T-Cell Response to Viral Hemorrhagic Fevers
Abstract
1. Introduction
2. Epidemiology
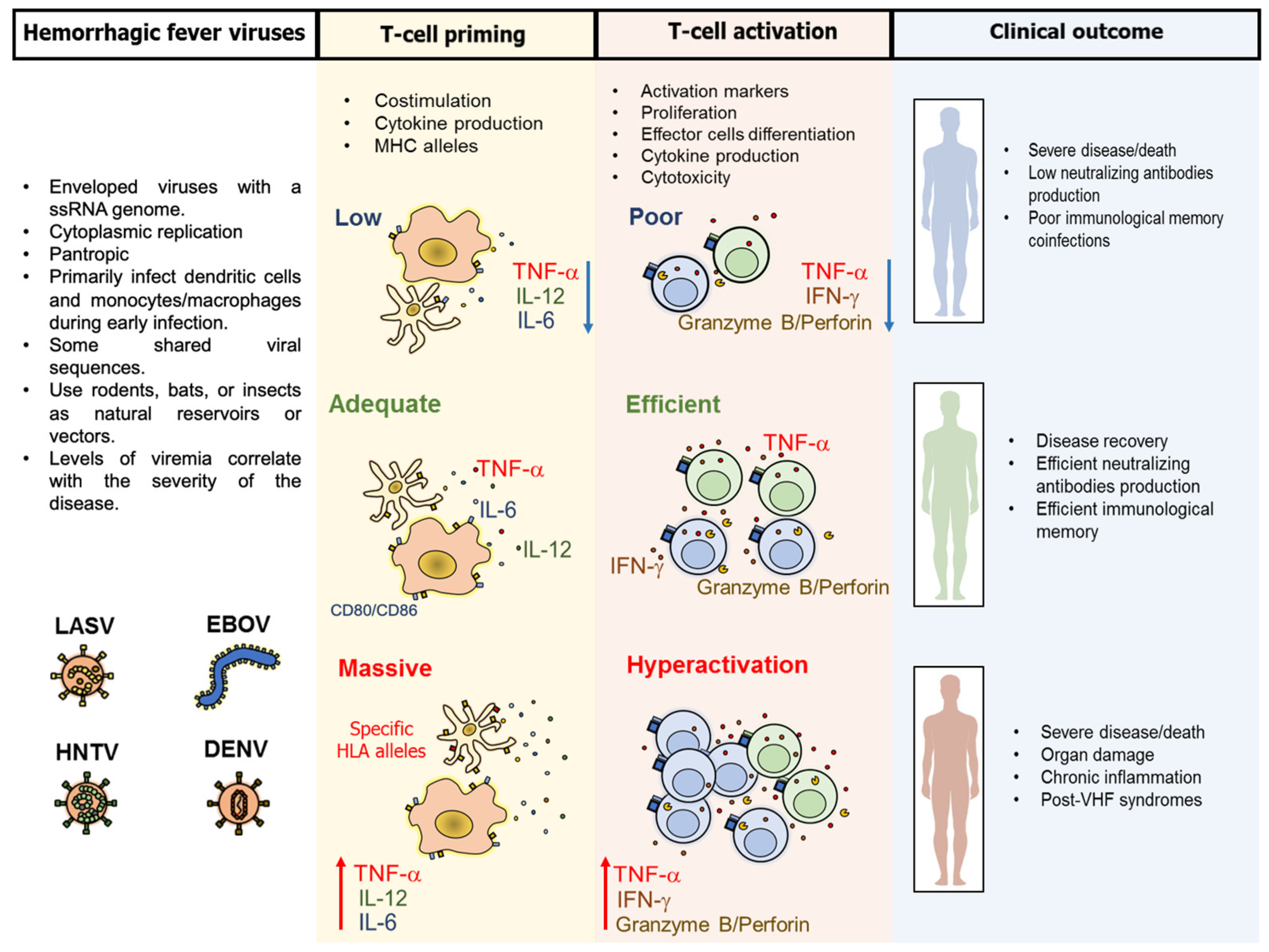
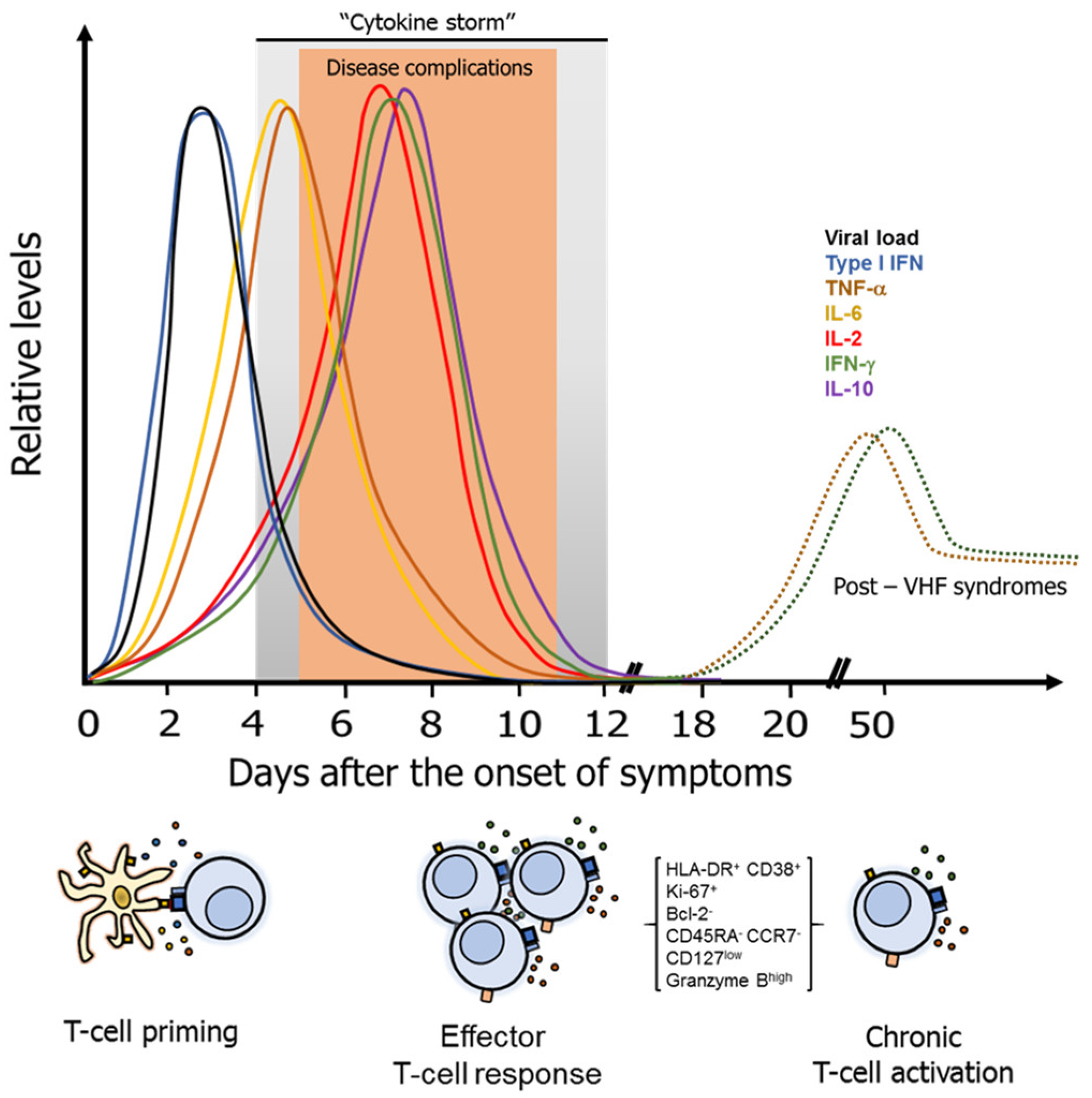
3. Pathogenesis and Common Characteristics of the T-Cell Response Against VHF
Regulatory and Other T-Cell Subsets in VHF
4. Particular Characteristics of the T-Cell Response Against VHF
4.1. T-Cell Response Against LASV
4.2. T-Cell Response Against EBOV Infection
4.3. T-Cell Response Against Hantaviruses
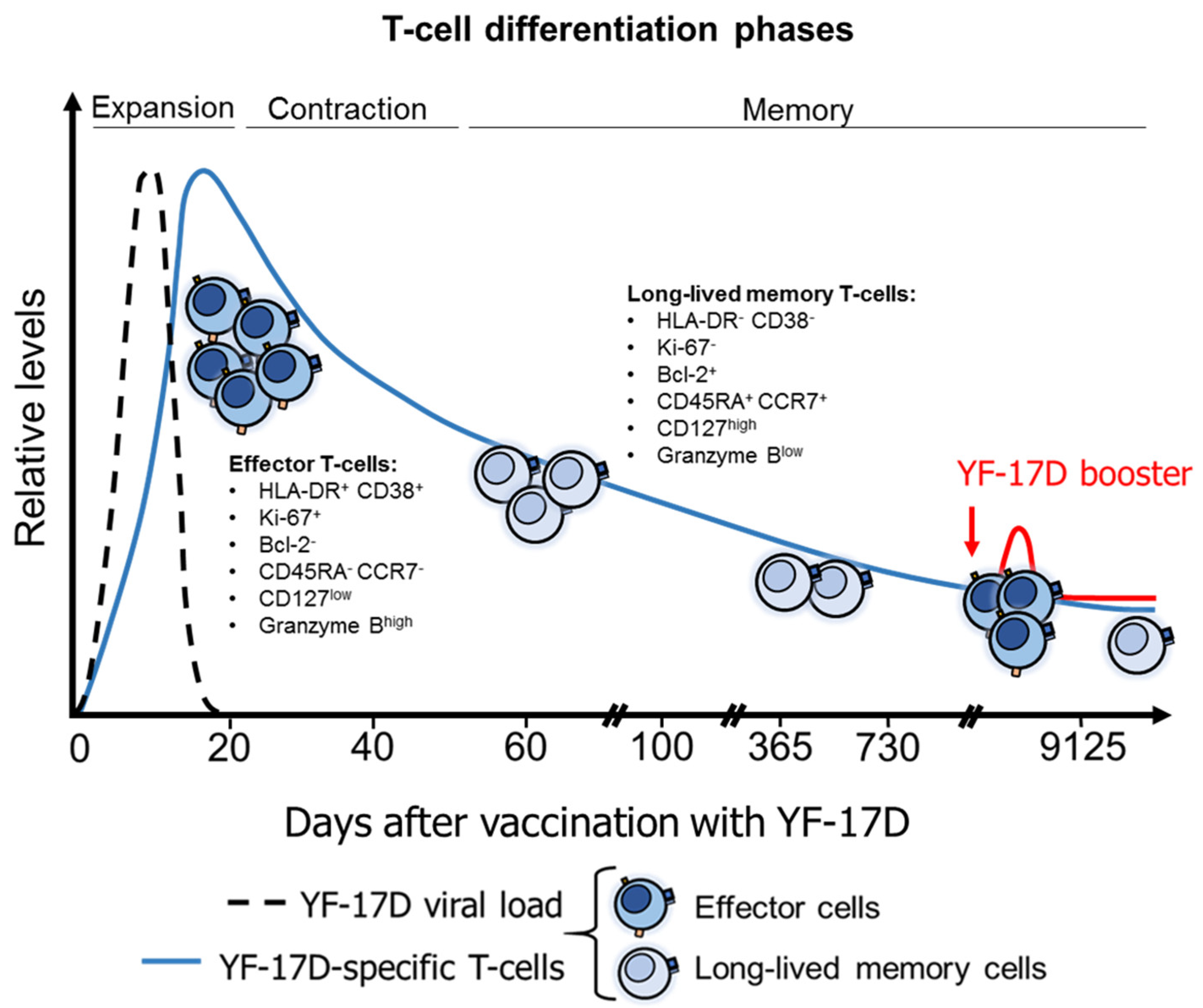
4.4. T-Cell Response after YFV Vaccination
4.5. DENV and Other Flaviviruses Elicit Cross-Reactive T-Cell Responses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Basler, C.F. Molecular pathogenesis of viral hemorrhagic fever. Semin. Immunopathol. 2017. [Google Scholar] [CrossRef]
- Salvato, M.S. Hemorrhagic Fever Viruses, 1st ed.; Springer Nature: New York, NY, USA, 2018; ISBN 9781493969814. [Google Scholar]
- Maes, P.; Alkhovsky, S.V.; Bào, Y.; Beer, M.; Birkhead, M.; Briese, T.; Buchmeier, M.J.; Calisher, C.H.; Charrel, R.N.; Choi, I.R.; et al. Taxonomy of the family Arenaviridae and the order Bunyavirales: Update 2018. Arch. Virol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Marty, A.M.; Jahrling, P.B.; Geisbert, T.W. Viral hemorrhagic fevers. Clin. Lab. Med. 2006. [Google Scholar] [CrossRef]
- Racsa, L.D.; Kraft, C.S.; Olinger, G.G.; Hensley, L.E. Viral Hemorrhagic Fever Diagnostics. Clin. Infect. Dis. 2016. [Google Scholar] [CrossRef]
- Ippolito, G.; Feldmann, H.; Lanini, S.; Vairo, F.; Di Caro, A.; Capobianchi, M.R.; Nicastri, E. Viral hemorrhagic fevers: Advancing the level of treatment. BMC Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Zapata, J.C.; Cox, D.; Salvato, M.S. The Role of Platelets in the Pathogenesis of Viral Hemorrhagic Fevers. PLoS Negl. Trop. Dis. 2014. [Google Scholar] [CrossRef] [PubMed]
- Hallam, H.J.; Hallam, S.; Rodriguez, S.E.; Barrett, A.D.T.; Beasley, D.W.C.; Chua, A.; Ksiazek, T.G.; Milligan, G.N.; Sathiyamoorthy, V.; Reece, L.M. Baseline mapping of Lassa fever virology, epidemiology and vaccine research and development review-article. Vaccines 2018. [Google Scholar] [CrossRef]
- Epidemic focus: Lassa Fever. Relev. Epidemiol. Hebd. 2016, 91, 265–266.
- Shaffer, J.G.; Grant, D.S.; Schieffelin, J.S.; Boisen, M.L.; Goba, A.; Hartnett, J.N.; Levy, D.C.; Yenni, R.E.; Moses, L.M.; Fullah, M.; et al. Lassa Fever in Post-Conflict Sierra Leone. PLoS Negl. Trop. Dis. 2014. [Google Scholar] [CrossRef]
- Ngatu, N.R.; Kayembe, N.J.-M.; Phillips, E.K.; Okech-Ojony, J.; Patou-Musumari, M.; Gaspard-Kibukusa, M.; Madone-Mandina, N.; Godefroid-Mayala, M.; Mutaawe, L.; Manzengo, C.; et al. Epidemiology of ebolavirus disease (EVD) and occupational EVD in health care workers in Sub-Saharan Africa: Need for strengthened public health preparedness. J. Epidemiol. 2017. [Google Scholar] [CrossRef]
- World Health Organization. Ebola virus disease–Democratic Republic of the Congo. Available online: https://www.who.int/csr/don/29-november-2018-ebola-drc/en/ (accessed on 7 January 2019).
- Ebola situation reports: Democratic Republic of the Congo. Available online: https://www.who.int/ebola/situation-reports/drc-2018/en/ (accessed on 7 January 2019).
- Annual, U.S. Hantavirus Disease and HPS Case Fatality, 1993-2016. Available online: https://www.cdc.gov/hantavirus/surveillance/annual-cases.html (accessed on 13 December 2018).
- Jonsson, C.B.; Figueiredo, L.T.M.; Vapalahti, O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin. Microbiol. Rev. 2010. [Google Scholar] [CrossRef] [PubMed]
- Barnett, E.D. Yellow Fever: Epidemiology and Prevention. Clin. Infect. Dis. 2007. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P. Review of the risks and benefits of yellow fever vaccination including some new analyses. Expert Rev. Vaccines 2012. [Google Scholar] [CrossRef] [PubMed]
- Garske, T.; Van Kerkhove, M.D.; Yactayo, S.; Ronveaux, O.; Lewis, R.F.; Staples, J.E.; Perea, W.; Ferguson, N.M.; Burke, D.; De La Hoz, F.; et al. Yellow Fever in Africa: Estimating the Burden of Disease and Impact of Mass Vaccination from Outbreak and Serological Data. PLoS Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.P.; Chippaux, A. Yellow fever in Africa and the Americas: A historical and epidemiological perspective. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- WHO. Weekly Epidemiological Record, 10 August 2018, vol. 93, 32 (pp. 409–416). Available online: https://www.who.int/wer/2018/wer9332/en/ (accessed on 7 January 2019).
- Yellow Fever–Brazil, Disease Outbreak News. 27 February 2018. Available online: http://www.who.int/csr/don/27-february-2018-yellow-fever-brazil/en/ (accessed on 13 December 2018).
- Hamer, D.H.; Angelo, K.; Caumes, E.; van Genderen, P.J.J.; Florescu, S.A.; Popescu, C.P.; Perret, C.; McBride, A.; Checkley, A.; Ryan, J.; et al. Fatal Yellow Fever in Travelers to Brazil, 2018. MMWR. Morb. Mortal. Wkly. Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the Global Spatial Limits of Dengue Virus Transmission by Evidence-Based Consensus. PLoS Negl. Trop. Dis. 2012. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, Z.; Wen, Z.; Liu, Y.; Zeng, C.; Xiao, D.; Ou, M.; Han, Y.; Huang, S.; Liu, D.; et al. Global Epidemiology of Dengue Outbreaks in 1990–2015: A Systematic Review and Meta-Analysis. Front. Cell. Infect. Microbiol. 2017. [Google Scholar] [CrossRef]
- WHO. Dengue: Guidelines for Diagnosis, Treatment, Prevention, and Control. Available online: https://www.who.int/rpc/guidelines/9789241547871/en/ (accessed on 7 January 2019).
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013. [Google Scholar] [CrossRef]
- Murray, N.E.A.; Quam, M.B.; Wilder-Smith, A. Epidemiology of dengue: Past, present and future prospects. Clin. Epidemiol. 2013. [Google Scholar] [CrossRef]
- Dahlke, C.; Lunemann, S.; Kasonta, R.; Kreuels, B.; Schmiedel, S.; Ly, M.L.; Fehling, S.K.; Strecker, T.; Becker, S.; Altfeld, M.; et al. Comprehensive characterization of cellular immune responses following ebola virus infection. J. Infect. Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; van der Most, R.G.; Akondy, R.S.; Glidewell, J.T.; Albott, S.; Masopust, D.; Murali-Krishna, K.; Mahar, P.L.; Edupuganti, S.; Lalor, S.; et al. Human Effector and Memory CD8+T Cell Responses to Smallpox and Yellow Fever Vaccines. Immunity 2008. [Google Scholar] [CrossRef] [PubMed]
- Akondy, R.S.; Monson, N.D.; Miller, J.D.; Edupuganti, S.; Teuwen, D.; Wu, H.; Quyyumi, F.; Garg, S.; Altman, J.D.; Del Rio, C.; et al. The Yellow Fever Virus Vaccine Induces a Broad and Polyfunctional Human Memory CD8+ T Cell Response. J. Immunol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Farooq, F.; Beck, K.; Paolino, K.M.; Phillips, R.; Waters, N.C.; Regules, J.A.; Bergmann-Leitner, E.S. Circulating follicular T helper cells and cytokine profile in humans following vaccination with the rVSV-ZEBOV Ebola vaccine. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J.; Ahmed, R. Memory CD8 T-Cell Differentiation during Viral Infection. J. Virol. 2004. [Google Scholar] [CrossRef] [PubMed]
- McElroy, A.K.; Akondy, R.S.; Harmon, J.R.; Ellebedy, A.H.; Cannon, D.; Klena, J.D.; Sidney, J.; Sette, A.; Mehta, A.K.; Kraft, C.S.; et al. A Case of Human Lassa Virus Infection with Robust Acute T-Cell Activation and Long-Term Virus-Specific T-Cell Responses. J. Infect. Dis. 2017. [Google Scholar] [CrossRef]
- Scott, J.T.; Sesay, F.R.; Massaquoi, T.A.; Idriss, B.R.; Sahr, F.; Semple, M.G. Post-ebola syndrome, Sierra Leone. Emerg. Infect. Dis. 2016. [Google Scholar] [CrossRef]
- Kilpatrick, E.D.; Terajima, M.; Koster, F.T.; Catalina, M.D.; Cruz, J.; Ennis, F.A. Role of Specific CD8+ T Cells in the Severity of a Fulminant Zoonotic Viral Hemorrhagic Fever, Hantavirus Pulmonary Syndrome. J. Immunol. 2004. [Google Scholar] [CrossRef]
- Mathew, A.; Rothman, A.L. Understanding the contribution of cellular immunity to dengue disease pathogenesis. Immunol. Rev. 2008. [Google Scholar] [CrossRef]
- Zinkernagel, R.M.; Haenseler, E.; Leist, T.; Cerny, A.; Hengartner, H.; Althage, A. T cell-mediated hepatitis in mice infected with lymphocytic choriomeningitis virus. Liver cell destruction by H-2 class I-restricted virus-specific cytotoxic T cells as a physiological correlate of the 51Cr-release assay? J. Exp. Med. 1986, 164, 1075–1092. [Google Scholar] [CrossRef]
- Beier, J.I.; Jokinen, J.D.; Holz, G.E.; Whang, P.S.; Martin, A.M.; Warner, N.L.; Arteel, G.E.; Lukashevich, I.S. Novel mechanism of arenavirus-induced liver pathology. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Lukashevich, I.S.; Tikhonov, I.I.; Rodas, J.; Zapata, J.C.; Yang, Y.; Djavani, M.; Salvato, M.S. Arenavirus-mediated liver pathology: Acute lymphocytic choriomeningitis virus infection of rhesus macaques is characterized by high-level interleukin-6 expression and hepatocyte proliferation. J. Virol. 2003. [Google Scholar] [CrossRef]
- Sung, J.M.; Lee, C.K.; Wu-Hsieh, B.A. Intrahepatic Infiltrating NK and CD8 T Cells Cause Liver Cell Death in Different Phases of Dengue Virus Infection. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, J.A.S.; Barros, V.L.R.S.; Pagliari, C.; Fernandes, E.R.; Guedes, F.; Takakura, C.F.H.; Andrade, H.F.; Vasconcelos, P.F.C.; Duarte, M.I.S. Revisiting the liver in human yellow fever: Virus-induced apoptosis in hepatocytes associated with TGF-β, TNF-α and NK cells activity. Virology 2006. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, J.A.S.; Barros, V.L.R.S.; Pagliari, C.; Fernandes, E.R.; Andrade, H.F.; Vasconcelos, P.F.C.; Duarte, M.I.S. Hepatocyte lesions and cellular immune response in yellow fever infection. Trans. R. Soc. Trop. Med. Hyg. 2007. [Google Scholar] [CrossRef] [PubMed]
- Ruibal, P.; Oestereich, L.; Ludtke, A.; Becker-Ziaja, B.; Wozniak, D.M.; Kerber, R.; Korva, M.; Cabeza-Cabrerizo, M.; Bore, J.A.; Koundouno, F.R.; et al. Unique human immune signature of Ebola virus disease in Guinea. Nature 2016. [Google Scholar] [CrossRef] [PubMed]
- Speranza, E.; Ruibal, P.; Port, J.R.; Feng, F.; Burkhardt, L.; Grundhoff, A.; Gunther, S.; Oestereich, L.; Hiscox, J.A.; Connor, J.H.; et al. T-Cell Receptor Diversity and the Control of T-Cell Homeostasis Mark Ebola Virus Disease Survival in Humans. J. Infect. Dis. 2018, 218, S508–S518. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, J.; Zhu, Y.; Xu, Z.; Yang, K.; Yang, A.; Jin, B. Cellular Immune Response to Hantaan Virus Nucleocapsid Protein in the Acute Phase of Hemorrhagic Fever with Renal Syndrome: Correlation with Disease Severity. J. Infect. Dis. 2009. [Google Scholar] [CrossRef] [PubMed]
- Chandele, A.; Sewatanon, J.; Gunisetty, S.; Singla, M.; Onlamoon, N.; Akondy, R.S.; Kissick, H.T.; Nayak, K.; Reddy, E.S.; Kalam, H.; et al. Characterization of Human CD8 T Cell Responses in Dengue Virus-Infected Patients from India. J. Virol. 2016. [Google Scholar] [CrossRef] [PubMed]
- ter Meulen, J.; Badusche, M.; Kuhnt, K.; Doetze, A.; Satoguina, J.; Marti, T.; Loeliger, C.; Koulemou, K.; Koivogui, L.; Schmitz, H.; et al. Characterization of Human CD4+ T-Cell Clones Recognizing Conserved and Variable Epitopes of the Lassa Virus Nucleoprotein. J. Virol. 2000. [Google Scholar] [CrossRef]
- McElroy, A.K.; Akondy, R.S.; Davis, C.W.; Ellebedy, A.H.; Mehta, A.K.; Kraft, C.S.; Lyon, G.M.; Ribner, B.S.; Varkey, J.; Sidney, J.; et al. Human Ebola virus infection results in substantial immune activation. Proc. Natl. Acad. Sci. USA 2015. [Google Scholar] [CrossRef] [PubMed]
- Duangchinda, T.; Dejnirattisai, W.; Vasanawathana, S.; Limpitikul, W.; Tangthawornchaikul, N.; Malasit, P.; Mongkolsapaya, J.; Screaton, G. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc. Natl. Acad. Sci. USA 2010. [Google Scholar] [CrossRef] [PubMed]
- Sakabe, S.; Sullivan, B.M.; Hartnett, J.N.; Robles-Sikisaka, R.; Gangavarapu, K.; Cubitt, B.; Ware, B.C.; Kotliar, D.; Branco, L.M.; Goba, A.; et al. Analysis of CD8+ T cell response during the 2013–2016 Ebola epidemic in West Africa. Proc. Natl. Acad. Sci. USA 2018. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yuan, B.; Zhuang, R.; Zhang, Y.; Liu, B.; Zhang, C.; Zhang, Y.; Yu, H.; Yi, J.; Yang, A.; et al. Hantaan Virus Infection Induces Both Th1 and ThGranzyme B+ Cell Immune Responses That Associated with Viral Control and Clinical Outcome in Humans. PLoS Pathog. 2015. [Google Scholar] [CrossRef] [PubMed]
- Carrion, R.; Brasky, K.; Mansfield, K.; Johnson, C.; Gonzales, M.; Ticer, A.; Lukashevich, I.; Tardif, S.; Patterson, J. Lassa Virus Infection in Experimentally Infected Marmosets: Liver Pathology and Immunophenotypic Alterations in Target Tissues. J. Virol. 2007. [Google Scholar] [CrossRef] [PubMed]
- Agrati, C.; Castilletti, C.; Casetti, R.; Sacchi, A.; Falasca, L.; Turchi, F.; Tumino, N.; Bordoni, V.; Cimini, E.; Viola, D.; et al. Longitudinal characterization of dysfunctional T cell-activation during human acute Ebola infection. Cell Death Dis. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hammerbeck, C.D.; Hooper, J.W. T Cells Are Not Required for Pathogenesis in the Syrian Hamster Model of Hantavirus Pulmonary Syndrome. J. Virol. 2011. [Google Scholar] [CrossRef]
- Mongkolsapaya, J.; Dejnirattisai, W.; Xu, X.N.; Vasanawathana, S.; Tangthawornchaikul, N.; Chairunsri, A.; Sawasdivorn, S.; Duangchinda, T.; Dong, T.; Rowland-Jones, S.; et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 2003. [Google Scholar] [CrossRef]
- Mahanty, S.; Hutchinson, K.; Agarwal, S.; Mcrae, M.; Rollin, P.E.; Pulendran, B. Cutting Edge: Impairment of Dendritic Cells and Adaptive Immunity by Ebola and Lassa Viruses. J. Immunol. 2003. [Google Scholar] [CrossRef]
- Gupta, S.; Braun, M.; Tischler, N.D.; Stoltz, M.; Sundström, K.B.; Björkström, N.K.; Ljunggren, H.G.; Klingström, J. Hantavirus-infection Confers Resistance to Cytotoxic Lymphocyte-Mediated Apoptosis. PLoS Pathog. 2013. [Google Scholar] [CrossRef]
- Palmer, D.R.; Sun, P.; Celluzzi, C.; Pang, S.; Sun, W.; Marovich, M.A.; Burgess, T.; Bisbing, J. Differential Effects of Dengue Virus on Infected and Bystander Dendritic Cells Differential Effects of Dengue Virus on Infected and Bystander Dendritic Cells. J. Virol. 2005. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.E.; Varga, S.M. The CD8 T Cell Response to Respiratory Virus Infections. Front. Immunol. 2018, 9, 678. [Google Scholar] [CrossRef] [PubMed]
- Cerwenka, A.; Morgan, T.M.; Dutton, R.W. Naive, effector, and memory CD8 T cells in protection against pulmonary influenza virus infection: Homing properties rather than initial frequencies are crucial. J. Immunol. 1999. [Google Scholar] [CrossRef]
- Hogan, R.J.; Usherwood, E.J.; Zhong, W.; Roberts, A.D.; Dutton, R.W.; Harmsen, A.G.; Woodland, D.L. Activated Antigen-Specific CD8+ T Cells Persist in the Lungs Following Recovery from Respiratory Virus Infections. J. Immunol. 2001. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, L.; Nguyen, T.H.O.; Wan, Y.; Sant, S.; Quiñones-Parra, S.M.; Crawford, J.C.; Eltahla, A.A.; Rizzetto, S.; Bull, R.A.; et al. Clonally diverse CD38+HLA-DR+CD8+ T cells persist during fatal H7N9 disease. Nat. Commun. 2018. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, H.; Bugany, H.; Mahner, F.; Klenk, H.D.; Drenckhahn, D.; Schnittler, H.J. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J. Virol. 1996, 70, 2208–2214. [Google Scholar]
- Lukashevich, I.S.; Maryankova, R.; Vladyko, A.S.; Nashkevich, N.; Koleda, S.; Djavani, M.; Horejsh, D.; Voitenok, N.N.; Salvato, M.S. Lassa and Mopeia virus replication in human monocytes/macrophages and in endothelial cells: Different effects on IL-8 and TNF-alpha gene expression. J. Med. Virol. 1999, 59, 552–560. [Google Scholar] [CrossRef]
- Djavani, M.M.; Crasta, O.R.; Zapata, J.C.; Fei, Z.; Folkerts, O.; Sobral, B.; Swindells, M.; Bryant, J.; Davis, H.; Pauza, C.D.; et al. Early Blood Profiles of Virus Infection in a Monkey Model for Lassa Fever. J. Virol. 2007. [Google Scholar] [CrossRef]
- Ly, H. Differential immune responses to new world and old world mammalian arenaviruses. Int. J. Mol. Sci. 2017, 18, 1040. [Google Scholar] [CrossRef]
- Libraty, D.H.; Endy, T.P.; Houng, H.-S.H.; Green, S.; Kalayanarooj, S.; Suntayakorn, S.; Chansiriwongs, W.; Vaughn, D.W.; Nisalak, A.; Ennis, F.A.; et al. Differing Influences of Virus Burden and Immune Activation on Disease Severity in Secondary Dengue-3 Virus Infections. J. Infect. Dis. 2002. [Google Scholar] [CrossRef]
- Baize, S.; Leroy, E.M.; Georges, A.J.; Georges-Courbot, M.C.; Capron, M.; Bedjabaga, I.; Lansoud-Soukate, J.; Mavoungou, E. Inflammatory responses in Ebola virus-infected patients. Clin. Exp. Immunol. 2002. [Google Scholar] [CrossRef]
- Azeredo, E.L.; Zagne, S.M.O.; Santiago, M.A.; Gouvea, A.S.; Santana, A.A.; Neves-Souza, P.C.F.; Nogueira, R.M.R.; Miagostovich, M.P.; Kubelka, C.F. Characterisation of lymphocyte response and cytokine patterns with dengue fever. Immunobiology 2001. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, K.L.; Rollin, P.E. Cytokine and Chemokine Expression in Humans Infected with Sudan Ebola Virus. J. Infect. Dis. 2007. [Google Scholar] [CrossRef] [PubMed]
- Miller, Y.I.; Choi, S.H.; Wiesner, P.; Fang, L.; Harkewicz, R.; Hartvigsen, K.; Boullier, A.; Gonen, A.; Diehl, C.J.; Que, X.; et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.; Gilroy, D.W. Lipid Mediators in Inflammation. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Yiu, H.H.; Graham, A.L.; Stengel, R.F. Dynamics of a Cytokine Storm. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Kaiko, G.E.; Horvat, J.C.; Beagley, K.W.; Hansbro, P.M. Immunological decision-making: How does the immune system decide to mount a helper T-cell response? Immunology 2008. [Google Scholar] [CrossRef] [PubMed]
- Rathakrishnan, A.; Wang, S.M.; Hu, Y.; Khan, A.M.; Ponnampalavanar, S.; Lum, L.C.S.; Manikam, R.; Sekaran, S.D. Cytokine Expression Profile of Dengue Patients at Different Phases of Illness. PLoS ONE 2012. [Google Scholar] [CrossRef]
- Younan, P.; Iampietro, M.; Nishida, A.; Ramanathan, P.; Santos, R.I.; Dutta, M.; Lubaki, N.M.; Koup, R.A.; Katze, M.G.; Bukreyev, A. Ebola virus binding to Tim-1 on T lymphocytes induces a cytokine storm. mBio 2017. [Google Scholar] [CrossRef]
- Flatz, L.; Rieger, T.; Merkler, D.; Bergthaler, A.; Regen, T.; Schedensack, M.; Bestmann, L.; Verschoor, A.; Kreutzfeldt, M.; Brück, W.; et al. T cell-dependence of lassa fever pathogenesis. PLoS Pathog. 2010. [Google Scholar] [CrossRef]
- Khaiboullina, S.F.; Levis, S.; Morzunov, S.P.; Martynova, E.V.; Anokhin, V.A.; Gusev, O.A.; St Jeor, S.C.; Lombardi, V.C.; Rizvanov, A.A. Serum cytokine profiles differentiating hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Front. Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Vignali, D.A.A.; Collison, L.W.; Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008. [Google Scholar] [CrossRef]
- Lindgren, T.; Ahlm, C.; Mohamed, N.; Evander, M.; Ljunggren, H.-G.; Bjorkstrom, N.K. Longitudinal Analysis of the Human T Cell Response during Acute Hantavirus Infection. J. Virol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Jayaratne, H.E.; Wijeratne, D.; Fernando, S.; Kamaladasa, A.; Gomes, L.; Wijewickrama, A.; Ogg, G.S.; Malavige, G.N. Regulatory T-cells in acute dengue viral infection. Immunology 2018, 154, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Lühn, K.; Simmons, C.P.; Moran, E.; Dung, N.T.P.; Chau, T.N.B.; Quyen, N.T.H.; Thao, L.T.T.; Van Ngoc, T.; Dung, N.M.; Wills, B.; et al. Increased frequencies of CD4+ CD25 high regulatory T cells in acute dengue infection. J. Exp. Med. 2007. [Google Scholar] [CrossRef]
- Tillu, H.; Tripathy, A.S.; Reshmi, P.V.; Cecilia, D. Altered profile of regulatory T cells and associated cytokines in mild and moderate dengue. Eur. J. Clin. Microbiol. Infect. Dis. 2016. [Google Scholar] [CrossRef]
- Blom, K.; Braun, M.; Ivarsson, M.A.; Gonzalez, V.D.; Falconer, K.; Moll, M.; Ljunggren, H.-G.; Michaelsson, J.; Sandberg, J.K. Temporal Dynamics of the Primary Human T Cell Response to Yellow Fever Virus 17D As It Matures from an Effector- to a Memory-Type Response. J. Immunol. 2013. [Google Scholar] [CrossRef]
- Martins, M.A.; Silva, M.L.; Marciano, A.P.V.; Peruhype-Magalhaes, V.; Eloi-Santos, S.M.; Ribeiro, J.G.L.; Correa-Oliveira, R.; Homma, A.; Kroon, E.G.; Teixeira-Carvalho, A.; et al. Activation/modulation of adaptive immunity emerges simultaneously after 17DD yellow fever first-time vaccination: Is this the key to prevent severe adverse reactions following immunization? Clin. Exp. Immunol. 2007, 148, 90–100. [Google Scholar] [CrossRef] [PubMed]
- de Alwis, R.; Bangs, D.J.; Angelo, M.A.; Cerpas, C.; Fernando, A.; Sidney, J.; Peters, B.; Gresh, L.; Balmaseda, A.; de Silva, A.D.; et al. Immunodominant Dengue Virus-Specific CD8 + T Cell Responses Are Associated with a Memory PD-1 + Phenotype. J. Virol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Green, S.; Vaughn, D.W.; Kalayanarooj, S.; Nimmannitya, S.; Suntayakorn, S.; Nisalak, A.; Rothman, A.L.; Ennis, F.A. Elevated plasma interleukin-10 levels in acute dengue correlate with disease severity. J. Med. Virol. 1999. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Liong, K.H.; Gunalan, M.G.; Li, N.; Lim, D.S.L.; Fisher, D.A.; MacAry, P.A.; Leo, Y.S.; Wong, S.-C.; Puan, K.J.; et al. Type I IFNs and IL-18 Regulate the Antiviral Response of Primary Human γδ T Cells against Dendritic Cells Infected with Dengue Virus. J. Immunol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, D.; Peng, A.; Li, L.; Cha, H.; Qu, J.; Xie, H.; Wang, Y.; Xia, Y.; Huang, J. Cellular immune response of dengue virus infection at different phases. Int. J. Clin. Exp. Med. 2016, 9, 19373–19380. [Google Scholar]
- Meyer, M.; Garron, T.; Lubaki, N.M.; Mire, C.E.; Fenton, K.A.; Klages, C.; Olinger, G.G.; Geisbert, T.W.; Collins, P.L.; Bukreyev, A. Aerosolized Ebola vaccine protects primates and elicits lung-resident T cell responses. J. Clin. Investig. 2015. [Google Scholar] [CrossRef]
- Schountz, T.; Quackenbush, S.; Rovnak, J.; Haddock, E.; Black, W.C.; Feldmann, H.; Prescott, J. Differential Lymphocyte and Antibody Responses in Deer Mice Infected with Sin Nombre Hantavirus or Andes Hantavirus. J. Virol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Pandey, N.; Garg, R.K.; Kumar, R. IL-17 level in patients with dengue virus infection & its association with severity of illness. J. Clin. Immunol. 2013. [Google Scholar] [CrossRef]
- Zapata, J.C.; Medina-Moreno, S.; Guzmán-Cardozo, C.; Salvato, M.S. Improving the Breadth of the Host’s Immune Response to Lassa Virus. Pathogens 2018, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Warner, B.M.; Safronetz, D.; Stein, D.R. Current research for a vaccine against Lassa hemorrhagic fever virus. Drug Des. Devel. Ther. 2018, 12, 2519–2527. [Google Scholar] [CrossRef]
- Fisher-Hoch, S.P.; Mitchell, S.W.; Sasso, D.R.; Lange, J.V.; Ramsey, R.; McCormick, J.B. Physiological and Immunologic Disturbances Associated with Shock in a Primate Model of Lassa Fever. J. Infect. Dis. 1987. [Google Scholar] [CrossRef]
- Walker, D.H.; McCormick, J.B.; Johnson, K.M.; Webb, P.A.; Komba-Kono, G.; Elliott, L.H.; Gardner, J.J. Pathologic and virologic study of fatal Lassa fever in man. Am. J. Pathol. 1982. [Google Scholar] [CrossRef]
- Baize, S.; Marianneau, P.; Loth, P.; Reynard, S.; Journeaux, A.; Chevallier, M.; Tordo, N.; Deubel, V.; Contamin, H. Early and Strong Immune Responses Are Associated with Control of Viral Replication and Recovery in Lassa Virus-Infected Cynomolgus Monkeys. J. Virol. 2009. [Google Scholar] [CrossRef]
- Geisbert, T.W.; Jones, S.; Fritz, E.A.; Shurtleff, A.C.; Geisbert, J.B.; Liebscher, R.; Grolla, A.; Ströher, U.; Fernando, L.; Daddario, K.M.; et al. Development of a new vaccine for the prevention of Lassa fever. PLoS Med. 2005. [Google Scholar] [CrossRef] [PubMed]
- Zapata, J.C.; Pauza, C.D.; Djavani, M.M.; Rodas, J.D.; Moshkoff, D.; Bryant, J.; Ateh, E.; Garcia, C.; Lukashevich, I.S.; Salvato, M.S. Lymphocytic choriomeningitis virus (LCMV) infection of macaques: A model for Lassa fever. Antiviral Res. 2011. [Google Scholar] [CrossRef] [PubMed]
- Pannetier, D.; Reynard, S.; Russier, M.; Journeaux, A.; Tordo, N.; Deubel, V.; Baize, S. Human Dendritic Cells Infected with the Nonpathogenic Mopeia Virus Induce Stronger T-Cell Responses than Those Infected with Lassa Virus. J. Virol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Rodas, J.D.; Cairo, C.; Djavani, M.; Zapata, J.C.; Ruckwardt, T.; Bryant, J.; Pauza, C.D.; Lukashevich, I.S.; Salvato, M.S. Circulating natural killer and γδ T cells decrease soon after infection of rhesus macaques with lymphocytic choriomeningitis virus. Mem. Inst. Oswaldo Cruz 2009. [Google Scholar] [CrossRef]
- Marta, R.F.; Montero, V.S.; Hack, C.E.; Sturk, A.; Maiztegui, J.I.; Molinas, F.C. Proinflammatory cytokines and elastase-α-1-antitrypsin in Argentine hemorrhagic fever. Am. J. Trop. Med. Hyg. 1999, 60, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Maiztegui, J.; Fernandez, N.; De Damilano, A. Efficacy of immune plasma in treatment of argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet 1979. [Google Scholar] [CrossRef]
- Fisher-Hoch, S.P.; Hutwagner, L.; Brown, B.; McCormick, J.B. Effective Vaccine for Lassa Fever. J. Virol. 2000. [Google Scholar] [CrossRef]
- Lukashevich, I.S.; Patterson, J.; Carrion, R.; Moshkoff, D.; Ticer, A.; Zapata, J.; Brasky, K.; Geiger, R.; Hubbard, G.B.; Bryant, J.; et al. A Live Attenuated Vaccine for Lassa Fever Made by Reassortment of Lassa and Mopeia Viruses. J. Virol. 2005. [Google Scholar] [CrossRef]
- Zapata, J.C.; Poonia, B.; Bryant, J.; Davis, H.; Ateh, E.; George, L.; Crasta, O.; Zhang, Y.; Slezak, T.; Jaing, C.; et al. An attenuated Lassa vaccine in SIV-infected rhesus macaques does not persist or cause arenavirus disease but does elicit Lassa virus-specific immunity. Virol. J. 2013. [Google Scholar] [CrossRef]
- Goicochea, M.A.; Zapata, J.C.; Bryant, J.; Davis, H.; Salvato, M.S.; Lukashevich, I.S. Evaluation of Lassa virus vaccine immunogenicity in a CBA/J-ML29 mouse model. Vaccine 2012. [Google Scholar] [CrossRef]
- Morrison, H.G.; Bauer, S.P.; Lange, J.V.; Esposito, J.I.; McCormick, J.B.; Auperin, D.D. Protection of guinea pigs from lassa fever by vaccinia virus recombinants expressing the nucleoprotein or the envelope glycoproteins of lassa virus. Virology 1989. [Google Scholar] [CrossRef]
- Rodas, J.D.; Lukashevich, I.S.; Zapata, J.C.; Cairo, C.; Tikhonov, I.; Djavani, M.; Pauza, C.D.; Salvato, M.S. Mucosal Arenavirus Infection of Primates Can Protect Them from Lethal Hemorrhagic Fever. J. Med. Virol. 2004. [Google Scholar] [CrossRef] [PubMed]
- Boesen, A.; Sundar, K.; Coico, R. Lassa fever virus peptides predicted by computational analysis induce epitope-specific cytotoxic-T-lymphocyte responses in HLA-A2.1 transgenic mice. Clin. Diagn. Lab. Immunol. 2005. [Google Scholar] [CrossRef] [PubMed]
- Botten, J.; Alexander, J.; Pasquetto, V.; Sidney, J.; Barrowman, P.; Ting, J.; Peters, B.; Southwood, S.; Stewart, B.; Rodriguez-Carreno, M.P.; et al. Identification of Protective Lassa Virus Epitopes That Are Restricted by HLA-A2. J. Virol. 2006. [Google Scholar] [CrossRef] [PubMed]
- Ter Meulen, J.; Badusche, M.; Satoguina, J.; Strecker, T.; Lenz, O.; Loeliger, C.; Sakho, M.; Koulemou, K.; Koivogui, L.; Hoerauf, A. Old and New World arenaviruses share a highly conserved epitope in the fusion domain of the glycoprotein 2, which is recognized by Lassa virus-specific human CD4+ T-cell clones. Virology 2004. [Google Scholar] [CrossRef] [PubMed]
- Salvato, M.; Domi, A.; Guzman-Cardozo, C.; Zapata, J.; Medina-Moreno, S.; Hsu, H.; McCurley, N.; Basu, R.; Hauser, M.; Hellerstein, M. A single dose of Modified Vaccinia Ankara expressing Lassa Virus-like particles protects mice from lethal intracerebral virus challenge. Sci. Rep. 2018. Under Review. [Google Scholar]
- Baize, S.; Leroy, E.M.; Georges-Courbot, M.C.; Capron, M.; Lansoud-Soukate, J.; Debré, P.; Fisher-Hoch, S.P.; McCormick, J.B.; Georges, A.J. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat. Med. 1999. [Google Scholar] [CrossRef]
- Wauquier, N.; Becquart, P.; Padilla, C.; Baize, S.; Leroy, E.M. Human fatal zaire ebola virus infection is associated with an aberrant innate immunity and with massive lymphocyte apoptosis. PLoS Negl. Trop. Dis. 2010. [Google Scholar] [CrossRef]
- Younan, P.; Iampietro, M.; Bukreyev, A. Disabling of lymphocyte immune response by Ebola virus. PLoS Pathog. 2018. [Google Scholar] [CrossRef]
- Iampietro, M.; Younan, P.; Nishida, A.; Dutta, M.; Lubaki, N.M.; Santos, R.I.; Koup, R.A.; Katze, M.G.; Bukreyev, A. Ebola virus glycoprotein directly triggers T lymphocyte death despite of the lack of infection. PLoS Pathog. 2017. [Google Scholar] [CrossRef]
- Bradfute, S.B.; Warfield, K.L.; Bavari, S. Functional CD8+ T Cell Responses in Lethal Ebola Virus Infection. J. Immunol. 2008. [Google Scholar] [CrossRef]
- Gupta, M.; Mahanty, S.; Greer, P.; Towner, J.S.; Shieh, W.-J.; Zaki, S.R.; Ahmed, R.; Rollin, P.E. Persistent Infection with Ebola Virus under Conditions of Partial Immunity. J. Virol. 2004. [Google Scholar] [CrossRef]
- Zeng, X.; Blancett, C.D.; Koistinen, K.A.; Schellhase, C.W.; Bearss, J.J.; Radoshitzky, S.R.; Honnold, S.P.; Chance, T.B.; Warren, T.K.; Froude, J.W.; et al. Identification and pathological characterization of persistent asymptomatic Ebola virus infection in rhesus monkeys. Nat. Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kreuels, B.; Wichmann, D.; Emmerich, P.; Schmidt-Chanasit, J.; de Heer, G.; Kluge, S.; Sow, A.; Renné, T.; Günther, S.; Lohse, A.W.; et al. A Case of Severe Ebola Virus Infection Complicated by Gram-Negative Septicemia. N. Engl. J. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Changula, K.; Yoshida, R.; Noyori, O.; Marzi, A.; Miyamoto, H.; Ishijima, M.; Yokoyama, A.; Kajihara, M.; Feldmann, H.; Mweene, A.S.; et al. Mapping of conserved and species-specific antibody epitopes on the Ebola virus nucleoprotein. Virus Res. 2013. [Google Scholar] [CrossRef] [PubMed]
- Marzi, A.; Feldmann, H. Ebola virus vaccines: An overview of current approaches. Expert Rev. Vaccines 2014. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.A.; Hart, M.K. Protection from Ebola virus mediated by cytotoxic T lymphocytes specific for the viral nucleoprotein. J. Virol. 2001. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, P.; Ebihara, H.; Marzi, A.; Hatta, Y.; Watanabe, S.; Suresh, M.; Neumann, G.; Feldmann, H.; Kawaoka, Y. Replication-Deficient Ebolavirus as a Vaccine Candidate. J. Virol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, Y.; Caposio, P.; Parkins, C.J.; Botto, S.; Messaoudi, I.; Cicin-Sain, L.; Feldmann, H.; Jarvis, M.A. A replicating cytomegalovirus-based vaccine encoding a single Ebola virus nucleoprotein CTL epitope confers protection against Ebola virus. PLoS Negl. Trop. Dis. 2011. [Google Scholar] [CrossRef] [PubMed]
- Vanderzanden, L.; Bray, M.; Fuller, D.; Roberts, T.; Custer, D.; Spik, K.; Jahrling, P.; Huggins, J.; Schmaljohn, A.; Schmaljohn, C. DNA vaccines expressing either the GP or NP genes of Ebola virus protect mice from lethal challenge. Virology 1998. [Google Scholar] [CrossRef] [PubMed]
- Simmons, G.; Lee, A.; Rennekamp, A.J.; Fan, X.; Bates, P.; Shen, H. Identification of murine T-cell epitopes in Ebola virus nucleoprotein. Virology 2004. [Google Scholar] [CrossRef] [PubMed]
- Warfield, K.L.; Swenson, D.L.; Olinger, G.G.; Kalina, W.V.; Aman, M.J.; Bavari, S. Ebola Virus-Like Particle–Based Vaccine Protects Nonhuman Primates against Lethal Ebola Virus Challenge. J. Infect. Dis. 2007. [Google Scholar] [CrossRef] [PubMed]
- Domi, A.; Feldmann, F.; Basu, R.; McCurley, N.; Shifflett, K.; Emanuel, J.; Hellerstein, M.S.; Guirakhoo, F.; Orlandi, C.; Flinko, R.; et al. A Single Dose of Modified Vaccinia Ankara expressing Ebola Virus Like Particles Protects Nonhuman Primates from Lethal Ebola Virus Challenge. Sci. Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.K. Vaccine research efforts for filoviruses. Int. J. Parasitol. 2003. [Google Scholar] [CrossRef]
- Sullivan, N.J.; Martin, J.E.; Graham, B.S.; Nabel, G.J. Correlates of protective immunity for Ebola vaccines: Implications for regulatory approval by the animal rule. Nat. Rev. Microbiol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, C.G.; Tangye, S.G.; Moser, B.; Mackay, C.R. Follicular B helper T cells in antibody responses and autoimmunity. Nat. Rev. Immunol. 2005. [Google Scholar] [CrossRef]
- Martins, K.A.O.; Cooper, C.L.; Stronsky, S.M.; Norris, S.L.W.; Kwilas, S.A.; Steffens, J.T.; Benko, J.G.; van Tongeren, S.A.; Bavari, S. Adjuvant-enhanced CD4 T Cell Responses are Critical to Durable Vaccine Immunity. EBioMedicine 2016. [Google Scholar] [CrossRef]
- Cooper, C.L.; Martins, K.A.; Stronsky, S.M.; Langan, D.P.; Steffens, J.; Van Tongeren, S.; Bavari, S. T-cell-dependent mechanisms promote Ebola VLP-induced antibody responses, but are dispensable for vaccine-mediated protection. Emerg. Microbes Infect. 2017. [Google Scholar] [CrossRef]
- Nakamura, T.; Yanagihara, R.; Gibbs, C.J.; Gajdusek, D.C. Immune spleen cell-mediated protection against fatal Hantaan virus infection in infant mice. J. Infect. Dis. 1985. [Google Scholar] [CrossRef]
- Asada, H.; Tamura, M.; Kondo, K.; Okuno, Y.; Takahashi, Y.; Dohi, Y.; Nagai, T.; Kurata, T.; Yamanishi, K. Role of T lymphocyte subsets in protection and recovery from Hantaan virus infection in mice. J. Gen. Virol. 1987. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Yuan, B.; Wang, M.; Zhang, Y.; Xu, Z.; Zhang, C.; Zhang, Y.; Liu, B.; Yi, J.; et al. HLA-A2 and B35 Restricted Hantaan Virus Nucleoprotein CD8+T-Cell Epitope-Specific Immune Response Correlates with Milder Disease in Hemorrhagic Fever with Renal Syndrome. PLoS Negl. Trop. Dis. 2013. [Google Scholar] [CrossRef] [PubMed]
- Manigold, T.; Martinez, J.; Lazcano, X.; Ye, C.; Schwartz, S.; Cuiza, A.; Valdivieso, F.; Hjelle, B.; Vial, P. Case report: T-cell responses during clearance of Andes virus from blood cells 2 months after severe hantavirus cardiopulmonary syndrome. J. Med. Virol. 2008. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Kang, Z.; Zhao, Q.; Wang, X.; Hui, L. Kinetics and Immunodominance of Virus-Specific T Cell Responses During Hantaan Virus Infection. Viral Immunol. 2015, 28, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.; Yoshimatsu, K.; Lee, B.-H.; Kariwa, H.; Takashima, I.; Arikawa, J. Hantavirus-specific CD8(+)-T-cell responses in newborn mice persistently infected with Hantaan virus. J. Virol. 2003. [Google Scholar] [CrossRef]
- Araki, K.; Yoshimatsu, K.; Lee, B.H.; Kariwa, H.; Takashima, I.; Arikawa, J. A new model of Hantaan virus persistence in mice: The balance between HTNV infection and CD8+T-cell responses. Virology 2004. [Google Scholar] [CrossRef] [PubMed]
- Easterbrook, J.D.; Klein, S.L. Immunological mechanisms mediating hantavirus persistence in rodent reservoirs. PLoS Pathog. 2008. [Google Scholar] [CrossRef] [PubMed]
- Spengler, J.R.; Kelly Keating, M.; McElroy, A.K.; Zivcec, M.; Coleman-McCray, J.A.D.; Harmon, J.R.; Bollweg, B.C.; Goldsmith, C.S.; Bergeron, É.; Keck, J.G.; Zaki, S.R.; et al. Crimean-Congo Hemorrhagic Fever in Humanized Mice Reveals Glial Cells as Primary Targets of Neurological Infection. J. Infect. Dis. 2017. [Google Scholar] [CrossRef]
- Harmon, J.R.; Spengler, J.R.; Coleman-McCray, J.D.; Nichol, S.T.; Spiropoulou, C.F.; McElroy, A.K. CD4 T cells, CD8 T cells, and monocytes coordinate to prevent Rift Valley fever virus encephalitis. J. Virol. 2018. [Google Scholar] [CrossRef]
- Dodd, K.A.; McElroy, A.K.; Jones, M.E.B.; Nichol, S.T.; Spiropoulou, C.F. Rift Valley Fever Virus Clearance and Protection from Neurologic Disease Are Dependent on CD4+ T Cell and Virus-Specific Antibody Responses. J. Virol. 2013. [Google Scholar] [CrossRef]
- Goedhals, D.; Paweska, J.T.; Burt, F.J. Long-lived CD8+ T cell responses following Crimean-Congo haemorrhagic fever virus infection. PLoS Negl. Trop. Dis. 2017. [Google Scholar] [CrossRef]
- Huang, C.; Jin, B.; Wang, M.; Li, E.; Sun, C. Hemorrhagic fever with renal syndrome: Relationship between pathogenesis and cellular immunity. J. Infect. Dis. 1994. [Google Scholar] [CrossRef]
- Ennis, F.A.; Cruz, J.; Spiropoulou, C.F.; Waite, D.; Peters, C.J.; Nichol, S.T.; Kariwa, H.; Koster, F.T. Hantavirus pulmonary syndrome: CD8+and CD4+cytotoxic t lymphocytes to epitopes on Sin Nombre virus nucleocapsid protein isolated during acute illness. Virology 1997. [Google Scholar] [CrossRef] [PubMed]
- Van Epps, H.; Schmaljohn, C.; Ennis, F. Human memory cytotoxic T-lymphocyte (CTL) responses to Hantaan virus infection: Identification of virus-specific and cross-reactive CD8+ CTL epitopes on nucleocapsid. J. Virol. 1999, 73, 5301–5308. [Google Scholar] [PubMed]
- Van Epps, H.L.; Terajima, M.; Mustonen, J.; Arstila, T.P.; Corey, E.A.; Vaheri, A.; Ennis, F.A. Long-lived Memory T Lymphocyte Responses After Hantavirus Infection. J. Exp. Med. 2002. [Google Scholar] [CrossRef]
- Manigold, T.; Mori, A.; Graumann, R.; Llop, E.; Simon, V.; Ferrés, M.; Valdivieso, F.; Castillo, C.; Hjelle, B.; Vial, P. Highly differentiated, resting Gn-specific memory CD8+T cells persist years after infection by Andes hantavirus. PLoS Pathog. 2010. [Google Scholar] [CrossRef] [PubMed]
- Terajima, M.; Van Epps, H.L.; Li, D.; Leporati, A.M.; Juhlin, S.E.; Mustonen, J.; Vaheri, A.; Ennis, F.A. Generation of recombinant vaccinia viruses expressing Puumala virus proteins and use in isolating cytotoxic T cells specific for Puumala virus. Virus Res. 2002. [Google Scholar] [CrossRef]
- Sen, N.; Sen, A.; Mackow, E.R. Degrons at the C Terminus of the Pathogenic but Not the Nonpathogenic Hantavirus G1 Tail Direct Proteasomal Degradation. J. Virol. 2007. [Google Scholar] [CrossRef]
- Safronetz, D.; Hegde, N.R.; Ebihara, H.; Denton, M.; Kobinger, G.P.; St. Jeor, S.; Feldmann, H.; Johnson, D.C. Adenovirus Vectors Expressing Hantavirus Proteins Protect Hamsters against Lethal Challenge with Andes Virus. J. Virol. 2009. [Google Scholar] [CrossRef]
- Dargeviciute, A.; Brus Sjölander, K.; Sasnauskas, K.; Krüger, D.H.; Meisel, H.; Ulrich, R.; Lundkvist, Å. Yeast-expressed Puumala hantavirus nucleocapsid protein induces protection in a bank vole model. Vaccine 2002. [Google Scholar] [CrossRef]
- Ulrich, R.; Lundkvist, Å.; Meisel, H.; Koletzki, D.; Brus Sjölander, K.; Gelderblom, H.R.; Borisova, G.; Schnitzler, P.; Darai, G.; Krüger, D.H. Chimaeric HBV core particles carrying a defined segment of Puumala hantavirus nucleocapsid protein evoke protective immunity in an animal model. Vaccine 1998. [Google Scholar] [CrossRef]
- de Carvalho Nicacio, C.; Gonzalez Della Valle, M.; Padula, P.; Bjorling, E.; Plyusnin, A.; Lundkvist, A. Cross-protection against challenge with Puumala virus after immunization with nucleocapsid proteins from different hantaviruses. J. Virol. 2002. [Google Scholar] [CrossRef]
- Nakamura, T.; Yanagihara, R.; Gibbs, C.J.; Amyx, H.L.; Gajdusek, D.C. Differential susceptibility and resistance of immunocompetent and immunodeficient mice to fatal Hantaan virus infection. Arch. Virol. 1985. [Google Scholar] [CrossRef]
- Mustonen, J.; Partanen, J.; Kanerva, M.; Pietilä, K.; Vapalahti, O.; Pasternack, A.; Vaheri, A. Genetic susceptibility to severe course of nephropathia epidemica caused by Puumala hantavirus. Kidney Int. 1996. [Google Scholar] [CrossRef]
- Mäkelä, S.; Mustonen, J.; Ala-Houhala, I.; Hurme, M.; Partanen, J.; Vapalahti, O.; Vaheri, A.; Pasternack, A. Human leukocyte antigen-B8-DR3 is a more important risk factor for severe Puumala hantavirus infection than the tumor necrosis factor-alpha(-308) G/A polymorphism. J. Infect. Dis. 2002. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Rothman, A.L.; Kurane, I.; Montoya, J.M.; Nolte, K.B.; Norman, J.E.; Waite, D.C.; Koster, F.T.; Ennis, F.A. High levels of cytokine-producing cells in the lung tissues of patients with fatal hantavirus pulmonary syndrome. J. Infect. Dis. 1999. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Eckerle, I.; Daniel, V.; Burkhardt, U.; Opelz, G.; Schnitzler, P. Cytokine expression during early and late phase of acute Puumala hantavirus infection. BMC Immunol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Gotuzzo, E.; Yactayo, S.; Córdova, E. Review article: Efficacy and duration of immunity after yellow fever vaccination: Systematic review on the need for a booster every 10 years. Am. J. Trop. Med. Hyg. 2013. [Google Scholar] [CrossRef]
- Fuertes Marraco, S.A.; Soneson, C.; Cagnon, L.; Gannon, P.O.; Allard, M.; Maillard, S.A.; Montandon, N.; Rufer, N.; Waldvogel, S.; Delorenzi, M.; et al. Long-lasting stem cell-like memory CD8+ T cells with a naïve-like profile upon yellow fever vaccination. Sci. Transl. Med. 2015. [Google Scholar] [CrossRef]
- Barba-Spaeth, G.; Longman, R.S.; Albert, M.L.; Rice, C.M. Live attenuated yellow fever 17D infects human DCs and allows for presentation of endogenous and recombinant T cell epitopes. J. Exp. Med. 2005. [Google Scholar] [CrossRef]
- Querec, T.; Bennouna, S.; Alkan, S.; Laouar, Y.; Gorden, K.; Flavell, R.; Akira, S.; Ahmed, R.; Pulendran, B. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med. 2006. [Google Scholar] [CrossRef]
- Kohler, S.; Bethke, N.; Böthe, M.; Sommerick, S.; Frentsch, M.; Romagnani, C.; Niedrig, M.; Thiel, A. The early cellular signatures of protective immunity induced by live viral vaccination. Eur. J. Immunol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.R.; Fernandez, S.; Bisbing, J.; Peachman, K.K.; Rao, M.; Barvir, D.; Gunther, V.; Burgess, T.; Kohno, Y.; Padmanabhan, R.; et al. Restricted replication and lysosomal trafficking of yellow fever 17D vaccine virus in human dendritic cells. J. Gen. Virol. 2007. [Google Scholar] [CrossRef] [PubMed]
- Bassi, M.R.; Kongsgaard, M.; Steffensen, M.A.; Fenger, C.; Rasmussen, M.; Skjødt, K.; Finsen, B.; Stryhn, A.; Buus, S.; Christensen, J.P.; et al. CD8 + T Cells Complement Antibodies in Protecting against Yellow Fever Virus. J. Immunol. 2015. [Google Scholar] [CrossRef]
- Watson, A.M.; Lam, L.K.M.; Klimstra, W.B.; Ryman, K.D. The 17D-204 Vaccine Strain-Induced Protection against Virulent Yellow Fever Virus Is Mediated by Humoral Immunity and CD4+ but not CD8+ T Cells. PLoS Pathog. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.K.M.; Watson, A.M.; Ryman, K.D.; Klimstra, W.B. Gamma-interferon exerts a critical early restriction on replication and dissemination of yellow fever virus vaccine strain. Vaccines 2018. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, R.; Benson, M.J.; de Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, B.; Jaspert, R.; Niedrig, M.; Kostner, C.; L’age-Stehr, J. Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: A model of human flavivirus infection. J. Med. Virol. 1998. [Google Scholar] [CrossRef]
- Kongsgaard, M.; Bassi, M.R.; Rasmussen, M.; Skjødt, K.; Thybo, S.; Gabriel, M.; Hansen, M.B.; Christensen, J.P.; Thomsen, A.R.; Buus, S.; et al. Adaptive immune responses to booster vaccination against yellow fever virus are much reduced compared to those after primary vaccination. Sci. Rep. 2017. [Google Scholar] [CrossRef]
- Wieten, R.W.; Jonker, E.F.F.; Van Leeuwen, E.M.M.; Remmerswaal, E.B.M.; Ten Berge, I.J.M.; De Visser, A.W.; Van Genderen, P.J.J.; Goorhuis, A.; Visser, L.G.; Grobusch, M.P.; et al. A single 17D yellow fever vaccination provides lifelong immunity; characterization of yellow-fever-specific neutralizing antibody and T-cell responses after vaccination. PLoS ONE 2016. [Google Scholar] [CrossRef]
- James, E.A.; LaFond, R.E.; Gates, T.J.; Mai, D.T.; Malhotra, U.; Kwok, W.W. Yellow Fever Vaccination Elicits Broad Functional CD4+ T Cell Responses That Recognize Structural and Nonstructural Proteins. J. Virol. 2013. [Google Scholar] [CrossRef]
- Co, M.D.T.; Kilpatrick, E.D.; Rothman, A.L. Dynamics of the CD8 T-cell response following yellow fever virus 17D immunization. Immunology 2009. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, W.S.; Emerson, R.O.; Lindau, P.; Vignali, M.; Snyder, T.M.; Desmarais, C.; Sanders, C.; Utsugi, H.; Warren, E.H.; McElrath, J.; et al. Dynamics of the Cytotoxic T Cell Response to a Model of Acute Viral Infection. J. Virol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Co, M.D.T.; Terajima, M.; Cruz, J.; Ennis, F.A.; Rothman, A.L. Human cytotoxic T lymphocyte responses to live attenuated 17D yellow fever vaccine: Identification of HLA-B35-restricted CTL epitopes on nonstructural proteins NS1, NS2b, NS3, and the structural protein E. Virology 2002. [Google Scholar] [CrossRef]
- de Melo, A.B.; Nascimento, E.J.M.; Braga-Neto, U.; Dhalia, R.; Silva, A.M.; Oelke, M.; Schneck, J.P.; Sidney, J.; Sette, A.; Montenegro, S.M.L.; et al. T-cell memory responses elicited by yellow fever vaccine are targeted to overlapping epitopes containing multiple HLA-I and -II binding motifs. PLoS Negl. Trop. Dis. 2013, 7, e1938. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, D.; Therrien, R.; Kettaf, N.; Angermann, B.R.; Boucher, G.; Filali-Mouhim, A.; Moser, J.M.; Mehta, R.S.; Drake, D.R.; Castro, E.; et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J. Exp. Med. 2008. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.L.; Martins, M.A.; Espírito-Santo, L.R.; Campi-Azevedo, A.C.; Silveira-Lemos, D.; Ribeiro, J.G.L.; Homma, A.; Kroon, E.G.; Teixeira-Carvalho, A.; Elói-Santos, S.M.; et al. Characterization of main cytokine sources from the innate and adaptive immune responses following primary 17DD yellow fever vaccination in adults. Vaccine 2011. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.P.; Matos, D.C.S.; Bertho, A.L.; Mendonca, S.C.F.; Marcovistz, R. Detection of Th1/Th2 cytokine signatures in yellow fever 17DD first-time vaccinees through ELISpot assay. Cytokine 2008, 42, 152–155. [Google Scholar] [CrossRef]
- Akondy, R.S.; Fitch, M.; Edupuganti, S.; Yang, S.; Kissick, H.T.; Li, K.W.; Youngblood, B.A.; Abdelsamed, H.A.; McGuire, D.J.; Cohen, K.W.; et al. Origin and differentiation of human memory CD8 T cells after vaccination. Nature 2017. [Google Scholar] [CrossRef]
- Akondy, R.S.; Johnson, P.L.F.; Nakaya, H.I.; Edupuganti, S.; Mulligan, M.J.; Lawson, B.; Miller, J.D.; Pulendran, B.; Antia, R.; Ahmed, R. Initial viral load determines the magnitude of the human CD8 T cell response to yellow fever vaccination. Proc. Natl. Acad. Sci. USA 2015. [Google Scholar] [CrossRef]
- Sangkawibha, N.; Rojanasuphot, S.; Ahandrik, S.; Viriyapongse, S.; Jatanasen, S.; Salitul, V.; Phanthumachinda, B.; Halstead, S.B. Risk factors in dengue shock syndrome: A prospective epidemiologic study in Rayong, Thailand: I. THE 1980 outbreak. Am. J. Epidemiol. 1984. [Google Scholar] [CrossRef]
- Perdomo-Celis, F.; Salgado, D.M.; Narváez, C.F. Magnitude of viremia, antigenemia and infection of circulating monocytes in children with mild and severe dengue. Acta Trop. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chiewsilp, P.; Scott, R.M.; Bhamarapravati, N. Histocompatibility antigens and dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 1981. [Google Scholar] [CrossRef]
- Alagarasu, K.; Mulay, A.P.; Sarikhani, M.; Rashmika, D.; Shah, P.S.; Cecilia, D. Profile of human leukocyte antigen class I alleles in patients with dengue infection from Western India. Hum. Immunol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Loke, H.; Bethell, D.B.; Phuong, C.X.T.; Dung, M.; Schneider, J.; White, N.J.; Day, N.P.; Farrar, J.; Hill, A.V.S. Strong HLA Class I–Restricted T Cell Responses in Dengue Hemorrhagic Fever: A Double-Edged Sword? J. Infect. Dis. 2001. [Google Scholar] [CrossRef] [PubMed]
- Xavier Eurico De Alencar, L.; De Mendonça Braga-Neto, U.; José Moura Do Nascimento, E.; Tenório Cordeiro, M.; Maria Silva, A.; Alexandre Antunes De Brito, C.; Da Silva, M.D.P.C.; Gil, L.H.V.G.; Montenegro, S.M.L.; Marques, E.T.D.A. HLA-B*44 is associated with dengue severity caused by DENV-3 in a brazilian population. J. Trop. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.P.; do Brasil, P.E.A.A.; Cabello, G.M.K.; de Souz, R.V.; Brasil, P.; Georg, I.; Cabello, P.H.; de Castro, L. HLA-A*01 allele: A risk factor for dengue haemorrhagic fever in Brazil’s population. Mem. Inst. Oswaldo Cruz 2012. [Google Scholar] [CrossRef]
- Green, S.; Pichyangkul, S.; Vaughn, D.W.; Kalayanarooj, S.; Nimmannitya, S.; Nisalak, A.; Kurane, I.; Rothman, A.L.; Ennis, F.A. Early CD69 Expression on Peripheral Blood Lymphocytes from Children with Dengue Hemorrhagic Fever. J. Infect. Dis. 1999. [Google Scholar] [CrossRef]
- Arias, J.; Valero, N.; Mosquera, J.; Montiel, M.; Reyes, E.; Larreal, Y.; Alvarez-Mon, M. Increased expression of cytokines, soluble cytokine receptors, soluble apoptosis ligand and apoptosis in dengue. Virology 2014. [Google Scholar] [CrossRef]
- Butthep, P.; Chunhakan, S.; Yoksan, S.; Tangnararatchakit, K.; Chuansumrit, A. Alteration of cytokines and chemokines during febrile episodes associated with endothelial cell damage and plasma leakage in dengue hemorrhagic fever. Pediatr. Infect. Dis. J. 2012. [Google Scholar] [CrossRef]
- Bozza, F.A.; Cruz, O.G.; Zagne, S.M.O.; Azeredo, E.L.; Nogueira, R.M.R.; Assis, E.F.; Bozza, P.T.; Kubelka, C.F. Multiplex cytokine profile from dengue patients: MIP-1beta and IFN-gamma as predictive factors for severity. BMC Infect. Dis. 2008. [Google Scholar] [CrossRef]
- Jeewandara, C.; Adikari, T.N.; Gomes, L.; Fernando, S.; Fernando, R.H.; Perera, M.K.T.; Ariyaratne, D.; Kamaladasa, A.; Salimi, M.; Prathapan, S.; et al. Functionality of Dengue Virus Specific Memory T Cell Responses in Individuals Who Were Hospitalized or Who Had Mild or Subclinical Dengue Infection. PLoS Negl. Trop. Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Medin, C.; Fitzgerald, K.; Rothman, A. Dengue Virus Nonstructural Protein NS5 Induces Interleukin-8 Transcription and Secretion. Am. Soc. Microbiol. 2005. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Wang, S.-Y. Activation of terminally differentiated human monocytes/macrophages by dengue virus: Productive infection, hierarchical production of innate cytokines and chemokines, and the synergistic effect of lipopolysaccharide. J. Virol. 2002, 76, 9877–9887. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Duangchinda, T.; Lin, C.-L.S.; Vasanawathana, S.; Jones, M.; Jacobs, M.; Malasit, P.; Xu, X.-N.; Screaton, G.; Mongkolsapaya, J. A Complex Interplay among Virus, Dendritic Cells, T Cells, and Cytokines in Dengue Virus Infections. J. Immunol. 2008. [Google Scholar] [CrossRef]
- Perdomo-Celis, F.; Romero, F.; Salgado, D.M.; Vega, R.; Rodríguez, J.; Angel, J.; Franco, M.A.; Greenberg, H.B.; Narváez, C.F. Identification and characterization at the single-cell level of cytokine-producing circulating cells in children with dengue. J. Infect. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.-J.; Wang, J.-J.; Shaio, M.-F.; Kao, C.-L.; Chang, D.-M.; Han, S.-W.; Lai, J.-H. Infection of Human Dendritic Cells by Dengue Virus Causes Cell Maturation and Cytokine Production. J. Immunol. 2001. [Google Scholar] [CrossRef]
- Kurane, I.; Innis, B.L.; Nisalak, A.; Hoke, C.; Nimmannitya, S.; Meager, A.; Ennis, F.A. Human T cell response to dengue virus antigens. Proliferative responses and interferon gamma production. J. Clin. Investig. 1989. [Google Scholar] [CrossRef]
- Gagnon, S.J.; Ennis, F.A.; Rothman, A.L. Bystander target cell lysis and cytokine production by dengue virus-specific human CD4(+) cytotoxic T-lymphocyte clones. J. Virol. 1999. [Google Scholar] [CrossRef]
- Green, S.; Vaughn, D.W.; Kalayanarooj, S.; Nimmannitya, S.; Suntayakorn, S.; Nisalak, A.; Lew, R.; Innis, B.L.; Kurane, I.; Rothman, A.L.; et al. Early Immune Activation in Acute Dengue Illness Is Related to Development of Plasma Leakage and Disease Severity. J. Infect. Dis. 1999. [Google Scholar] [CrossRef]
- Pichyangkul, S.; Endy, T.P.; Kalayanarooj, S.; Nisalak, A.; Yongvanitchit, K.; Green, S.; Rothman, A.L.; Ennis, F.A.; Libraty, D.H. A Blunted Blood Plasmacytoid Dendritic Cell Response to an Acute Systemic Viral Infection Is Associated with Increased Disease Severity. J. Immunol. 2003. [Google Scholar] [CrossRef]
- Torres, S.; Hernández, J.C.; Giraldo, D.; Arboleda, M.; Rojas, M.; Smit, J.M.; Urcuqui-Inchima, S. Differential Expression of Toll-like Receptors in Dendritic Cells of Patients with Dengue during Early and Late Acute Phases of the Disease. PLoS Negl. Trop. Dis. 2013. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Celluzzi, C.M.; Marovich, M.; Subramanian, H.; Eller, M.; Widjaja, S.; Palmer, D.; Porter, K.; Sun, W.; Burgess, T. CD40 Ligand Enhances Dengue Viral Infection of Dendritic Cells: A Possible Mechanism for T Cell-Mediated Immunopathology. J. Immunol. 2006. [Google Scholar] [CrossRef]
- Dung, N.T.; Duyen, H.T.; Thuy, N.T.; Ngoc, T.V.; Chau, N.V.; Hien, T.T.; Rowland-Jones, S.L.; Dong, T.; Farrar, J.; Wills, B.; et al. Timing of CD8+ T cell responses in relation to commencement of capillary leakage in children with dengue. J. Immunol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.N.B.; Quyen, N.T.H.; Thuy, T.T.; Tuan, N.M.; Hoang, D.M.; Dung, N.T.P.; Lien, L.B.; Quy, N.T.; Hieu, N.T.; Hieu, L.T.M.; et al. Dengue in Vietnamese Infants—Results of Infection-Enhancement Assays Correlate with Age-Related Disease Epidemiology, and Cellular Immune Responses Correlate with Disease Severity. J. Infect. Dis. 2008. [Google Scholar] [CrossRef] [PubMed]
- Perdomo-Celis, F.; Salgado, D.M.; Narváez, C.F. Selective dysfunction of subsets of peripheral blood mononuclear cells during pediatric dengue and its relationship with clinical outcome. Virology 2017. [Google Scholar] [CrossRef] [PubMed]
- Rivino, L.; Kumaran, E.A.; Thein, T.L.; Too, C.T.; Gan, V.C.H.; Hanson, B.J.; Wilder-Smith, A.; Bertoletti, A.; Gascoigne, N.R.J.; Lye, D.C.; et al. Virus-specific T lymphocytes home to the skin during natural dengue infection. Sci. Transl. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Elong Ngono, A.; Chen, H.W.; Tang, W.W.; Joo, Y.; King, K.; Weiskopf, D.; Sidney, J.; Sette, A.; Shresta, S. Protective Role of Cross-Reactive CD8 T Cells Against Dengue Virus Infection. EBioMedicine 2016. [Google Scholar] [CrossRef] [PubMed]
- Malavige, G.N.; McGowan, S.; Atukorale, V.; Salimi, M.; Peelawatta, M.; Fernando, N.; Jayaratne, S.D.; Ogg, G. Identification of serotype-specific T cell responses to highly conserved regions of the dengue viruses. Clin. Exp. Immunol. 2012. [Google Scholar] [CrossRef]
- Hatch, S.; Endy, T.P.; Thomas, S.; Mathew, A.; Potts, J.; Pazoles, P.; Libraty, D.H.; Gibbons, R.; Rothman, A.L. Intracellular cytokine production by dengue virus-specific T cells correlates with subclinical secondary infection. J. Infect. Dis. 2011. [Google Scholar] [CrossRef]
- Weiskopf, D.; Angelo, M.A.; de Azeredo, E.L.; Sidney, J.; Greenbaum, J.A.; Fernando, A.N.; Broadwater, A.; Kolla, R.V.; De Silva, A.D.; de Silva, A.M.; et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc. Natl. Acad. Sci. USA 2013. [Google Scholar] [CrossRef]
- Wijeratne, D.T.; Fernando, S.; Gomes, L.; Jeewandara, C.; Ginneliya, A.; Samarasekara, S.; Wijewickrama, A.; Hardman, C.S.; Ogg, G.S.; Malavige, G.N. Quantification of dengue virus specific T cell responses and correlation with viral load and clinical disease severity in acute dengue infection. PLoS Negl. Trop. Dis. 2018, 12, e0006540. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Bangs, D.J.; Sidney, J.; Kolla, R.V.; De Silva, A.D.; de Silva, A.M.; Crotty, S.; Peters, B.; Sette, A. Dengue virus infection elicits highly polarized CX3CR1 + cytotoxic CD4 + T cells associated with protective immunity. Proc. Natl. Acad. Sci. USA 2015. [Google Scholar] [CrossRef]
- Yauch, L.E.; Prestwood, T.R.; May, M.M.; Morar, M.M.; Zellweger, R.M.; Peters, B.; Sette, A.; Shresta, S. CD4+ T cells are not required for the induction of dengue virus-specific CD8+ T cell or antibody responses but contribute to protection after vaccination. J. Immunol. 2010. [Google Scholar] [CrossRef] [PubMed]
- LaFleur, C.; Granados, J.; Vargas-Alarcon, G.; Ruíz-Morales, J.; Villarreal-Garza, C.; Higuera, L.; Hernández-Pacheco, G.; Cutiño-Moguel, T.; Rangel, H.; Figueroa, R.; et al. HLA-DR antigen frequencies in Mexican patients with dengue virus infection: HLA-DR4 as a possible genetic resistance factor for dengue hemorrhagic fever. Hum. Immunol. 2002. [Google Scholar] [CrossRef]
- Sierra, B.; Alegre, R.; Perez, A.B.; Garcia, G.; Sturn-Ramirez, K.; Obasanjo, O.; Aguirre, E.; Alvarez, M.; Rodriguez-Roche, R.; Valdes, L.; et al. HLA-A, -B, -C, and -DRB1 allele frequencies in Cuban individuals with antecedents of dengue 2 disease: Advantages of the Cuban population for HLA studies of dengue virus infection. Hum. Immunol. 2007, 68, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Angelo, M.A.; Lopez, B.; O’Rourke, P.H.; Sidney, J.; Cerpas, C.; Balmaseda, A.; Silveira, C.G.T.; Maestri, A.; Costa, P.R.; et al. Global assessment of dengue virus-specific CD4+T Cell responses in dengue-endemic areas. Front. Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Harris, E.; Baric, R.; Coller, B.A.; Coloma, J.; Crowe, J.E.; Cummings, D.A.T.; Dean, H.; De Silva, A.; Diamond, M.S.; et al. Immune correlates of protection for dengue: State of the art and research Agenda. Vaccine 2017. [Google Scholar] [CrossRef]
- Wen, J.; Elong Ngono, A.; Angel Regla-Nava, J.; Kim, K.; Gorman, M.J.; Diamond, M.S.; Shresta, S. Dengue virus-reactive CD8+T cells mediate cross-protection against subsequent Zika virus challenge. Nat. Commun. 2017. [Google Scholar] [CrossRef]
- Regla-Nava, J.A.; Elong Ngono, A.; Viramontes, K.M.; Huynh, A.T.; Wang, Y.T.; Nguyen, A.V.T.; Salgado, R.; Mamidi, A.; Kim, K.; Diamond, M.S.; et al. Cross-reactive Dengue virus-specific CD8+T cells protect against Zika virus during pregnancy. Nat. Commun. 2018. [Google Scholar] [CrossRef]
- Wen, J.; Tang, W.W.; Sheets, N.; Ellison, J.; Sette, A.; Kim, K.; Shresta, S. Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8+T cells. Nat. Microbiol. 2017. [Google Scholar] [CrossRef]
- Saron, W.A.A.; Rathore, A.P.S.; Ting, L.; Ooi, E.E.; Low, J.; Abraham, S.N.; St. John, A.L. Flavivirus serocomplex cross-reactive immunity is protective by activating heterologous memory CD4 T cells. Sci. Adv. 2018. [Google Scholar] [CrossRef]
- Grifoni, A.; Pham, J.; Sidney, J.; O’Rourke, P.H.; Paul, S.; Peters, B.; Martini, S.R.; de Silva, A.D.; Ricciardi, M.J.; Magnani, D.M.; et al. Prior Dengue virus exposure shapes T cell immunity to Zika virus in humans. J. Virol. 2017. [Google Scholar] [CrossRef]
- Xu, X.; Vaughan, K.; Weiskopf, D.; Grifoni, A.; Diamond, M.S.; Sette, A.; Peters, B. Identifying Candidate Targets of Immune Responses in Zika Virus Based on Homology to Epitopes in Other Flavivirus Species. PLoS Curr. 2016. [Google Scholar] [CrossRef]
- Simmons, C.P.; Dong, T.; Chau, N.V.; Dung, N.T.P.; Chau, T.N.B.; Thao, L.T.T.; Dung, N.T.; Hien, T.T.; Rowland-Jones, S.; Farrar, J. Early T-Cell Responses to Dengue Virus Epitopes in Vietnamese Adults with Secondary Dengue Virus Infections. J. Virol. 2005. [Google Scholar] [CrossRef] [PubMed]
- Kurane, I.; Brinton, M.A.; Samson, A.L.; Ennis, F.A. Dengue virus-specific, human CD4+ CD8- cytotoxic T-cell clones: Multiple patterns of virus cross-reactivity recognized by NS3-specific T-cell clones. J. Virol. 1991, 65, 1823–1828. [Google Scholar] [PubMed]
- Weiskopf, D.; Cerpas, C.; Angelo, M.A.; Bangs, D.J.; Sidney, J.; Paul, S.; Peters, B.; Sanches, F.P.; Silvera, C.G.T.; Costa, P.R.; et al. Human CD8<>+<> T-Cell Responses Against the 4 Dengue Virus Serotypes Are Associated with Distinct Patterns of Protein Targets. J. Infect. Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Guy, B.; Lang, J.; Saville, M.; Jackson, N. Vaccination Against Dengue: Challenges and Current Developments. Annu. Rev. Med. 2016. [Google Scholar] [CrossRef]
- Guirakhoo, F.; Arroyo, J.; Pugachev, K.V.; Miller, C.; Zhang, Z.-X.; Weltzin, R.; Georgakopoulos, K.; Catalan, J.; Ocran, S.; Soike, K.; et al. Construction, Safety, and Immunogenicity in Nonhuman Primates of a Chimeric Yellow Fever-Dengue Virus Tetravalent Vaccine. J. Virol. 2001. [Google Scholar] [CrossRef]
- Guirakhoo, F.; Kitckener, S.; Morrison, D.; Forrat, R.; McCarthy, K.; Nichols, R.; Yoksan, S.; Duan, X.; Ermak, T.H.; Kanesa-Thasan, N.; et al. Live attenuated chimeric yellow fever dengue type 2 (ChimeriVaxTM- DEN2) vaccine: Phase I clinical trial for safety and immunogenicity—Effect of yellow fever pre-immunity in induction of cross neutralizing antibody responses to all 4 dengue serotypes. Hum. Vaccin. 2006. [Google Scholar] [CrossRef]
- Villar, L.; Dayan, G.H.; Arredondo-Garcia, J.L.; Rivera, D.M.; Cunha, R.; Deseda, C.; Reynales, H.; Costa, M.S.; Morales-Ramirez, J.O.; Carrasquilla, G.; et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N. Engl. J. Med. 2015, 372, 113–123. [Google Scholar] [CrossRef]
- van der Most, R.G.; Murali-Krishna, K.; Ahmed, R.; Strauss, J.H. Chimeric Yellow Fever/Dengue Virus as a Candidate Dengue Vaccine: Quantitation of the Dengue Virus-Specific CD8 T-Cell Response. J. Virol. 2000, 74, 8094–9101. [Google Scholar] [CrossRef] [PubMed]
- Guy, B.; Nougarede, N.; Begue, S.; Sanchez, V.; Souag, N.; Carre, M.; Chambonneau, L.; Morrisson, D.N.; Shaw, D.; Qiao, M.; et al. Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine 2008. [Google Scholar] [CrossRef] [PubMed]
- Hadinegoro, S.R.; Arredondo-García, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Hj Muhammad Ismail, H.I.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N. Engl. J. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Angelo, M.A.; Grifoni, A.; O’Rourke, P.H.; Sidney, J.; Paul, S.; Peters, B.; de Silva, A.D.; Phillips, E.; Mallal, S.; Diehl, S.A.; et al. Human CD4 + T Cell Responses to an Attenuated Tetravalent Dengue Vaccine Parallel Those Induced by Natural Infection in Magnitude, HLA Restriction, and Antigen Specificity. J. Virol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Angelo, M.A.; Bangs, D.J.; Sidney, J.; Paul, S.; Peters, B.; de Silva, A.D.; Lindow, J.C.; Diehl, S.A.; Whitehead, S.; et al. The Human CD8 + T Cell Responses Induced by a Live Attenuated Tetravalent Dengue Vaccine Are Directed against Highly Conserved Epitopes. J. Virol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Mangada, M.M.; Ennis, F.A.; Rothman, A.L. Quantitation of dengue virus specific CD4+ T cells by intracellular cytokine staining. J. Immunol. Methods 2004. [Google Scholar] [CrossRef]
- Alexander-Miller, M.A.; Leggatt, G.R.; Berzofsky, J.A. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc. Natl. Acad. Sci. USA 1996. [Google Scholar] [CrossRef]
- Slifka, M.K.; Whitton, J.L. Functional avidity maturation of CD8+ T cells without selection of higher affinity TCR. Nat. Immunol. 2001. [Google Scholar] [CrossRef]
- Sun, P.; García, J.; Comach, G.; Vahey, M.T.; Wang, Z.; Forshey, B.M.; Morrison, A.C.; Sierra, G.; Bazan, I.; Rocha, C.; et al. Sequential Waves of Gene Expression in Patients with Clinically Defined Dengue Illnesses Reveal Subtle Disease Phases and Predict Disease Severity. PLoS Negl. Trop. Dis. 2013. [Google Scholar] [CrossRef]
- Dong, T.; Moran, E.; Vinh Chau, N.; Simmons, C.; Luhn, K.; Peng, Y.; Wills, B.; Phuong Dung, N.; Thi Thu Thao, L.; Hien, T.T.; et al. High pro-inflammatory cytokine secretion and loss of high avidity cross-reactive cytotoxic T-cells during the course of secondary dengue virus infection. PLoS ONE 2007. [Google Scholar] [CrossRef]
- Mongkolsapaya, J.; Duangchinda, T.; Dejnirattisai, W.; Vasanawathana, S.; Avirutnan, P.; Jairungsri, A.; Khemnu, N.; Tangthawornchaikul, N.; Chotiyarnwong, P.; Sae-Jang, K.; et al. T Cell Responses in Dengue Hemorrhagic Fever: Are Cross-Reactive T Cells Suboptimal? J. Immunol. 2006. [Google Scholar] [CrossRef]
- Mangada, M.; Endy, T.; Nisalak, A.; Chunsuttiwat, S.; Vaughn, D.; Libraty, D.; Green, S.; Ennis, F.; Rothman, A. Dengue-specific T cell responses in peripheral blood mononuclear cells obtained prior to secondary dengue virus infections in Thai schoolchildren. J. Infect. Dis. 2002. [Google Scholar] [CrossRef] [PubMed]
| Feature | Viral Hemorrhagic Fever Families | References | |||
|---|---|---|---|---|---|
| Arenaviridae LASV | Filoviridae EBOV | Hantaviridae Hantaviruses | Flaviviridae DENV | ||
| Most prominent T-cell role | More protective than pathogenic | More protective than pathogenic | More protective than pathogenic | More pathogenic than protective | [33,43,45,46] |
| T-cell immunodominant viral protein | Nucleoprotean glycoprotein | Nucleoprotein | Nucleocapsid protein; glycoprotein | Non-structural proteins | [45,47,48,49] |
| Reported effector protective mechanisms | IFN-γ, TNF-α, IL-2 | IFN-γ, TNF-α, production | IFN-γ, TNF-α, production cytotoxicity | TNF-γ, production cytotoxicity | [33,46,50,51] |
| Apoptosis of T-cells | Yes | High | Yes | Yes, particularly of antigen-specific cells | [52,53,54,55] |
| Defective T-cell activation or dysfunction | Low activation capacity by antigen-presenting cells; low proliferative and cytokine T-cell response | Low activation capacity by antigen-presenting cells | Not reported Hantavirus nucleocapsid protein inhibits granzyme B-mediated apoptosis | Low activation capacity by infected antigen-presenting cells | [56,57,58] |
| Molecule | Function | T-Cell Subset Marker |
|---|---|---|
| HLA-DR | Class II major histocompatibility complex molecule | Effector cells |
| CD38 | ADP-ribosyl cyclase ectoenzyme | Effector cells |
| Ki-67 | Nuclear protein expressed during active phases of cell cycle and associated with cell proliferative activity | Effector cells |
| B-cell lymphoma (Bcl)-2 | Anti-apoptotic protein that prevents the release of cytochrome C and oxygen reactive species from mitochondria | Naïve and central memory cells |
| CCR7 | Chemokine receptor for the CCL19 and CCL21 chemokines. Involved in the homing of T-cells to secondary lymphoid organs | Naïve and central memory cells |
| CD45RA | Phosphatase involved in T-cell receptor signal transduction | Naïve and central memory cells and terminally-differentiated cells |
| CD45RO | Phosphatase involved in T-cell receptor signal transduction | Memory cells |
| CD28 | Receptor for costimulatory molecules, involved in the amplificationof T-cell receptor signalir | Naïve and memory cells, decreasing cell differentiation |
| CD127 | Alpha chain of the II-7-receptor; IL-7 supports the survival of mature T-cells | Naïve and memory cells, decreasing along cell differentiation |
| Programmed death 1 protein (PD-1) | Regulatory receptor that inhibits T-cell receptor signaling | Memory cells |
| Perforin/Granzyme B | Cytotoxic molecules induce apoptosis intarget cells | Effector cells |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perdomo-Celis, F.; Salvato, M.S.; Medina-Moreno, S.; Zapata, J.C. T-Cell Response to Viral Hemorrhagic Fevers. Vaccines 2019, 7, 11. https://doi.org/10.3390/vaccines7010011
Perdomo-Celis F, Salvato MS, Medina-Moreno S, Zapata JC. T-Cell Response to Viral Hemorrhagic Fevers. Vaccines. 2019; 7(1):11. https://doi.org/10.3390/vaccines7010011
Chicago/Turabian StylePerdomo-Celis, Federico, Maria S. Salvato, Sandra Medina-Moreno, and Juan C. Zapata. 2019. "T-Cell Response to Viral Hemorrhagic Fevers" Vaccines 7, no. 1: 11. https://doi.org/10.3390/vaccines7010011
APA StylePerdomo-Celis, F., Salvato, M. S., Medina-Moreno, S., & Zapata, J. C. (2019). T-Cell Response to Viral Hemorrhagic Fevers. Vaccines, 7(1), 11. https://doi.org/10.3390/vaccines7010011






