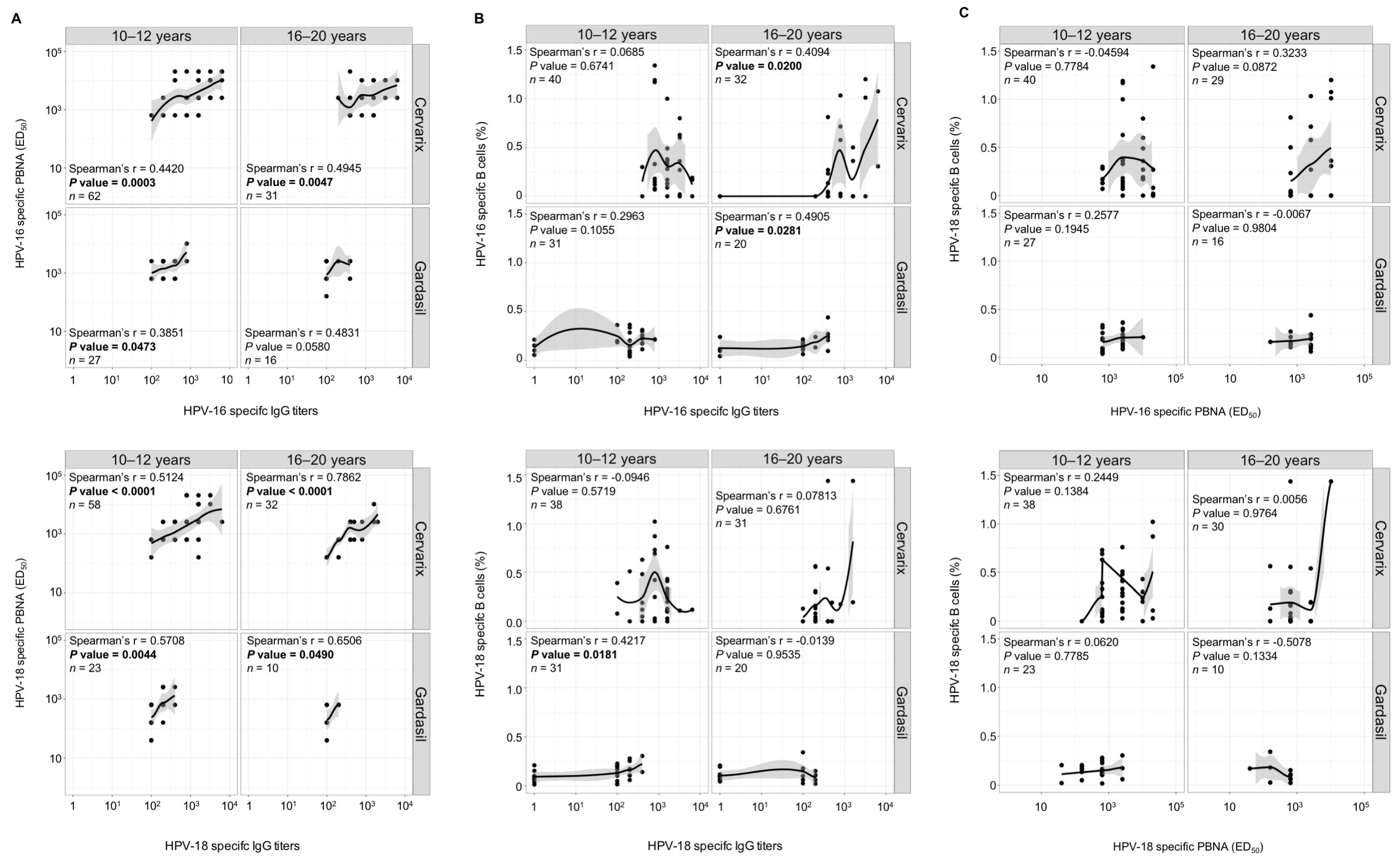
3. Results
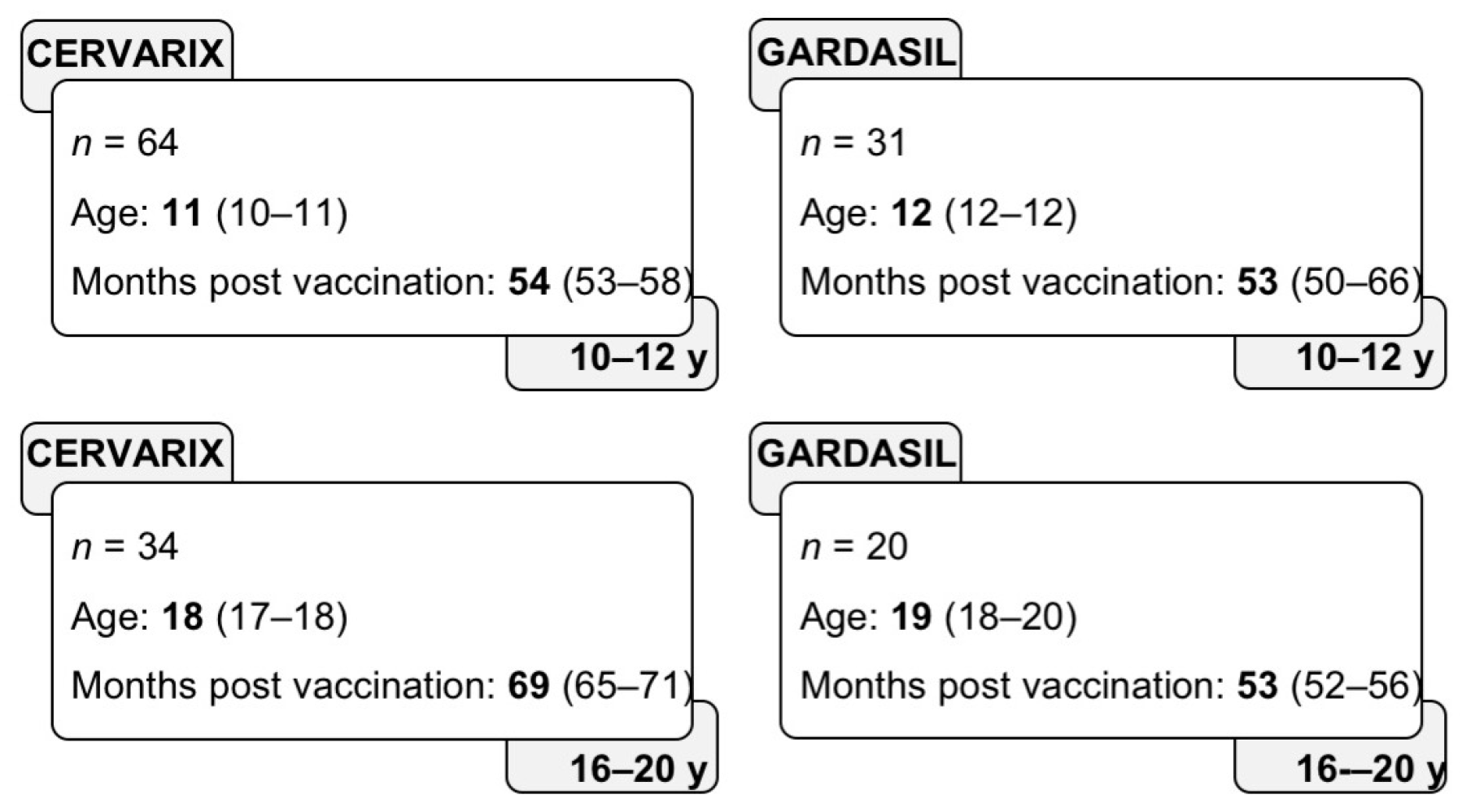
3.1. Study Subjects
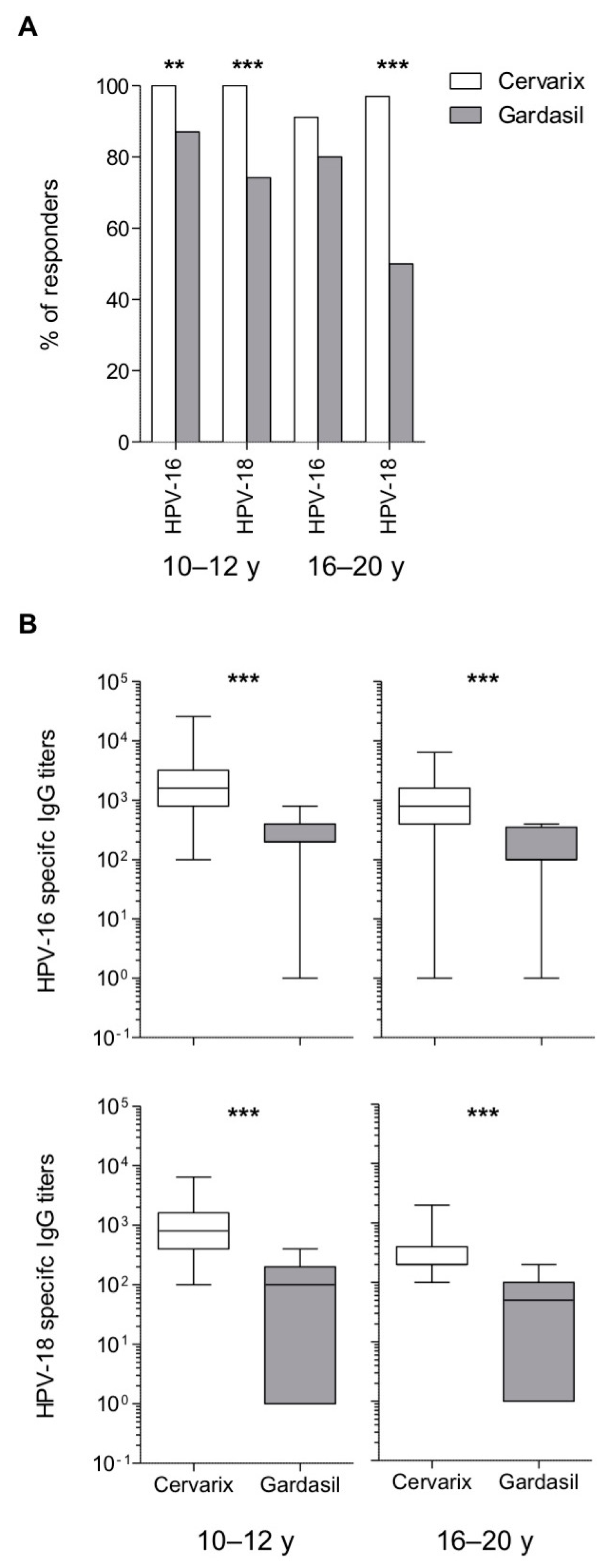
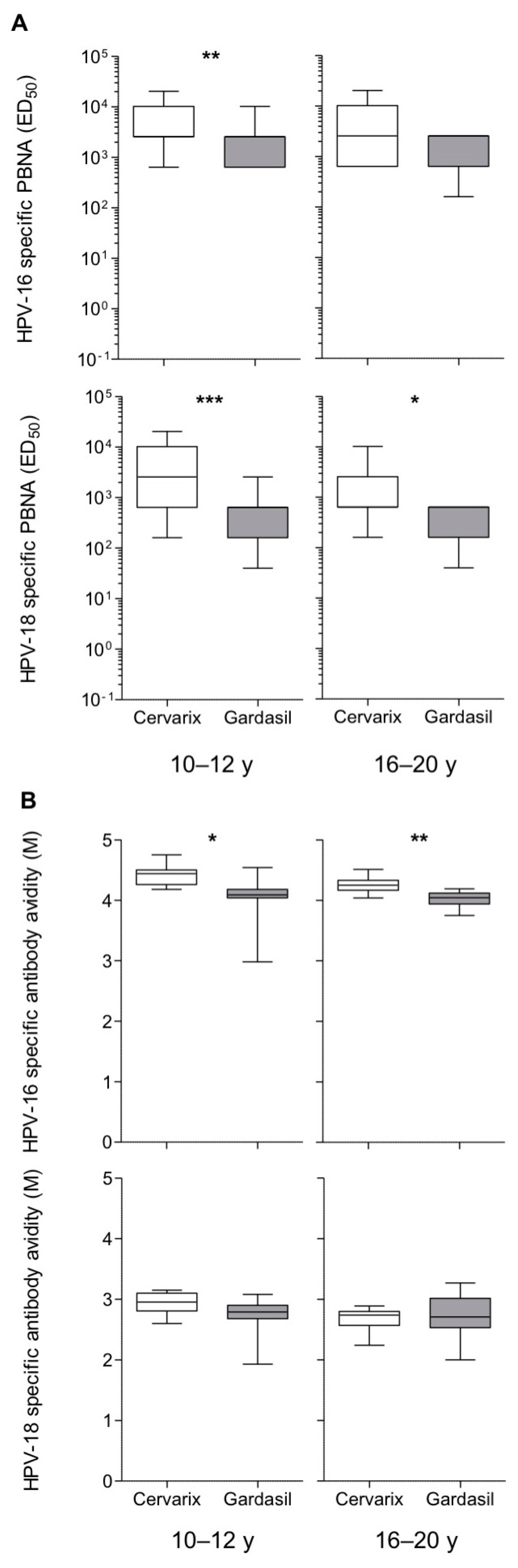
3.2. Long-Term Higher Antibody Levels in Cervarix-Vaccinated Women
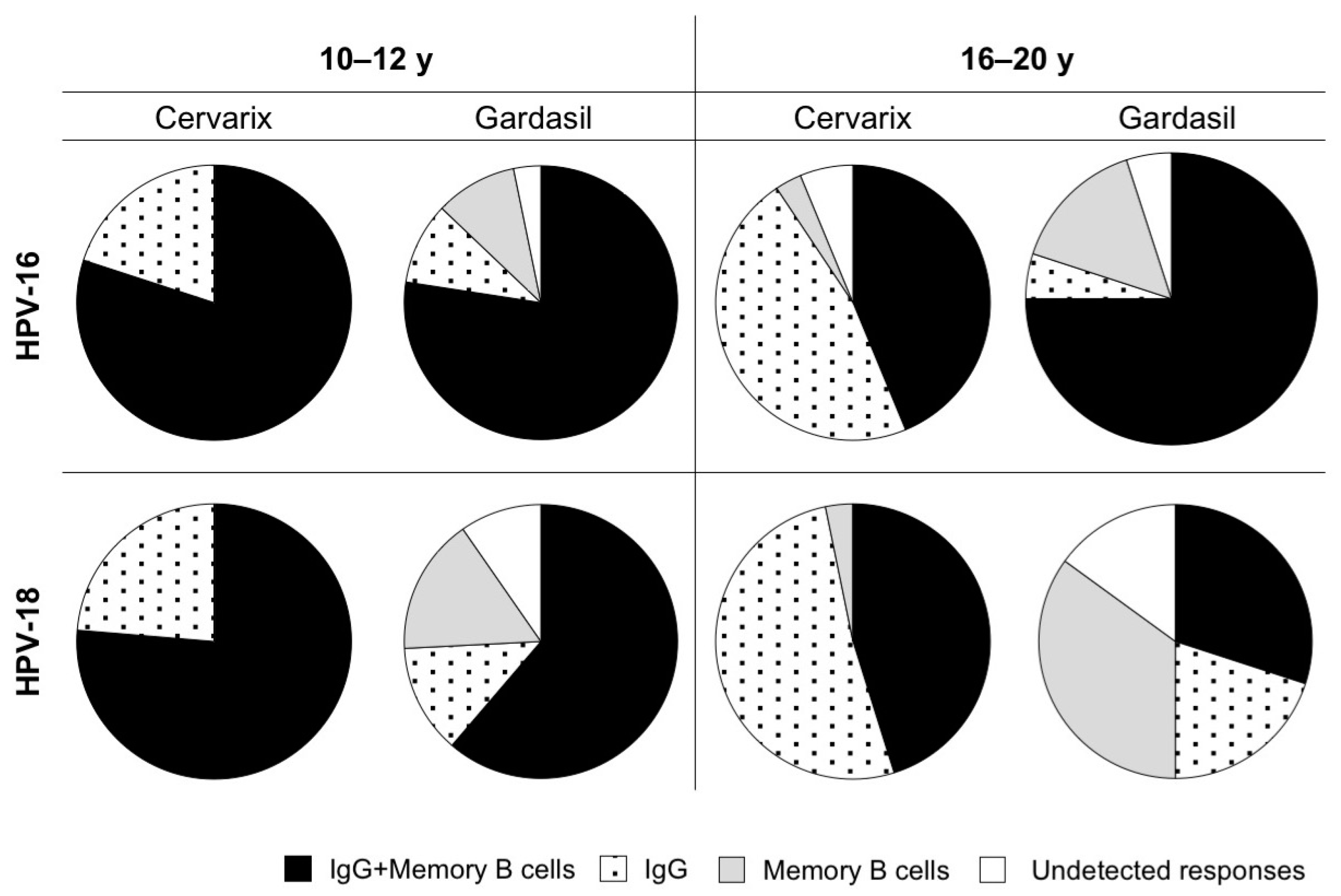
3.3. Maintenance of Long-Term Memory B Cells in Both Cervarix- and Gardasil-Vaccinated Women
3.4. Maintenance of HPV-6 and HPV-11-Specific Responses After Vaccination with Gardasil
3.5. Antibodies and Memory B Cells are Independently Maintained After Vaccination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhat, P.; Mattarollo, S.R.; Gosmann, C.; Frazer, I.H.; Leggatt, G.R. Regulation of immune responses to HPV infection and during HPV-directed immunotherapy. Immunol. Rev. 2011, 239, 85–98. [Google Scholar] [CrossRef]
- Dillner, J. The serological response to papillomaviruses. Semin. Cancer Biol. 1999, 9, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.Y.; Studentsov, Y.Y.; Bierman, R.; Burk, R.D. Natural history of human papillomavirus type 16 virus-like particle antibodies in young women. Cancer Epidemiol. Prev. Biomark. 2004, 13, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Aimagambetova, G.; Ukybassova, T.; Kongrtay, K.; Azizan, A. Human papillomavirus infection and cervical cancer: Epidemiology, screening, and vaccination-Review of current perspectives. J. Oncol. 2019, 2019, 3257939. [Google Scholar] [CrossRef]
- Nasman, A.; Du, J.; Dalianis, T. A global epidemic increase of an HPV induced tonsil and tongue-base cancer-potential benefit from a pan-gender use of HPV vaccine. J. Intern. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Human papillomavirus (HPV): Immunization, Vaccines and Biologicals. Available online: https://www.who.int/immunization/diseases/hpv/en/ (accessed on 11 December 2019).
- US Food and Drug Administration. Approval Letter: Human Papillomavirus Quadrivalent (Types 6, 11, 16, 18) Vaccine, Recombinant; US Food and Drug Administration: Washington, DC, USA, 2006. [Google Scholar]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S.; ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World; Summary report; HPV Information Centre: Barcelona, Spain, 17 June 2019. [Google Scholar]
- Schiller, J.T.; Castellsague, X.; Garland, S.M. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 2012, 30 (Suppl. 5), F123–F138. [Google Scholar] [CrossRef]
- Cutts, F.T.; Franceschi, S.; Goldie, S.; Castellsague, X.; de Sanjose, S.; Garnett, G.; Edmunds, W.J.; Claeys, P.; Goldenthal, K.L.; Harper, D.M.; et al. Human papillomavirus and HPV vaccines: A review. Bull. World Health Organ. 2007, 85, 719–726. [Google Scholar] [CrossRef]
- Draper, E.; Bissett, S.L.; Howell-Jones, R.; Edwards, D.; Munslow, G.; Soldan, K.; Beddows, S. Neutralization of non-vaccine human papillomavirus pseudoviruses from the A7 and A9 species groups by bivalent HPV vaccine sera. Vaccine 2011, 29, 8585–8590. [Google Scholar] [CrossRef]
- Wheeler, C.M.; Kjaer, S.K.; Sigurdsson, K.; Iversen, O.E.; Hernandez-Avila, M.; Perez, G.; Brown, D.R.; Koutsky, L.A.; Tay, E.H.; Garcia, P.; et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16–26 years. J. Infect. Dis. 2009, 199, 936–944. [Google Scholar] [CrossRef]
- Toh, Z.Q.; Kosasih, J.; Russell, F.M.; Garland, S.M.; Mulholland, E.K.; Licciardi, P.V. Recombinant human papillomavirus nonavalent vaccine in the prevention of cancers caused by human papillomavirus. Drug Infect. Resist. 2019, 12, 1951–1967. [Google Scholar] [CrossRef]
- Winer, R.L.; Lee, S.K.; Hughes, J.P.; Adam, D.E.; Kiviat, N.B.; Koutsky, L.A. Genital human papillomavirus infection: Incidence and risk factors in a cohort of female university students. Am. J. Epidemiol. 2003, 157, 218–226. [Google Scholar] [CrossRef]
- Moscicki, A.B.; Schiffman, M.; Kjaer, S.; Villa, L.L. Chapter 5: Updating the natural history of HPV and anogenital cancer. Vaccine 2006, 24 (Suppl. 3), S42–S51. [Google Scholar] [CrossRef]
- Stanley, M. HPV vaccination in boys and men. Hum. Vaccines Immunother. 2014, 10, 2109–2111. [Google Scholar] [CrossRef]
- Schmeler, K.M.; Sturgis, E.M. Expanding the benefits of HPV vaccination to boys and men. Lancet 2016, 387, 1798–1799. [Google Scholar] [CrossRef]
- Nardelli-Haefliger, D.; Wirthner, D.; Schiller, J.T.; Lowy, D.R.; Hildesheim, A.; Ponci, F.; De Grandi, P. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J. Natl. Cancer Inst. 2003, 95, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.N.; Buck, C.B.; Thompson, C.D.; Kines, R.; Bernardo, M.; Choyke, P.L.; Lowy, D.R.; Schiller, J.T. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 2007, 13, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, M.; Paavonen, J.; Wheeler, C.M.; Jaisamrarn, U.; Garland, S.M.; Castellsague, X.; Skinner, S.R.; Apter, D.; Naud, P.; Salmeron, J.; et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012, 13, 89–99. [Google Scholar] [CrossRef]
- Castellsague, X.; Munoz, N.; Pitisuttithum, P.; Ferris, D.; Monsonego, J.; Ault, K.; Luna, J.; Myers, E.; Mallary, S.; Bautista, O.M.; et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24-45 years of age. Br. J. Cancer 2011, 105, 28–37. [Google Scholar] [CrossRef]
- Kjaer, S.K.; Sigurdsson, K.; Iversen, O.E.; Hernandez-Avila, M.; Wheeler, C.M.; Perez, G.; Brown, D.R.; Koutsky, L.A.; Tay, E.H.; Garcia, P.; et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (Types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev. Res. 2009, 2, 868–878. [Google Scholar] [CrossRef]
- Munoz, N.; Kjaer, S.K.; Sigurdsson, K.; Iversen, O.E.; Hernandez-Avila, M.; Wheeler, C.M.; Perez, G.; Brown, D.R.; Koutsky, L.A.; Tay, E.H.; et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J. Natl. Cancer Inst. 2010, 102, 325–339. [Google Scholar] [CrossRef]
- Ferris, D.G.; Samakoses, R.; Block, S.L.; Lazcano-Ponce, E.; Restrepo, J.A.; Mehlsen, J.; Chatterjee, A.; Iversen, O.E.; Joshi, A.; Chu, J.L.; et al. 4-valent human papillomavirus (4vHPV) vaccine in preadolescents and adolescents after 10 years. Pediatrics 2017, 140, e20163947. [Google Scholar] [CrossRef] [PubMed]
- Nygard, M.; Saah, A.; Munk, C.; Tryggvadottir, L.; Enerly, E.; Hortlund, M.; Sigurdardottir, L.G.; Vuocolo, S.; Kjaer, S.K.; Dillner, J. Evaluation of the long-term anti-human papillomavirus 6 (HPV6), 11, 16, and 18 immune responses generated by the quadrivalent HPV vaccine. Clin. Vaccine Immunol. 2015, 22, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Artemchuk, H.; Eriksson, T.; Poljak, M.; Surcel, H.M.; Dillner, J.; Lehtinen, M.; Faust, H. Long-term antibody response to human papillomavirus vaccines: Up to 12 years of follow-up in the finnish maternity cohort. J. Infect. Dis. 2019, 219, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Guevara, A.; Cabello, R.; Woelber, L.; Moreira, E.D., Jr.; Joura, E.; Reich, O.; Shields, C.; Ellison, M.C.; Joshi, A.; Luxembourg, A. Antibody persistence and evidence of immune memory at 5years following administration of the 9-valent HPV vaccine. Vaccine 2017, 35, 5050–5057. [Google Scholar] [CrossRef] [PubMed]
- Lightman, S.M.; Utley, A.; Lee, K.P. Survival of long-lived plasma cells (LLPC): Piecing together the puzzle. Front. Immunol. 2019, 10, 965. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, F.; Gallerani, E.; Sforza, F.; Finessi, V.; Chachage, M.; Geldmacher, C.; Cafaro, A.; Ensoli, B.; Caputo, A.; Gavioli, R. The HIV-1 Tat protein affects human CD4+ T-cell programing and activation, and favors the differentiation of naive CD4+ T cells. AIDS 2018, 32, 575–581. [Google Scholar] [CrossRef]
- Telatin, V.; Nicoli, F.; Frasson, C.; Menegotto, N.; Barbaro, F.; Castelli, E.; Erne, E.; Palu, G.; Caputo, A. In chronic hepatitis C infection, myeloid-derived suppressor cell accumulation and T cell dysfunctions revert partially and late after successful direct-acting antiviral treatment. Front. Cell. Infect. Microbiol. 2019, 9, 190. [Google Scholar] [CrossRef]
- Buck, C.B.; Pastrana, D.V.; Lowy, D.R.; Schiller, J.T. Efficient intracellular assembly of papillomaviral vectors. J. Virol. 2004, 78, 751–757. [Google Scholar] [CrossRef]
- Barzon, L.; Squarzon, L.; Masiero, S.; Pacenti, M.; Marcati, G.; Mantelli, B.; Gabrielli, L.; Pascucci, M.G.; Lazzarotto, T.; Caputo, A.; et al. Neutralizing and cross-neutralizing antibody titres induced by bivalent and quadrivalent human papillomavirus vaccines in the target population of organized vaccination programmes. Vaccine 2014, 32, 5357–5362. [Google Scholar] [CrossRef]
- Nicoli, F.; Chachage, M.; Clowes, P.; Bauer, A.; Kowour, D.; Ensoli, B.; Cafaro, A.; Maboko, L.; Hoelscher, M.; Gavioli, R.; et al. Association between different anti-Tat antibody isotypes and HIV disease progression: Data from an African cohort. BMC Infect. Dis. 2016, 16, 344. [Google Scholar] [CrossRef]
- Nicoli, F.; Gallerani, E.; Skarlis, C.; Sicurella, M.; Cafaro, A.; Ensoli, B.; Caputo, A.; Marconi, P.C.; Gavioli, R. Systemic immunodominant CD8 responses with an effector-like phenotype are induced by intravaginal immunization with attenuated HSV vectors expressing HIV Tat and mediate protection against HSV infection. Vaccine 2016, 34, 2216–2224. [Google Scholar] [CrossRef] [PubMed]
- Sicurella, M.; Nicoli, F.; Gallerani, E.; Volpi, I.; Berto, E.; Finessi, V.; Destro, F.; Manservigi, R.; Cafaro, A.; Ensoli, B.; et al. An attenuated herpes simplex virus type 1 (HSV1) encoding the HIV-1 Tat protein protects mice from a deadly mucosal HSV1 challenge. PLoS ONE 2014, 9, e100844. [Google Scholar] [CrossRef] [PubMed]
- Dauner, J.G.; Pan, Y.; Hildesheim, A.; Kemp, T.J.; Porras, C.; Pinto, L.A. Development and application of a GuHCl-modified ELISA to measure the avidity of anti-HPV L1 VLP antibodies in vaccinated individuals. Mol. Cell. Probes 2012, 26, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Polanec, J.; Seppala, I.; Rousseau, S.; Hedman, K. Evaluation of protein-denaturing immunoassays for avidity of immunoglobulin G to rubella virus. J. Clin. Lab. Anal. 1994, 8, 16–21. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, Inc.: Boston, MA, USA, 2016. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Wickham, H. Scales: Scale Functions for Visualization. 2018. Available online: https://CRAN.R-project.org/package=scales (accessed on 11 December 2019).
- Amanna, I.J.; Slifka, M.K.; Crotty, S. Immunity and immunological memory following smallpox vaccination. Immunol. Rev. 2006, 211, 320–337. [Google Scholar] [CrossRef] [PubMed]
- Amanna, I.J.; Carlson, N.E.; Slifka, M.K. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 2007, 357, 1903–1915. [Google Scholar] [CrossRef] [PubMed]
- Einstein, M.H.; Baron, M.; Levin, M.J.; Chatterjee, A.; Edwards, R.P.; Zepp, F.; Carletti, I.; Dessy, F.J.; Trofa, A.F.; Schuind, A.; et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum. Vaccines 2009, 5, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.M.; DeMars, L.R. HPV vaccines - A review of the first decade. Gynecol. Oncol. 2017, 146, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Godi, A.; Panwar, K.; Haque, M.; Cocuzza, C.E.; Andrews, N.; Southern, J.; Turner, P.; Miller, E.; Beddows, S. Durability of the neutralizing antibody response to vaccine and non-vaccine HPV types 7 years following immunization with either Cervarix(R) or Gardasil(R) vaccine. Vaccine 2019, 37, 2455–2462. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Garcon, N.; Chomez, P.; Van Mechelen, M. GlaxoSmithKline Adjuvant Systems in vaccines: Concepts, achievements and perspectives. Expert Rev. Vaccines 2007, 6, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Morel, S.; Lockman, L.; Giannini, S.L.; Bisteau, M.; Carlsen, H.; Kielland, A.; Vosters, O.; Vanderheyde, N.; Schiavetti, F.; et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol. 2009, 183, 6186–6197. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J.; Harper, D.M. Human papillomavirus types 16 and 18 vaccine (recombinant, AS04 adjuvanted, adsorbed) [Cervarix]. Drugs 2008, 68, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.; Perry, C.M. Human papillomavirus quadrivalent (types 6, 11, 16, 18) recombinant vaccine (Gardasil). Drugs 2006, 66, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Petaja, T.; Keranen, H.; Karppa, T.; Kawa, A.; Lantela, S.; Siitari-Mattila, M.; Levanen, H.; Tocklin, T.; Godeaux, O.; Lehtinen, M.; et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10-18 years. J. Adolesc. Health 2009, 44, 33–40. [Google Scholar] [CrossRef]
- Rasheed, M.A.U.; Hickman, C.J.; McGrew, M.; Sowers, S.B.; Mercader, S.; Hopkins, A.; Grimes, V.; Yu, T.; Wrammert, J.; Mulligan, M.J.; et al. Decreased humoral immunity to mumps in young adults immunized with MMR vaccine in childhood. Proc. Natl. Acad. Sci. USA 2019, 116, 19071–19076. [Google Scholar] [CrossRef]
- WHO. Human papillomavirus vaccines. WHO Position Paper. Wkly. Epidemiol. Rec. 2009, 84, 118–131. [Google Scholar]
- Bernasconi, N.L.; Traggiai, E.; Lanzavecchia, A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 2002, 298, 2199–2202. [Google Scholar] [CrossRef]
- Crotty, S.; Aubert, R.D.; Glidewell, J.; Ahmed, R. Tracking human antigen-specific memory B cells: A sensitive and generalized ELISPOT system. J. Immunol. Methods 2004, 286, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Einstein, M.H.; Baron, M.; Levin, M.J.; Chatterjee, A.; Fox, B.; Scholar, S.; Rosen, J.; Chakhtoura, N.; Meric, D.; Dessy, F.J.; et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: Follow-up from months 12-24 in a Phase III randomized study of healthy women aged 18-45 years. Hum. Vaccines 2011, 7, 1343–1358. [Google Scholar] [CrossRef] [PubMed]
- Einstein, M.H.; Levin, M.J.; Chatterjee, A.; Chakhtoura, N.; Takacs, P.; Catteau, G.; Dessy, F.J.; Moris, P.; Lin, L.; Struyf, F.; et al. Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: Follow-up through Month 48 in a Phase III randomized study. Hum. Vaccines Immunother. 2014, 10, 3455–3465. [Google Scholar] [CrossRef] [PubMed]
- Smolen, K.K.; Gelinas, L.; Franzen, L.; Dobson, S.; Dawar, M.; Ogilvie, G.; Krajden, M.; Fortuno, E.S., III; Kollmann, T.R. Age of recipient and number of doses differentially impact human B and T cell immune memory responses to HPV vaccination. Vaccine 2012, 30, 3572–3579. [Google Scholar] [CrossRef]
- Pasmans, H.; Schurink-Van’t Klooster, T.M.; Bogaard, M.J.M.; van Rooijen, D.M.; de Melker, H.E.; Welters, M.J.P.; van der Burg, S.H.; van der Klis, F.R.M.; Buisman, A.M. Long-term HPV-specific immune response after one versus two and three doses of bivalent HPV vaccination in Dutch girls. Vaccine 2019, 37, 7280–7288. [Google Scholar] [CrossRef]
- Einstein, M.H.; Baron, M.; Levin, M.J.; Chatterjee, A.; Fox, B.; Scholar, S.; Rosen, J.; Chakhtoura, N.; Lebacq, M.; van der Most, R.; et al. Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18-45 years. Hum. Vaccines 2011, 7, 1359–1373. [Google Scholar] [CrossRef]
- Brynjolfsson, S.F.; Persson Berg, L.; Olsen Ekerhult, T.; Rimkute, I.; Wick, M.J.; Martensson, I.L.; Grimsholm, O. Long-lived plasma cells in mice and men. Front. Immunol. 2018, 9, 2673. [Google Scholar] [CrossRef]
- Hammarlund, E.; Thomas, A.; Amanna, I.J.; Holden, L.A.; Slayden, O.D.; Park, B.; Gao, L.; Slifka, M.K. Plasma cell survival in the absence of B cell memory. Nat. Commun. 2017, 8, 1781. [Google Scholar] [CrossRef]
- Landsverk, O.J.; Snir, O.; Casado, R.B.; Richter, L.; Mold, J.E.; Reu, P.; Horneland, R.; Paulsen, V.; Yaqub, S.; Aandahl, E.M.; et al. Antibody-secreting plasma cells persist for decades in human intestine. J. Exp. Med. 2017, 214, 309–317. [Google Scholar] [CrossRef]
- Herrera, D.; Rojas, O.L.; Duarte-Rey, C.; Mantilla, R.D.; Angel, J.; Franco, M.A. Simultaneous assessment of rotavirus-specific memory B cells and serological memory after B cell depletion therapy with rituximab. PLoS ONE 2014, 9, e97087. [Google Scholar] [CrossRef]
- Manz, R.A.; Lohning, M.; Cassese, G.; Thiel, A.; Radbruch, A. Survival of long-lived plasma cells is independent of antigen. Int. Immunol. 1998, 10, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Leyendeckers, H.; Odendahl, M.; Lohndorf, A.; Irsch, J.; Spangfort, M.; Miltenyi, S.; Hunzelmann, N.; Assenmacher, M.; Radbruch, A.; Schmitz, J. Correlation analysis between frequencies of circulating antigen-specific IgG-bearing memory B cells and serum titers of antigen-specific IgG. Eur. J. Immunol. 1999, 29, 1406–1417. [Google Scholar] [CrossRef]
- Crotty, S.; Felgner, P.; Davies, H.; Glidewell, J.; Villarreal, L.; Ahmed, R. Cutting edge: Long-term B cell memory in humans after smallpox vaccination. J. Immunol. 2003, 171, 4969–4973. [Google Scholar] [CrossRef] [PubMed]
- Godi, A.; Bissett, S.L.; Miller, E.; Beddows, S. Relationship between humoral immune responses against HPV16, HPV18, HPV31 and HPV45 in 12-15 year old girls receiving Cervarix® or Gardasil® vaccine. PLoS ONE 2015, 10, e0140926. [Google Scholar] [CrossRef]
- Valats, J.C.; Tuaillon, E.; Funakoshi, N.; Hoa, D.; Brabet, M.C.; Bollore, K.; Ducos, J.; Vendrell, J.P.; Blanc, P. Investigation of memory B cell responses to hepatitis B surface antigen in health care workers considered as non-responders to vaccination. Vaccine 2010, 28, 6411–6416. [Google Scholar] [CrossRef] [PubMed]
- Amanna, I.J.; Slifka, M.K. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol. Rev. 2010, 236, 125–138. [Google Scholar] [CrossRef]
- Andraud, M.; Lejeune, O.; Musoro, J.Z.; Ogunjimi, B.; Beutels, P.; Hens, N. Living on three time scales: The dynamics of plasma cell and antibody populations illustrated for hepatitis a virus. PLoS Comput. Biol. 2012, 8, e1002418. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Joyner, C.J.; Sanz, I.; Lee, F.E. Factors affecting early antibody secreting cell maturation into long-lived plasma cells. Front. Immunol. 2019, 10, 2138. [Google Scholar] [CrossRef]
- Giannini, S.L.; Hanon, E.; Moris, P.; Van Mechelen, M.; Morel, S.; Dessy, F.; Fourneau, M.A.; Colau, B.; Suzich, J.; Losonksy, G.; et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 2006, 24, 5937–5949. [Google Scholar] [CrossRef]
- Casella, C.R.; Mitchell, T.C. Putting endotoxin to work for us: Monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell. Mol. Life Sci. 2008, 65, 3231–3240. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, C.; Li, R.; Wang, Z.; Yuan, Y.; Li, H.; Fu, Z.; Zhou, M.; Zhao, L. Monophosphoryl-lipid A (MPLA) is an efficacious adjuvant for inactivated rabies vaccines. Viruses 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Adelsberger, J.W.; Kemp, T.J.; Baseler, M.W.; Ledgerwood, J.E.; Pinto, L.A. Circulating CXCR5(+)CD4(+) T follicular-like helper cell and memory B cell responses to human papillomavirus vaccines. PLoS ONE 2015, 10, e0137195. [Google Scholar] [CrossRef] [PubMed]
- Shinnakasu, R.; Kurosaki, T. Regulation of memory B and plasma cell differentiation. Curr. Opin. Immunol. 2017, 45, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Hipp, N.; Symington, H.; Pastoret, C.; Caron, G.; Monvoisin, C.; Tarte, K.; Fest, T.; Delaloy, C. IL-2 imprints human naive B cell fate towards plasma cell through ERK/ELK1-mediated BACH2 repression. Nat. Commun. 2017, 8, 1443. [Google Scholar] [CrossRef] [PubMed]

| Antigen | Time after Vaccination | N = | Spearman’s r; p Value | Slope (95% C.I.) | R2 |
|---|---|---|---|---|---|
| HPV-16 | <6 months | 53 | 0.4092; 0.0023 | 4892 (496; 9287) | 0.08932 |
| >2 years | 40 | 0.06859; 0.6741 | 75 (−106; 257) | 0.01839 | |
| HPV-18 | <6 months | 53 | 0.5310; <0.0001 | 3829 (1684; 5975) | 0.2014 |
| >2 years | 38 | −0.09464; 0.5719 | −97 (−407; 213) | 0.01109 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicoli, F.; Mantelli, B.; Gallerani, E.; Telatin, V.; Bonazzi, I.; Marconi, P.; Gavioli, R.; Gabrielli, L.; Lazzarotto, T.; Barzon, L.; et al. HPV-Specific Systemic Antibody Responses and Memory B Cells are Independently Maintained up to 6 Years and in a Vaccine-Specific Manner Following Immunization with Cervarix and Gardasil in Adolescent and Young Adult Women in Vaccination Programs in Italy. Vaccines 2020, 8, 26. https://doi.org/10.3390/vaccines8010026
Nicoli F, Mantelli B, Gallerani E, Telatin V, Bonazzi I, Marconi P, Gavioli R, Gabrielli L, Lazzarotto T, Barzon L, et al. HPV-Specific Systemic Antibody Responses and Memory B Cells are Independently Maintained up to 6 Years and in a Vaccine-Specific Manner Following Immunization with Cervarix and Gardasil in Adolescent and Young Adult Women in Vaccination Programs in Italy. Vaccines. 2020; 8(1):26. https://doi.org/10.3390/vaccines8010026
Chicago/Turabian StyleNicoli, Francesco, Barbara Mantelli, Eleonora Gallerani, Valentina Telatin, Irene Bonazzi, Peggy Marconi, Riccardo Gavioli, Liliana Gabrielli, Tiziana Lazzarotto, Luisa Barzon, and et al. 2020. "HPV-Specific Systemic Antibody Responses and Memory B Cells are Independently Maintained up to 6 Years and in a Vaccine-Specific Manner Following Immunization with Cervarix and Gardasil in Adolescent and Young Adult Women in Vaccination Programs in Italy" Vaccines 8, no. 1: 26. https://doi.org/10.3390/vaccines8010026
APA StyleNicoli, F., Mantelli, B., Gallerani, E., Telatin, V., Bonazzi, I., Marconi, P., Gavioli, R., Gabrielli, L., Lazzarotto, T., Barzon, L., Palù, G., & Caputo, A. (2020). HPV-Specific Systemic Antibody Responses and Memory B Cells are Independently Maintained up to 6 Years and in a Vaccine-Specific Manner Following Immunization with Cervarix and Gardasil in Adolescent and Young Adult Women in Vaccination Programs in Italy. Vaccines, 8(1), 26. https://doi.org/10.3390/vaccines8010026








