Novel Whole-Cell Inactivated Neisseria Gonorrhoeae Microparticles as Vaccine Formulation in Microneedle-Based Transdermal Immunization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the N. gonorrhoeae Vaccine Antigen
2.2. Preparation of the N. gonorrhoeae Vaccine Loaded Microparticles
2.3. Microparticle Recovery Yield
2.4. Particle Size Distribution
2.5. Zeta Potential Measurement
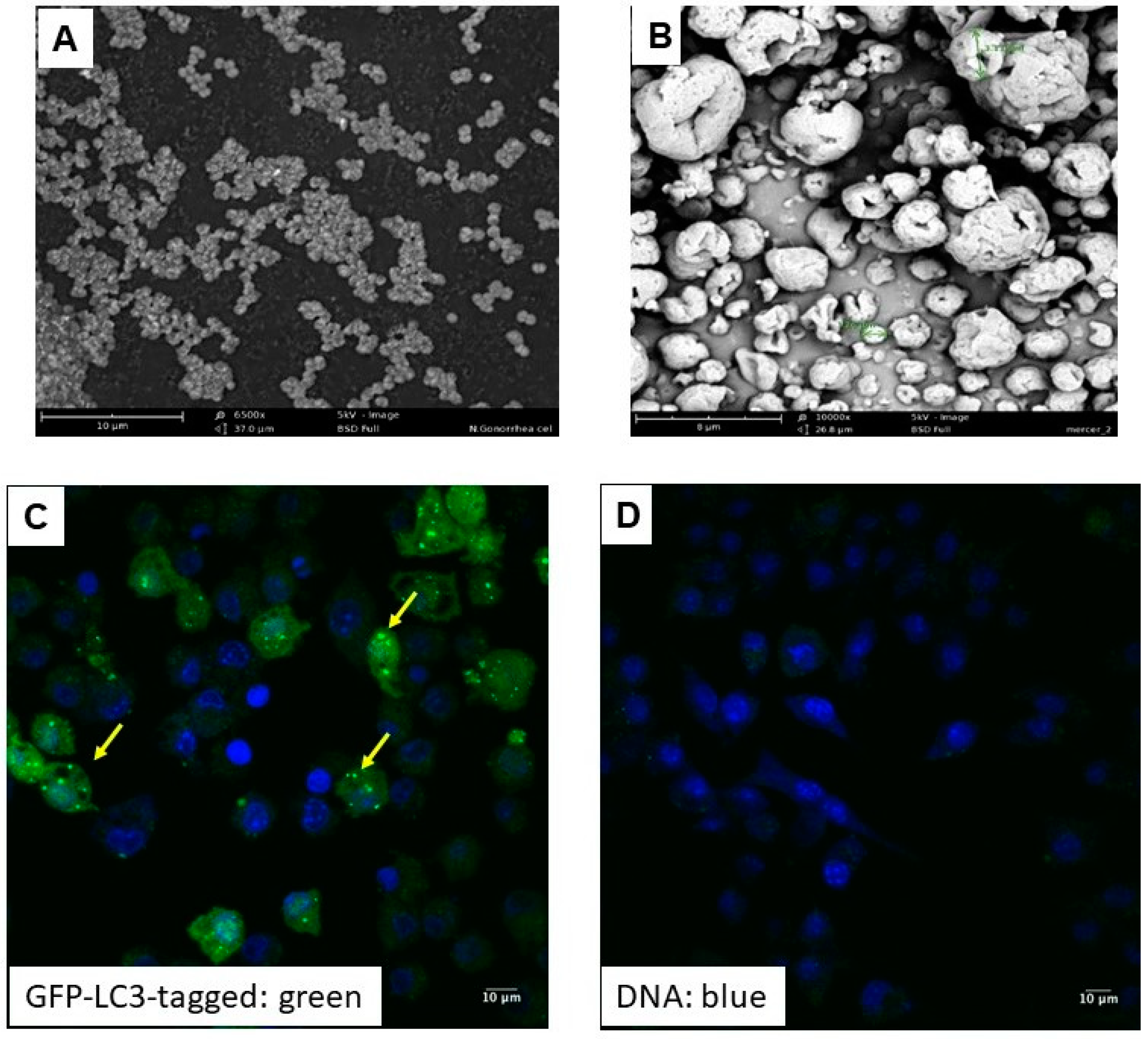
2.6. Scanning Electron Microscopy of the Microparticles
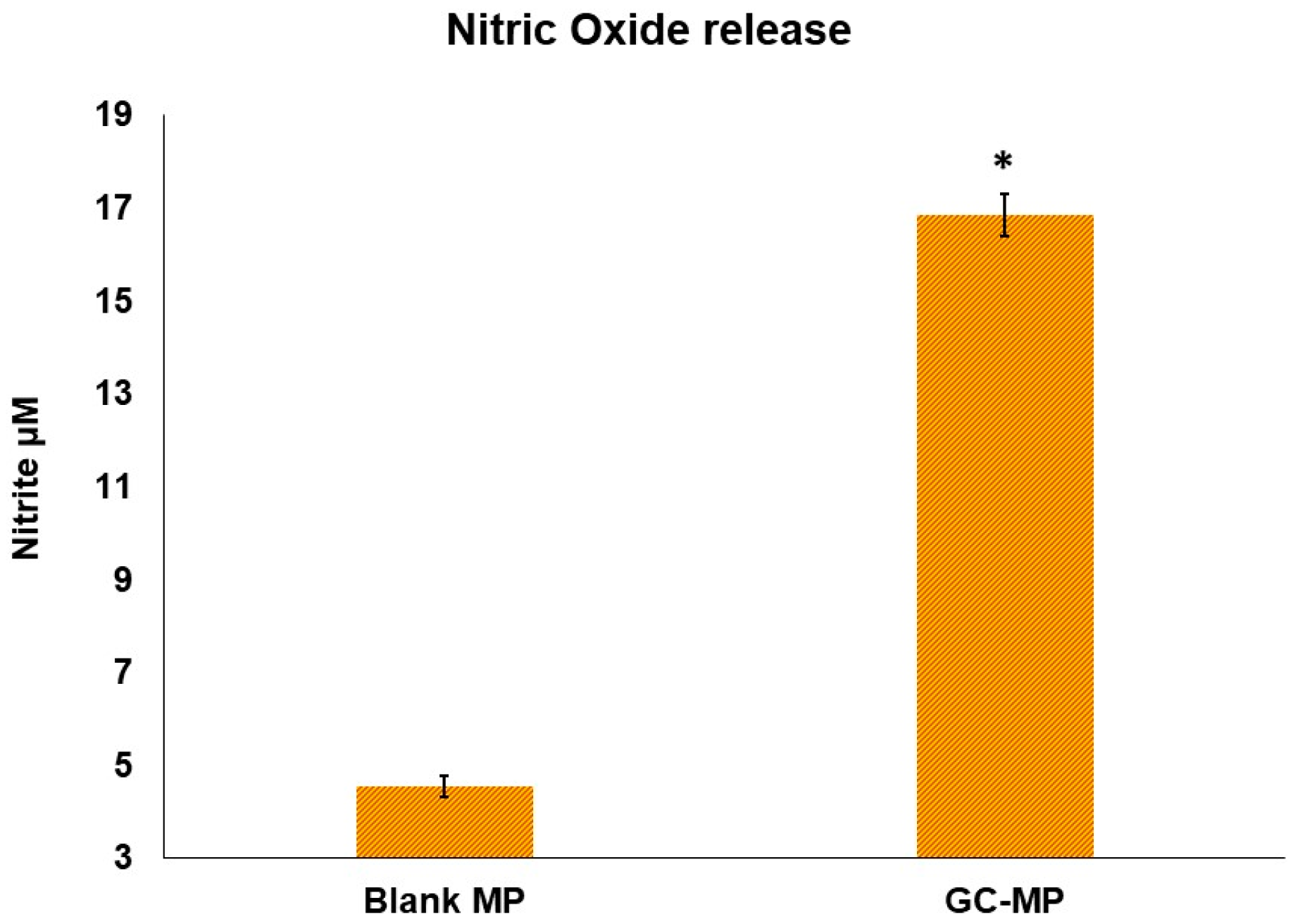
2.7. Nitric Oxide Release from Dendritic Cells DC2.4 Cell Line
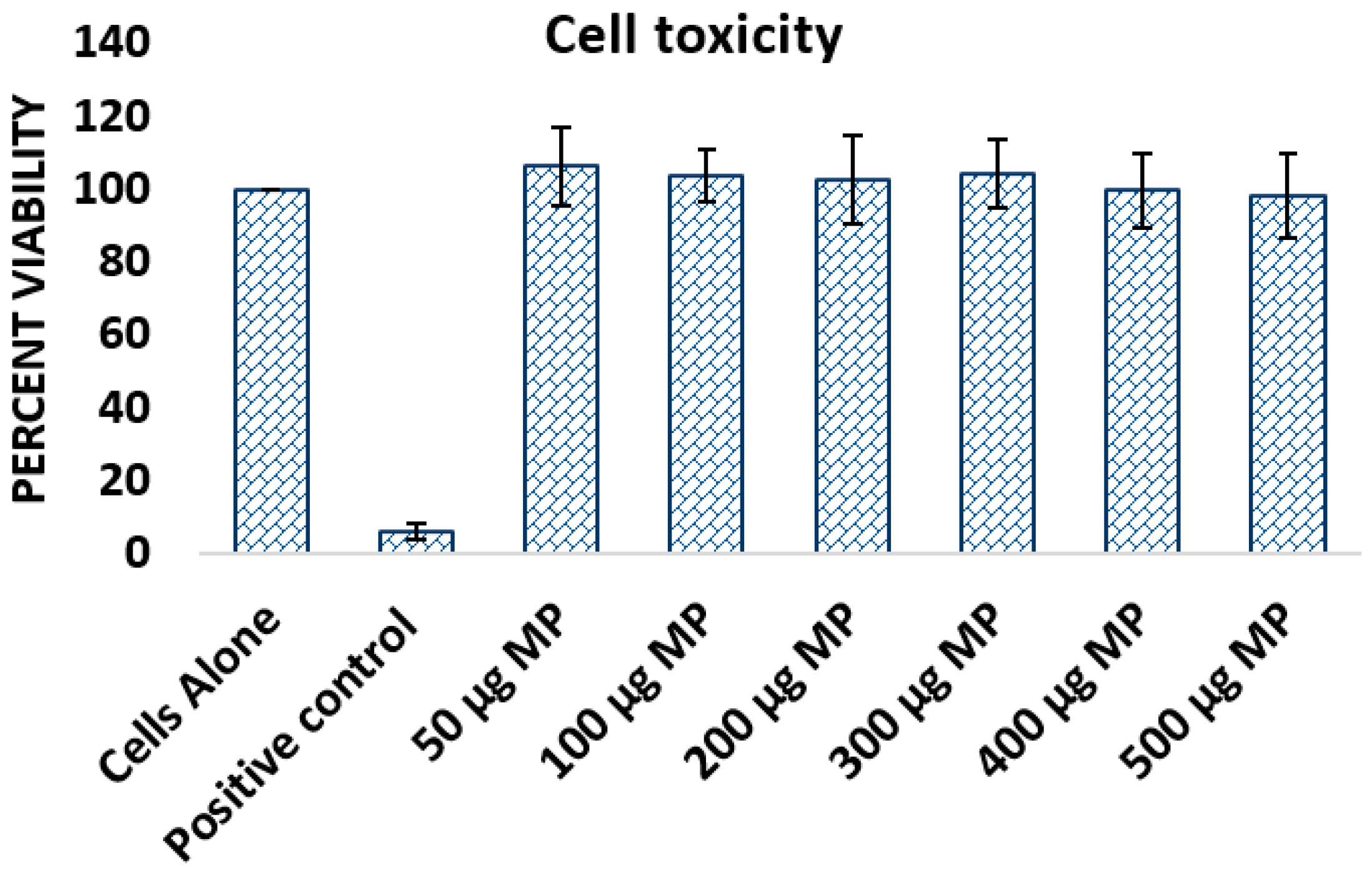
2.8. Cytotoxicity Study
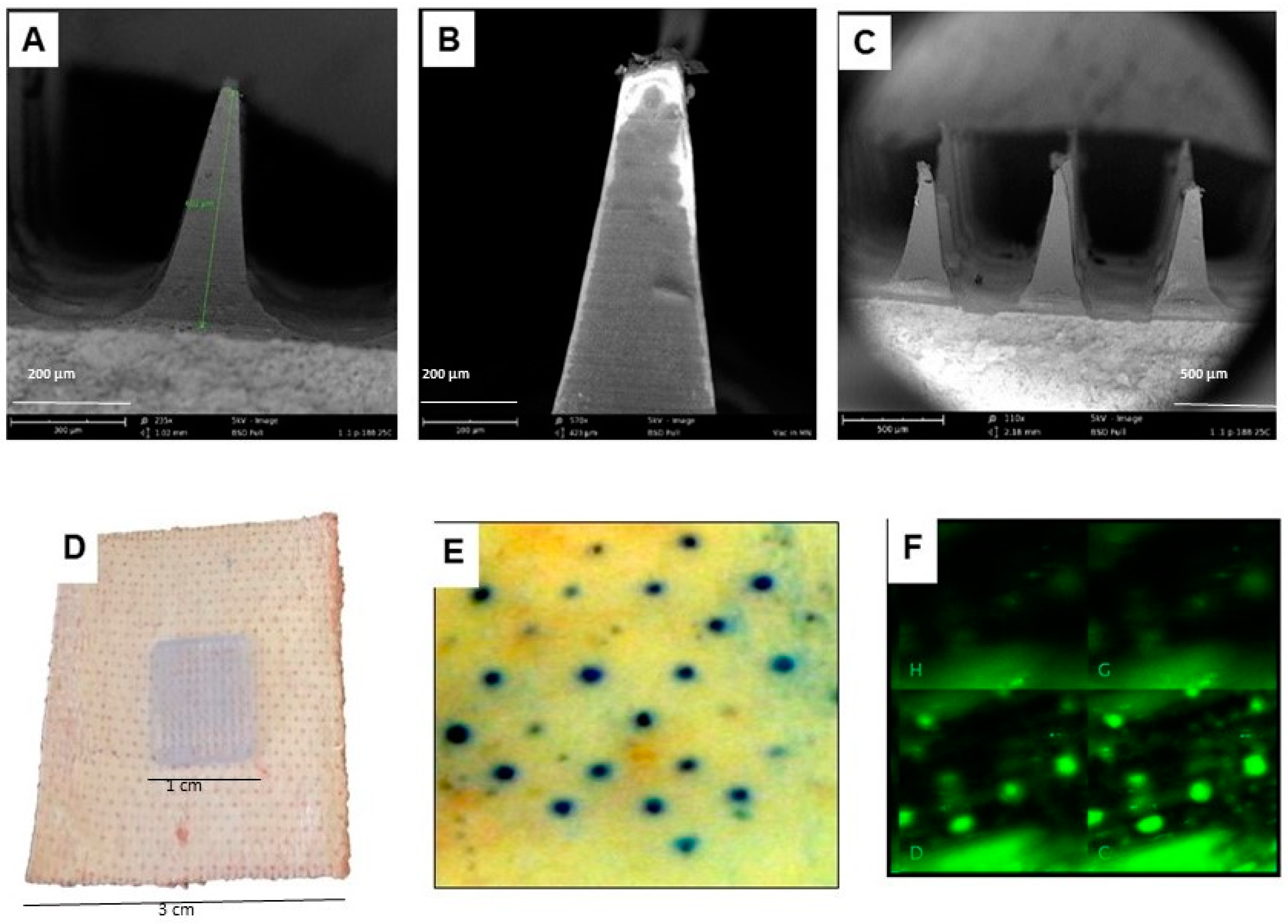
2.9. Formulation of Dissolvable Microneedles for Transdermal Delivery of Vaccine Particles
2.10. Mice Transdermal Immunization Using Dissolvable Microneedles
2.11. Quantification of Vaccine-Specific Serum Antibody Using ELISA
2.12. Determination of T-Cell and B-Cell-Based Immune Response in Lymphatic Organs
2.13. Statistical Analysis
3. Results
3.1. Characterization of Whole-Cell Formalin Fixed N. gonorrhoeae Microparticle Vaccine Loaded in Dissolvable Microneedles
3.1.1. Physical Characterization of N. gonorrhoeae Microparticle Vaccine
3.1.2. Cytotoxicity Study
3.1.3. Characterization of the Dissolvable Microneedles
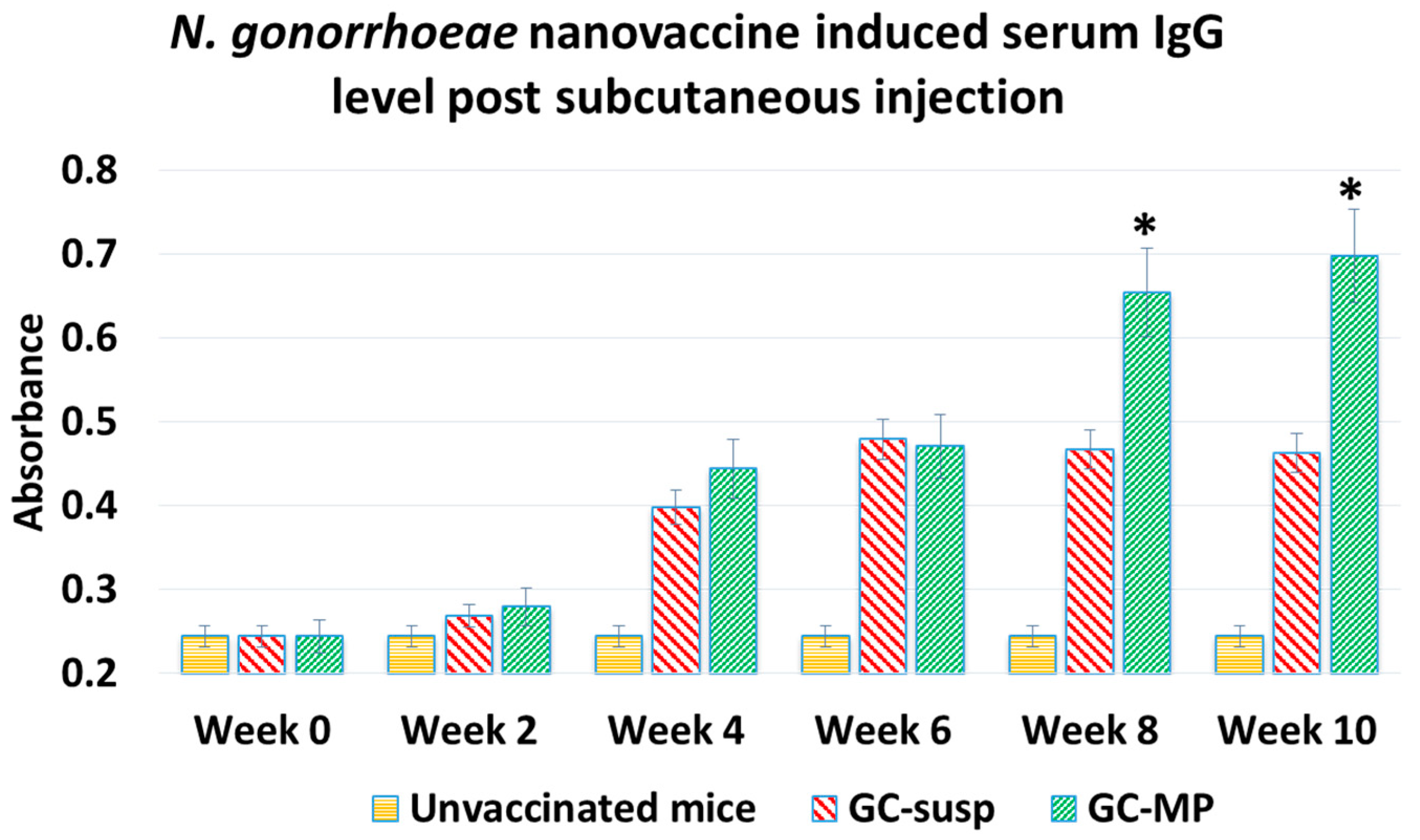
3.2. Immunogenicity of Whole-Cell N. gonorrhoeae Nanovaccine Administered via Subcutaneous Immunization in Mice
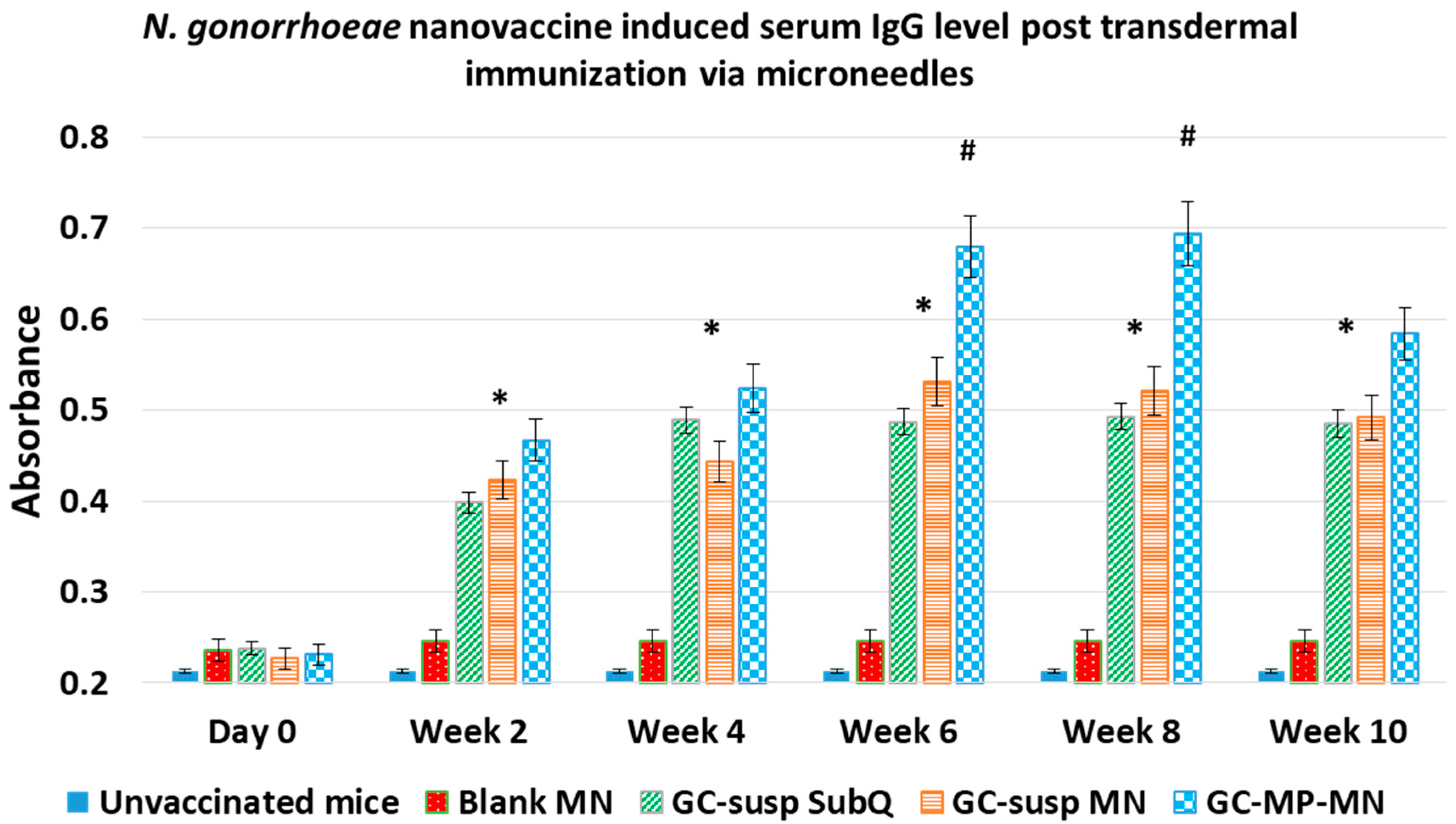
3.3. Mice Immunization Study Using Microneedles for Transdermal Vaccine Delivery
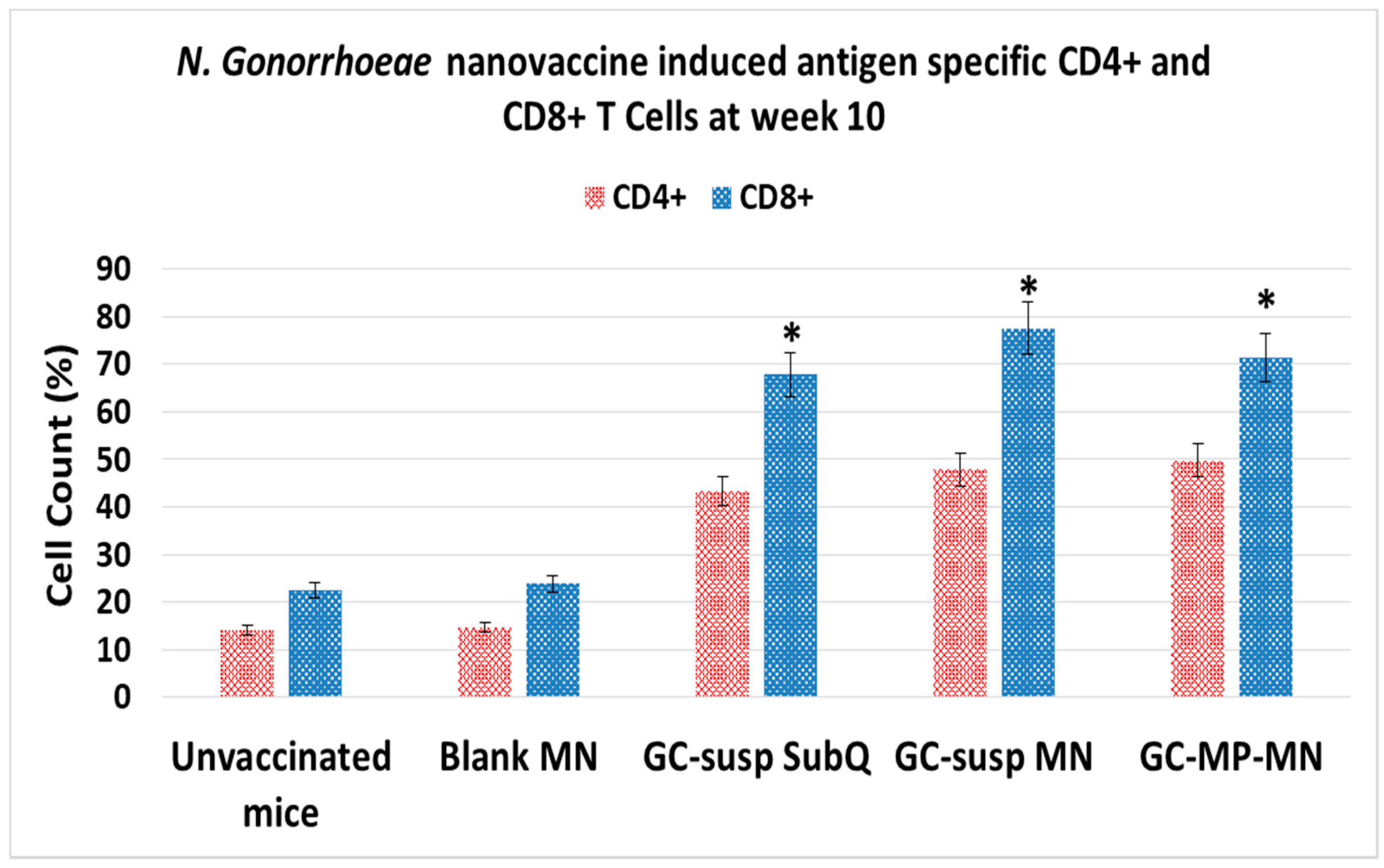
3.4. Induction of Antigen-Specific CD4 and CD8 T Lymphocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antibiotic Resistance Threats in the United States. 2013. Available online: https://www.cdc.gov/drugresistance/threat-report-2013/index.html (accessed on 19 October 2016).
- Kirkcaldy, R.D.; Harvey, A.; Papp, J.R.; del Rio, C.; Soge, O.O.; Holmes, K.K.; Hook, E.W., III; Kubin, G.; Riedel, S.; Zenilman, J.; et al. Neisseria gonorrhoeae antimicrobial susceptibility surveillance—The gonococcal isolate surveillance project, 27 sites, United States, 2014. MMWR Surveill. Summ. 2016, 65, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zughaier, S.M.; Kandler, J.L.; Balthazar, J.T.; Shafer, W.M. Phosphoethanolamine modification of Neisseria gonorrhoeae lipid a reduces autophagy flux in macrophages. PLoS ONE 2015, 10, e0144347. [Google Scholar] [CrossRef] [PubMed]
- Zughaier, S.M.; Kandler, J.L.; Shafer, W.M. Neisseria gonorrhoeae modulates iron-limiting innate immune defenses in macrophages. PLoS ONE 2014, 9, e87688. [Google Scholar] [CrossRef] [PubMed]
- STD Rates Reach Record High in United States. Available online: http://www.cnn.com/2016/10/20/health/std-statistics-record-high/index.html (accessed on 20 October 2016).
- Ison, C.A.; Deal, C.; Unemo, M. Current and future treatment options for gonorrhoea. Sex. Transm. Infect. 2013. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.L.; Jennings, M.P.; Apicella, M.A.; Seib, K.L. Is gonococcal disease preventable? The importance of understanding immunity and pathogenesis in vaccine development. Crit. Rev. Microbiol. 2016, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Del Rio, C.; Shafer, W.M. Antimicrobial resistance expressed by Neisseria gonorrhoeae: A major global public health problem in the 21st century. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Jerse, A.E.; Bash, M.C.; Russell, M.W. Vaccines against gonorrhea: Current status and future challenges. Vaccine 2014, 32, 1579–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetzler, L.M.; Feavers, I.M.; Gray-Owen, S.D.; Jerse, A.E.; Rice, P.A.; Deal, C.D. Summary and recommendations from the national institute of allergy and infectious diseases (NIAID) workshop “gonorrhea vaccines: The way forward”. Clin. Vaccine Immunol. CVI 2016, 23, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, S.L.; Deal, C.D.; Giersing, B.; Rees, H.; Bolan, G.; Johnston, C.; Timms, P.; Gray-Owen, S.D.; Jerse, A.E.; Cameron, C.E.; et al. The global roadmap for advancing development of vaccines against sexually transmitted infections: Update and next steps. Vaccine 2016, 34, 2939–2947. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, L. Field trials of a gonococcal vaccine. J. Reprod. Med. 1975, 14, 34–36. [Google Scholar] [PubMed]
- Greenberg, L.; Diena, B.B.; Ashton, F.A.; Wallace, R.; Kenny, C.P.; Znamirowski, R.; Ferrari, H.; Atkinson, J. Gonococcal vaccine studies in Inuvik. Can. J. Public Health 1974, 65, 29–33. [Google Scholar] [PubMed]
- Zhu, W.; Chen, C.-J.; Thomas, C.E.; Anderson, J.E.; Jerse, A.E.; Sparling, P.F. Vaccines for gonorrhea: Can We Rise to the Challenge? Front. Microbiol. 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ventevogel, M.S.; Knilans, K.J.; Anderson, J.E.; Oldach, L.M.; McKinnon, K.P.; Hobbs, M.M.; Sempowski, G.D.; Duncan, J.A. Neisseria gonorrhoeae suppresses dendritic cell-induced, antigen-dependent CD4 T Cell proliferation. PLoS ONE 2012, 7, e41260. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.A.; Gao, X.; Huang, M.T.-H.; O’Connor, B.P.; Thomas, C.E.; Willingham, S.B.; Bergstralh, D.T.; Jarvis, G.A.; Sparling, P.F.; Ting, J.P. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J. Immunol. 2009, 182, 6460–6469. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Islam, E.A.; Jarvis, G.A.; Gray-Owen, S.D.; Russell, M.W. Neisseria gonorrhoeae selectively suppresses the development of Th1 and Th2 cells, and enhances Th17 cell responses, through TGF-β-dependent mechanisms. Mucosal. Immunol. 2012, 5, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Feinen, B.; Jerse, A.E.; Gaffen, S.L.; Russell, M.W. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal Immunol. 2010, 3, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Shafer, W.M.; Onunka, V.C.; Jannoun, M.; Huthwaite, L.W. Molecular mechanism for the antigonococcal action of lysosomal cathepsin G. Mol. Microbiol. 1990, 4, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Shastri, P.N.; Kim, M.-C.; Quan, F.-S.; D’Souza, M.J.; Kang, S.-M. Immunogenicity and protection of oral influenza vaccines formulated into microparticles. J. Pharm. Sci. 2012, 101, 3623–3635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ubale, R.V.; D’Souza, M.J.; Infield, D.T.; McCarty, N.A.; Zughaier, S.M. Formulation of meningococcal capsular polysaccharide vaccine-loaded microparticles with robust innate immune recognition. J. Microencapsul. 2013, 30, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Bejugam, N.K.; Uddin, A.N.; Gayakwad, S.G.; D’Souza, M.J. Formulation and evaluation of albumin microspheres and its enteric coating using a spray-dryer. J. Microencapsul. 2008, 25, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Salnikova, M. Novel Approaches and Strategies for Biologics, Vaccines and Cancer Therapies; Academic Press: Cambridge, MA, USA, 2014; 520p. [Google Scholar]
- Ubale, R.V.; Gala, R.P.; Zughaier, S.M.; D’Souza, M.J. Induction of death receptor CD95 and co-stimulatory molecules CD80 and CD86 by meningococcal capsular polysaccharide-loaded vaccine nanoparticles. AAPS J. 2014, 16, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Uddin, A.N.; Bejugam, N.K.; Gayakwad, S.G.; Akther, P.; D’Souza, M.J. Oral delivery of gastro-resistant microencapsulated typhoid vaccine. J. Drug Target. 2009, 17, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Bejugam, N.K.; Gayakwad, S.G.; Uddin, A.N.; D’Souza, M.J. Microencapsulation of protein into biodegradable matrix: A smart solution cross-linking technique. J. Microencapsul. 2013, 30, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Chablani, L.; Tawde, S.A.; D’Souza, M.J. Spray-dried microparticles: A potential vehicle for oral delivery of vaccines. J. Microencapsul. 2012, 29, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Tawde, S.A.; Chablani, L.; Akalkotkar, A.; D’Souza, C.; Chiriva-Internati, M.; Selvaraj, P.; D’Souza, M.J. Formulation and evaluation of oral microparticulate ovarian cancer vaccines. Vaccine 2012, 30, 5675–5681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshmi Kolluru, P.; Gala, R.P.; D’Souza, M.J. Design of Experiments: A Valuable “Quality by Design” tool in formulation development. In Nanoparticulate Vaccine Delivery Systems, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015; p. 195. [Google Scholar]
- Gala, R.P.; Khan, I.; Elhissi, A.M.A.; Alhnan, M.A. A comprehensive production method of self-cryoprotected nano-liposome powders. Int. J. Pharm. 2015, 486, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Gala, R.P.; Ubale, R.V.; D’Souza, M.J.; Zughaier, S.M. Development of Non-Conjugated Meningitis Particulate vaccine. In Nanoparticulate Vaccine Delivery Systems, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015; p. 127. [Google Scholar]
- Zughaier, S.M.; Tzeng, Y.-L.; Zimmer, S.M.; Datta, A.; Carlson, R.W.; Stephens, D.S. Neisseria meningitidis lipooligosaccharide structure-dependent activation of the macrophage CD14/Toll-like receptor 4 pathway. Infect. Immun. 2004, 72, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Luo, H.; Lu, W.; Luan, H.; Wu, Y.; Luo, J.; Wang, Y.; Pi, J.; Lim, C.Y.; Wang, H. Rapidly dissolvable microneedle patches for transdermal delivery of exenatide. Pharm. Res. 2014, 31, 3348–3360. [Google Scholar] [CrossRef] [PubMed]
- Plans, P.; de Ory, F.; Campins, M.; Álvarez, E.; Payà, T.; Guisasola, E.; Compte, C.; Vellbé, K.; Sánchez, C.; Lozano, M.J.; et al. Prevalence of anti-rubella, anti-measles and anti-mumps IgG antibodies in neonates and pregnant women in Catalonia (Spain) in 2013: Susceptibility to measles increased from 2003 to 2013. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Singh, V.; Jurney, P.; Shi, L.; Sreenivasan, S.V.; Roy, K. Scalable imprinting of shape-specific polymeric nanocarriers using a release layer of switchable water solubility. ACS Nano 2012, 6, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Roy, K. Intracellular delivery of polymeric nanocarriers: A matter of size, shape, charge, elasticity and surface composition. Ther. Deliv. 2013, 4, 705–723. [Google Scholar] [CrossRef] [PubMed]
- Glangchai, L.C.; Caldorera-Moore, M.; Shi, L.; Roy, K. Nanoimprint lithography based fabrication of shape-specific, enzymatically-triggered smart nanoparticles. J. Control. Release 2008, 125, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Akalkotkar, A.; Chablani, L.; Tawde, S.A.; D’Souza, C.; D’Souza, M.J. Development of a microparticulate prostate cancer vaccine and evaluating the effect of route of administration on its efficacy via the skin. J. Microencapsul. 2015, 32, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Tawde, S.A.; Chablani, L.; Akalkotkar, A.; D’Souza, M.J. Evaluation of microparticulate ovarian cancer vaccine via transdermal route of delivery. J. Control. Release 2016, 235, 147–454. [Google Scholar] [CrossRef] [PubMed]
- Gala, R.P.; Popescu, C.; Knipp, G.T.; McCain, R.R.; Ubale, R.V.; Addo, R.; Bhowmik, T.; Kulczar, C.D.; D’Souza, M.J. Physicochemical and preclinical evaluation of a novel buccal measles vaccine. AAPS PharmSciTech. 2016, 18, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Chablani, L.; Tawde, S.A.; Akalkotkar, A.; D’Souza, C.; Selvaraj, P.; D’Souza, M.J. Formulation and evaluation of a particulate oral breast cancer vaccine. J. Pharm. Sci. 2012, 101, 3661–3671. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Marciani, D.; Piemonte, P.; Frascione, P. Topical anesthetics for cosmetic dermatologic procedures. G. Ital. Dermatol. Venereol. 2013, 148, 465–469. [Google Scholar] [PubMed]
- Gao, H.; Pan, J.-C.; Chen, B.; Xue, Z.-F.; Li, H.-D. The effect of HPV16E7 DNA vaccine transdermal delivery with microneedle array. Zhonghua Yu Fang Yi Xue Za Zhi 2008, 42, 663–666. [Google Scholar] [PubMed]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaveh, D.L.; Whelan, A.O.; Hograth, P.J. The duration of antigen-stimulation significantly alters the diversity of multifunctional CD4 T Cells measured by intracellular cytokine staining. PLoS ONE 2012, 7, e38926. [Google Scholar] [CrossRef] [PubMed]
- Petousis-Harris, H.; Paynter, J.; Morgan, J.; Saxton, P.; Mc Ardle, B.; Goodyear-Smith, F.; Black, S. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: A retrospective case-control study. Lancet 2017, 390, 1603–1610. [Google Scholar] [CrossRef]
- Craig, A.P.; Gray, R.T.; Edwards, J.L.; Apicella, M.A.; Jennings, M.P.; Wilson, D.P.; Seib, K.L. The potential impact of vaccination on the prevalence of gonorrhea. Vaccine 2015, 33, 4520–4525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, M.W. Could vaccination against Neisseria gonorrhoeae be on the horizon? Future Microbiol. 2018, 13, 495–497. [Google Scholar] [CrossRef] [PubMed]
| Material | Percent w/w | 200 mg Batch |
|---|---|---|
| Vaccine microparticles | 10% | 20 mg |
| Trehalose | 25% | 50 mg |
| Maltose | 25% | 50 mg |
| PVA | 20% | 40 mg |
| HPMC | 20% | 40 mg |
| Physical Characteristics | Range | Mean ± SD |
|---|---|---|
| Recovery yield (%) | 10 | 91.56 ± 5.3 |
| Particle Size (µm) | 4 | 3.65 ± 1.89 |
| Zeta Potential (mV) | 5 | −32.65 ± 2.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gala, R.P.; Zaman, R.U.; D’Souza, M.J.; Zughaier, S.M. Novel Whole-Cell Inactivated Neisseria Gonorrhoeae Microparticles as Vaccine Formulation in Microneedle-Based Transdermal Immunization. Vaccines 2018, 6, 60. https://doi.org/10.3390/vaccines6030060
Gala RP, Zaman RU, D’Souza MJ, Zughaier SM. Novel Whole-Cell Inactivated Neisseria Gonorrhoeae Microparticles as Vaccine Formulation in Microneedle-Based Transdermal Immunization. Vaccines. 2018; 6(3):60. https://doi.org/10.3390/vaccines6030060
Chicago/Turabian StyleGala, Rikhav P., Rokon Uz Zaman, Martin J. D’Souza, and Susu M. Zughaier. 2018. "Novel Whole-Cell Inactivated Neisseria Gonorrhoeae Microparticles as Vaccine Formulation in Microneedle-Based Transdermal Immunization" Vaccines 6, no. 3: 60. https://doi.org/10.3390/vaccines6030060
APA StyleGala, R. P., Zaman, R. U., D’Souza, M. J., & Zughaier, S. M. (2018). Novel Whole-Cell Inactivated Neisseria Gonorrhoeae Microparticles as Vaccine Formulation in Microneedle-Based Transdermal Immunization. Vaccines, 6(3), 60. https://doi.org/10.3390/vaccines6030060








