The Future of Influenza Vaccines: A Historical and Clinical Perspective
Abstract
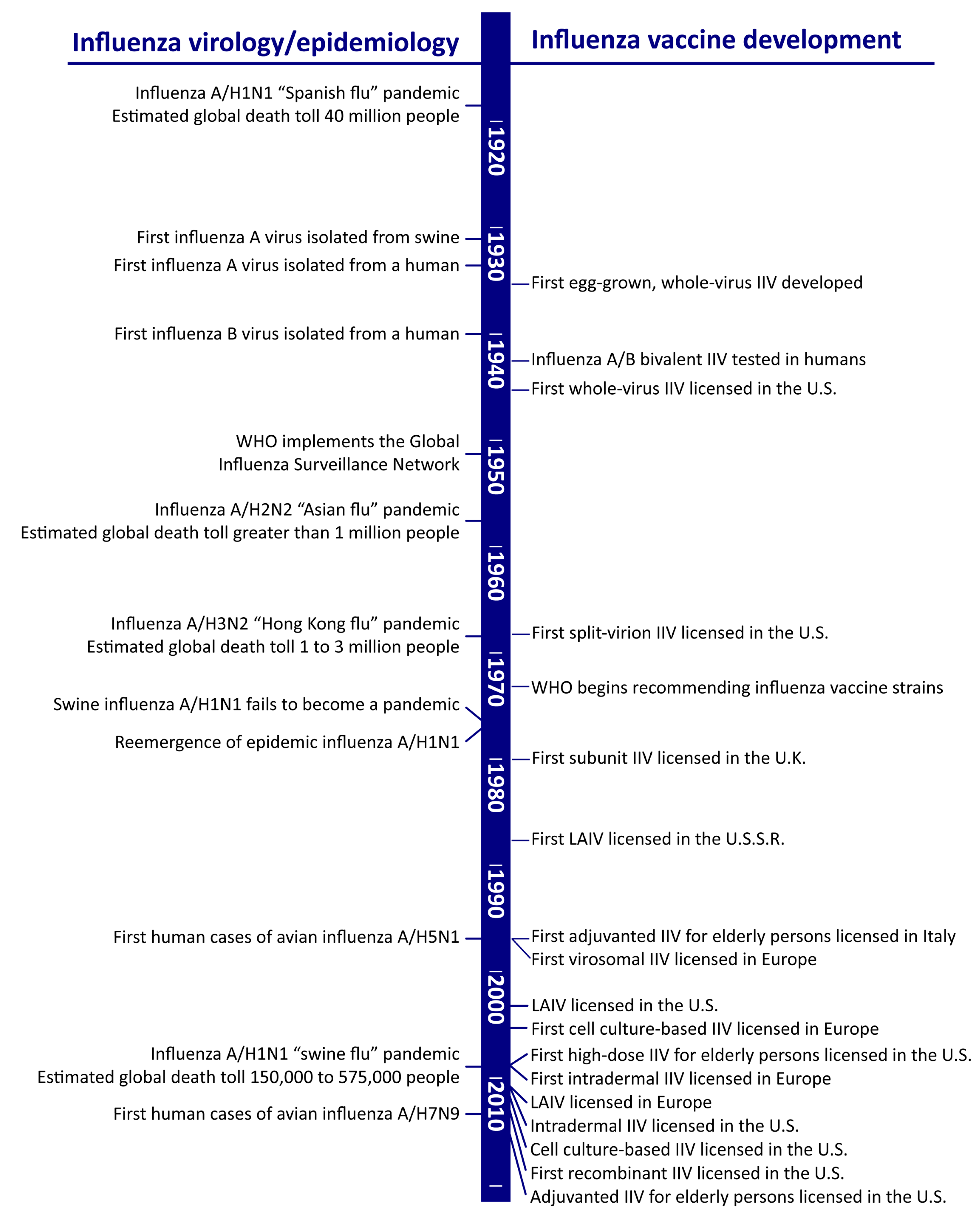
1. Introduction
2. A Vaccine That Prevents Infection
3. A Vaccine That Ameliorates Influenza Disease
4. A Vaccine That Prevents Influenza Virus Transmission
5. A Vaccine That Benefits Everyone
6. A Vaccine That is Accepted
7. Conclusions
Funding
Conflicts of Interest
References
- Grohskopf, L.A.; Sokolow, L.Z.; Broder, K.R.; Walter, E.B.; Bresee, J.S.; Fry, A.M.; Jernigan, D.B. Prevention and control of seasonal influenza with vaccines: Recommendations of the advisory committee on immunization practices—United States, 2017–2018 influenza season. MMWR Recomm. Rep. 2017, 66, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Francis, T.; Salk, J.E.; Pearson, H.E.; Brown, P.N. Protective effect of vaccination against induced influenza A. J. Clin. Investig. 1945, 24, 536–546. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, E.; Rappuoli, R. From empiricism to rational design: A personal perspective of the evolution of vaccine development. Nat. Rev. Immunol. 2014, 14, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Rueckert, C.; Guzman, C.A. Vaccines: From empirical development to rational design. PLoS Pathog. 2012, 8, e1003001. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.R.; McCraw, D.M.; Torian, U.; Gulati, N.M.; Myers, M.L.; Conlon, M.T.; Harris, A.K. Characterization of hemagglutinin antigens on influenza virus and within vaccines using electron microscopy. Vaccines 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, S.; Lambe, T. Clinical Advances in Viral-Vectored Influenza Vaccines. Vaccines 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Scorza, F.B.; Pardi, N. New Kids on the Block: RNA-Based Influenza Virus Vaccines. Vaccines 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.T.; Heaton, N.S. Efforts to Improve the Seasonal Influenza Vaccine. Vaccines 2018, 6. [Google Scholar] [CrossRef]
- Petrie, J.G.; Gordon, A. Epidemiological studies to support the development of next generation influenza vaccines. Vaccines 2018, 6. [Google Scholar] [CrossRef]
- Mohn, K.G.; Zhou, F. Clinical expectations for better influenza virus vaccines-perspectives from the young investigators’ point of view. Vaccines 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Clemens, E.B.; van de Sandt, C.; Wong, S.S.; Wakim, L.M.; Valkenburg, S.A. Harnessing the power of T Cells: The promising hope for a universal influenza vaccine. Vaccines 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Thulin, N.K.; Wang, T.T. The role of FC gamma receptors in broad protection against influenza viruses. Vaccines 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Stacey, H.D.; Barjesteh, N.; Mapletoft, J.P.; Miller, M.S. “Gnothi Seauton”: Leveraging the host response to improve influenza virus vaccine efficacy. Vaccines 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Center for Infectious Disease Research and Policy (CIDRAP). FDA Approves Sanofi’s Intradermal Flu Vaccine. Available online: http://www.cidrap.umn.edu/news-perspective/2011/05/fda-approves-sanofis-intradermal-flu-vaccine (accessed on 15 August 2018).
- Crovari, P.; Alberti, M.; Alicino, C. History and evolution of influenza vaccines. J. Prev. Med. Hyg. 2011, 52, 91–94. [Google Scholar] [PubMed]
- Dawood, F.S.; Iuliano, A.D.; Reed, C.; Meltzer, M.I.; Shay, D.K.; Cheng, P.Y.; Bandaranayake, D.; Breiman, R.F.; Brooks, W.A.; Buchy, P.; et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: A modelling study. Lancet Infect. Dis. 2012, 12, 687–695. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R. Inactivated and adjuvanted influenza vaccines. Curr. Top. Microbiol. Immunol. 2015, 386, 151–180. [Google Scholar] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Types of Seasonal Influenza Vaccine. Available online: https://ecdc.europa.eu/en/seasonal-influenza/prevention-and-control/vaccines/types-of-seasonal-influenza-vaccine (accessed on 15 August 2018).
- Hannoun, C. The evolving history of influenza viruses and influenza vaccines. Expert Rev. Vaccines 2013, 12, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Manini, I.; Trombetta, C.M.; Lazzeri, G.; Pozzi, T.; Rossi, S.; Montomoli, E. Egg-Independent influenza vaccines and vaccine candidates. Vaccines 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- National Museum of American History. Smithsonian Institution, the Antibody Initiative: Chasing Influenza. Available online: http://americanhistory.si.edu/collections/object-groups/antibody-initiative/influenza (accessed on 15 August 2018).
- Rudenko, L.; Yeolekar, L.; Kiseleva, I.; Isakova-Sivak, I. Development and approval of live attenuated influenza vaccines based on Russian master donor viruses: Process challenges and success stories. Vaccine 2016, 34, 5436–5441. [Google Scholar] [CrossRef] [PubMed]
- Sencer, D.J.; Millar, J.D. Reflections on the 1976 swine flu vaccination program. Emerg. Infect. Dis. 2006, 12, 29–33. [Google Scholar] [CrossRef] [PubMed]
- The Immunization Action Coalition (IAC). Vaccine Timeline: Historic Dates and Events Related to Vaccines and Immunization. Available online: http://www.immunize.org/timeline/ (accessed on 15 August 2018).
- World Health Organization (WHO). 70 Years of Influenza Control. Available online: http://www.who.int/influenza/gip-anniversary/en/ (accessed on 15 August 2018).
- Gross, C.P.; Sepkowitz, K.A. The myth of the medical breakthrough: Smallpox, vaccination, and Jenner reconsidered. Int. J. Infect. Dis. 1998, 3, 54–60. [Google Scholar] [CrossRef]
- Read, A.F.; Mackinnon, M.J. Pathogen evolution in a vaccinated world. In Evolution in Health and Disease, 2nd ed.; Stearns, S.C., Koella, J.C., Eds.; Oxford University Press, Inc.: New York, NY, USA, 2008. [Google Scholar]
- National Museum of American History. Smithsonian Institution, the Antibody Initiative: Measles, Mumps, and Rubella Vaccines. Available online: http://americanhistory.si.edu/collections/object-groups/antibody-initiative/mmr (accessed on 3 July 2018).
- Damaso, C.R. Revisiting Jenner’s mysteries, the role of the Beaugency lymph in the evolutionary path of ancient smallpox vaccines. Lancet Infect. Dis. 2018, 18, e55–e63. [Google Scholar] [CrossRef]
- Riedel, S. Edward Jenner and the history of smallpox and vaccination. Proc. Bayl. Univ. Med. Cent. 2005, 18, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Schrick, L.; Tausch, S.H.; Dabrowski, P.W.; Damaso, C.R.; Esparza, J.; Nitsche, A. An early American smallpox vaccine based on horsepox. N. Engl. J. Med. 2017, 377, 1491–1492. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Manzoor, S.; Saalim, M.; Resham, S.; Ashraf, J.; Javed, A.; Waqar, A.B. HIV-1 and hijacking of the host immune system: The current scenario. APMIS 2016, 124, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Moir, S.; Connors, M.; Fauci, A.S. The Immunology of Human Immunodeficiency Virus Infection. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Saunders, an Imprint of Elsevier Inc.: Philadelphia, PA, USA, 2015. [Google Scholar]
- Ortega-Prieto, A.M.; Dorner, M. Immune evasion strategies during chronic hepatitis B and C virus infection. Vaccines 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Radolf, J.D.; Deka, R.K.; Anand, A.; Smajs, D.; Norgard, M.V.; Yang, X.F. Treponema pallidum, the syphilis spirochete: Making a living as a stealth pathogen. Nat. Rev. Microbiol. 2016, 14, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Janoff, E.N.; Musher, D.M. Streptococcus pneumoniae. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Saunders, an Imprint of Elsevier Inc.: Philadelphia, PA, USA, 2015. [Google Scholar]
- Murphy, T.F. Haemophilus Species, Including H. influenzae and H. ducreyi (Chancroid). In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Saunders, an Imprint of Elsevier Inc.: Philadelphia, PA, USA, 2015. [Google Scholar]
- Bonnez, W. Papillomaviruses. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Saunders, an Imprint of Elsevier Inc.: Philadelphia, PA, USA, 2015. [Google Scholar]
- Romero, J.R.; Modlin, J.F. Introduction to the Human Enteroviruses and Parechoviruses. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Saunders, an Imprint of Elsevier Inc.: Philadelphia, PA, USA, 2015. [Google Scholar]
- Thomas, S.J.; Endy, T.P.; Rothman, A.L.; Barrett, A.D. Flaviviruses (Dengue, Yellow Fever, Japanese Encephalitis, West Nile Encephalitis, St. Louis Encephalitis, Tick-Borne Encephalitis, Kyasanur Forest Disease, Alkhurma Hemorrhagic Fever, Zika). In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Saunders, an imprint of Elsevier Inc.: Philadelphia, PA, USA, 2015. [Google Scholar]
- Heaton, N.S.; Sachs, D.; Chen, C.J.; Hai, R.; Palese, P. Genome-wide mutagenesis of influenza virus reveals unique plasticity of the hemagglutinin and NS1 proteins. Proc. Natl. Acad. Sci. USA 2013, 110, 20248–20253. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26. [Google Scholar] [CrossRef]
- Romero-Tejeda, A.; Capua, I. Virus-specific factors associated with zoonotic and pandemic potential. Influenza Other Respir. Viruses 2013, 7, S4–S14. [Google Scholar] [CrossRef] [PubMed]
- Brooks, W.A.; Zaman, K.; Lewis, K.D.; Ortiz, J.R.; Goswami, D.; Feser, J.; Sharmeen, A.T.; Nahar, K.; Rahman, M.; Rahman, M.Z.; et al. Efficacy of a Russian-backbone live attenuated influenza vaccine among young children in Bangladesh: A randomised, double-blind, placebo-controlled trial. Lancet Glob. Health 2016, 4, e946–e954. [Google Scholar] [CrossRef]
- DiazGranados, C.A.; Dunning, A.J.; Kimmel, M.; Kirby, D.; Treanor, J.; Collins, A.; Pollak, R.; Christoff, J.; Earl, J.; Landolfi, V.; et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N. Engl. J. Med. 2014, 371, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Dunkle, L.M.; Izikson, R.; Patriarca, P.; Goldenthal, K.L.; Muse, D.; Callahan, J.; Cox, M.M.J.; PSC12 Study Team. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N. Engl. J. Med. 2017, 376, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.; Roy-Ghanta, S.; Montellano, M.; Weckx, L.; Ulloa-Gutierrez, R.; Lazcano-Ponce, E.; Kerdpanich, A.; Safadi, M.A.; Cruz-Valdez, A.; Litao, S.; et al. Relative efficacy of AS03-adjuvanted pandemic influenza A(H1N1) vaccine in children: Results of a controlled, randomized efficacy trial. J. Infect. Dis. 2014, 210, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Victor, J.C.; Lewis, K.D.; Diallo, A.; Niang, M.N.; Diarra, B.; Dia, N.; Ortiz, J.R.; Widdowson, M.A.; Feser, J.; Hoagland, R.; et al. Efficacy of a Russian-backbone live attenuated influenza vaccine among children in Senegal: A randomised, double-blind, placebo-controlled trial. Lancet Glob. Health 2016, 4, e955–e965. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Seasonal Influenza Vaccine Effectiveness, 2005–2018. Available online: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm (accessed on 4 July 2018).
- Bonten, M.J.; Huijts, S.M.; Bolkenbaas, M.; Webber, C.; Patterson, S.; Gault, S.; van Werkhoven, C.H.; van Deursen, A.M.; Sanders, E.A.; Verheij, T.J.; et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N. Engl. J. Med. 2015, 372, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Moberley, S.; Holden, J.; Tatham, D.P.; Andrews, R.M. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst. Rev. 2013, 1. [Google Scholar] [CrossRef] [PubMed]
- Van Buynder, P.; Booy, R. Pneumococcal vaccination in older persons: Where are we today? Pneumonia 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, E.; Salemi, S.; D’Amelio, R. Anti-Infectious Human Vaccination in Historical Perspective. Int. Rev. Immunol. 2016, 35, 260–290. [Google Scholar] [CrossRef] [PubMed]
- Trunz, B.B.; Fine, P.; Dye, C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: A meta-analysis and assessment of cost-effectiveness. Lancet 2006, 367, 1173–1180. [Google Scholar] [CrossRef]
- Arriola, C.; Garg, S.; Anderson, E.J.; Ryan, P.A.; George, A.; Zansky, S.M.; Bennett, N.; Reingold, A.; Bargsten, M.; Miller, L.; et al. Influenza vaccination modifies disease severity among community-dwelling adults hospitalized with influenza. Clin. Infect. Dis. 2017, 65, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Arriola, C.S.; Anderson, E.J.; Baumbach, J.; Bennett, N.; Bohm, S.; Hill, M.; Lindegren, M.L.; Lung, K.; Meek, J.; Mermel, E.; et al. Does influenza vaccination modify influenza severity? Data on older adults hospitalized with influenza during the 2012–2013 season in the United States. J. Infect. Dis. 2015, 212, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Casado, I.; Dominguez, A.; Toledo, D.; Chamorro, J.; Force, L.; Soldevila, N.; Astray, J.; Egurrola, M.; Godoy, P.; Mayoral, J.M.; et al. Effect of influenza vaccination on the prognosis of hospitalized influenza patients. Expert Rev. Vaccines 2016, 15, 425–432. [Google Scholar] [PubMed]
- Cole, S.L.; Ho, L.P. Contribution of innate immune cells to pathogenesis of severe influenza virus infection. Clin. Sci. 2017, 131, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Thomas, P.G. Balancing Immune Protection and Immune Pathology by CD8(+) T-Cell Responses to Influenza Infection. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Han, S.N.; Meydani, S.N. Antioxidants, cytokines, and influenza infection in aged mice and elderly humans. J. Infect. Dis. 2000, 182, S74–S80. [Google Scholar] [CrossRef] [PubMed]
- Ramos, I.; Fernandez-Sesma, A. Modulating the innate immune response to influenza a virus: Potential therapeutic use of anti-inflammatory drugs. Front. Immunol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.; Kumar, V.; Netea, M.G.; Xavier, R.J. The causes and consequences of variation in human cytokine production in health. Curr. Opin. Immunol. 2018, 54, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, C.; Principi, N.; Giavoli, C.; Esposito, S. Obesity: Impact of infections and response to vaccines. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Ohmit, S.E.; Petrie, J.G.; Malosh, R.E.; Cowling, B.J.; Thompson, M.G.; Shay, D.K.; Monto, A.S. Influenza vaccine effectiveness in the community and the household. Clin. Infect. Dis. 2013, 56, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.K.; Lau, L.L.H.; Cauchemez, S.; Cowling, B.J. Household Transmission of Influenza Virus. Trends Microbiol. 2016, 24, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Pilishvili, T.; Lexau, C.; Farley, M.M.; Hadler, J.; Harrison, L.H.; Bennett, N.M.; Reingold, A.; Thomas, A.; Schaffner, W.; Craig, A.S.; et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J. Infect. Dis. 2010, 201, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Reichert, T.A.; Sugaya, N.; Fedson, D.S.; Glezen, W.P.; Simonsen, L.; Tashiro, M. The Japanese experience with vaccinating schoolchildren against influenza. N. Engl. J. Med. 2001, 344, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Paules, C.; Subbarao, K. Influenza. Lancet 2017, 390, 697–708. [Google Scholar] [CrossRef]
- Mubareka, S.; Lowen, A.C.; Steel, J.; Coates, A.L.; García-Sastre, A.; Palese, P. Transmission of influenza virus via aerosols and fomites in the guinea pig model. J. Infect. Dis. 2009, 199, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, N.M.; Rahmat, S.; Pica, N. Enhanced mammalian transmissibility of seasonal influenza A/H1N1 viruses encoding an oseltamivir-resistant neuraminidase. J. Virol. 2012, 86, 7268–7279. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.J.; Danzy, S.; Kyriakis, C.S.; Deymier, M.J.; Lowen, A.C.; Steel, J. The M segment of the 2009 pandemic influenza virus confers increased NA activity, filamentous morphology and efficient contact transmissibility to A/Puerto Rico/8/1934-based reassortant viruses. J. Virol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.L.; Liang, C.H.; Wu, C.Y.; Forrest, H.L.; Ferguson, A.; Choy, K.T.; Jones, J.; Wong, D.D.; Cheung, P.P.; Hsu, C.H.; et al. Hemagglutinin-neuraminidase balance confers respiratory-droplet transmissibility of the pandemic H1N1 influenza virus in ferrets. Proc. Natl. Acad. Sci. USA 2011, 108, 14264–14269. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Fouchier, R.A.M.; Eichelberger, M.C.; Webby, R.J.; Shaw-Saliba, K.; Wan, H.; Wilson, P.C.; Compans, R.W.; Skountzou, I.; Monto, A.S. NAction! How Can Neuraminidase-Based immunity contribute to better influenza virus vaccines? mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Center for Biologics Evaluation and Research; Food and Drug Administration. Guidance for Industry: Clinical Data Needed to Support the Licensure of Seasonal Inactivated Influenza Vaccines; U.S. Department of Health and Human Services: Rockville, MD, USA, 2007.
- Lowen, A.C.; Steel, J.; Mubareka, S.; Carnero, E.; García-Sastre, A.; Palese, P. Blocking interhost transmission of influenza virus by vaccination in the guinea pig model. J. Virol. 2009, 83, 2803–2818. [Google Scholar] [CrossRef] [PubMed]
- Seibert, C.W.; Rahmat, S.; Krause, J.C.; Eggink, D.; Albrecht, R.A.; Goff, P.H.; Krammer, F.; Duty, J.A.; Bouvier, N.M.; García-Sastre, A.; et al. Recombinant IgA is sufficient to prevent influenza virus transmission in guinea pigs. J. Virol. 2013, 87, 7793–7804. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zengel, J.R.; Suguitan, A.L., Jr.; Xu, Q.; Wang, W.; Lin, J.; Jin, H. Evaluation of the humoral and cellular immune responses elicited by the live attenuated and inactivated influenza vaccines and their roles in heterologous protection in ferrets. J. Infect. Dis. 2013, 208, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Hoft, D.F.; Lottenbach, K.R.; Blazevic, A.; Turan, A.; Blevins, T.P.; Pacatte, T.P.; Yu, Y.; Mitchell, M.C.; Hoft, S.G.; Belshe, R.B. Comparisons of the humoral and cellular immune responses induced by live attenuated influenza vaccine and inactivated influenza vaccine in adults. Clin. Vaccine Immunol. 2017, 24. [Google Scholar] [CrossRef] [PubMed]
- Ohmit, S.E.; Petrie, J.G.; Cross, R.T.; Johnson, E.; Monto, A.S. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J. Infect. Dis. 2011, 204, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Black, S.; Nicolay, U.; Vesikari, T.; Knuf, M.; Del Giudice, G.; Della Cioppa, G.; Tsai, T.; Clemens, R.; Rappuoli, R. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr. Infect. Dis. J. 2011, 30, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Ewen, C.; Zhou, X.; Kane, K.P.; Xie, D.; Hager, W.D.; Barry, M.B.; Kleppinger, A.; Wang, Y.; Bleackley, R.C. Granzyme B: Correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine 2009, 27, 2418–2425. [Google Scholar] [CrossRef] [PubMed]
- Neidich, S.D.; Green, W.D.; Rebeles, J.; Karlsson, E.A.; Schultz-Cherry, S.; Noah, T.L.; Chakladar, S.; Hudgens, M.G.; Weir, S.S.; Beck, M.A. Increased risk of influenza among vaccinated adults who are obese. Int. J. Obes. 2017, 41, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, E.; Sheikh, A.; Butler, C.; El Ferkh, K.; von Wissmann, B.; McMenamin, J.; Ritchie, L.; Schwarze, J.; Papadopoulos, N.G.; Johnston, S.L.; et al. effectiveness of influenza vaccines in asthma: A systematic review and meta-analysis. Clin. Infect. Dis. 2017, 65, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Bekkat-Berkani, R.; Wilkinson, T.; Buchy, P.; Dos Santos, G.; Stefanidis, D.; Devaster, J.M.; Meyer, N. Seasonal influenza vaccination in patients with COPD: A systematic literature review. BMC Pulm. Med. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Remschmidt, C.; Wichmann, O.; Harder, T. Influenza vaccination in patients with end-stage renal disease: Systematic review and assessment of quality of evidence related to vaccine efficacy, effectiveness, and safety. BMC Med. 2014, 12. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, V.; Jefferson, T.; Ferroni, E.; Rivetti, A.; Di Pietrantonj, C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst. Rev. 2018, 2. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.G.; Suarez, N.M.; Obermoser, G.; Lopez, S.M.; Flano, E.; Mertz, S.E.; Albrecht, R.A.; Garcia-Sastre, A.; Mejias, A.; Xu, H.; et al. Differences in antibody responses between trivalent inactivated influenza vaccine and live attenuated influenza vaccine correlate with the kinetics and magnitude of interferon signaling in children. J. Infect. Dis. 2014, 210, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.; Mai, L.Q.; Thanh, L.T.; Wolbers, M.; Hang, N.L.K.; Thai, P.Q.; Yen, N.T.T.; Hoa, L.N.M.; Bryant, J.E.; Duong, T.N.; et al. Hemagglutination inhibiting antibodies and protection against seasonal and pandemic influenza infection. J. Infect. 2015, 70, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Osterholm, M.T.; Kelley, N.S.; Sommer, A.; Belongia, E.A. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 36–44. [Google Scholar] [CrossRef]
- Rhorer, J.; Ambrose, C.S.; Dickinson, S.; Hamilton, H.; Oleka, N.A.; Malinoski, F.J.; Wittes, J. Efficacy of live attenuated influenza vaccine in children: A meta-analysis of nine randomized clinical trials. Vaccine 2009, 27, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, T.; Rivetti, A.; Di Pietrantonj, C.; Demicheli, V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst. Rev. 2018, 2. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, C.S.; Wu, X.; Knuf, M.; Wutzler, P. The efficacy of intranasal live attenuated influenza vaccine in children 2 through 17 years of age: A meta-analysis of 8 randomized controlled studies. Vaccine 2012, 30, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.D. Influenza and pneumonia excess mortality at specific ages in the epidemic of 1943–44, with comparative data for preceding epidemics (concluded). Public Health Rep. 1945, 60, 856–857. [Google Scholar]
- Pebody, R.G.; McLean, E.; Zhao, H.; Cleary, P.; Bracebridge, S.; Foster, K.; Charlett, A.; Hardelid, P.; Waight, P.; Ellis, J.; et al. Pandemic Influenza A (H1N1) 2009 and mortality in the United Kingdom: Risk factors for death, April 2009 to March 2010. Eurosurveillance 2010, 15, 19571. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention (CDC). 2009 H1N1 Flu in The News. Available online: https://www.cdc.gov/h1n1flu/in_the_news/age_group.htm (accessed on 5 July 2018).
- Howden, L.M.; Meyer, J.A. Age and Sex Composition: 2010. 2010 Census Briefs. Available online: https://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf (accessed on 5 July 2018).
- Office for National. Statistics Mid-2001 to Mid-2012 Population. Estimates: Single Year of Age and Sex for Local Authorities in the United Kingdom; Estimated. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland (accessed on 5 July 2018).
- Qin, Y.; Horby, P.W.; Tsang, T.K.; Chen, E.; Gao, L.; Ou, J.; Nguyen, T.H.; Duong, T.N.; Gasimov, V.; Feng, L.; et al. Differences in the epidemiology of human cases of avian influenza A(H7N9) and A(H5N1) Viruses Infection. Clin. Infect. Dis. 2015, 61, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Gostic, K.M.; Ambrose, M.; Worobey, M.; Lloyd-Smith, J.O. Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science 2016, 354, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Craig, R. Why Did the 1918 Flu Kill So Many Otherwise Healthy Young Adults? Available online: https://www.smithsonianmag.com/history/why-did-1918-flu-kill-so-many-otherwise-healthy-young-adults-180967178/ (accessed on 5 July 2018).
- Guthmiller, J.J.; Wilson, P.C. Harnessing immune history to combat influenza viruses. Curr. Opin. Immunol. 2018, 53, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Viboud, C.; Epstein, S.L. First flu is forever. Science 2016, 354, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Vaccination Coverage among Adults in the United States, National Health Interview Survey. 2016. Available online: https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/NHIS-2016.html (accessed on 6 July 2018).
- World Health Organization (WHO). Reported Estimates of PCV3 Coverage. Available online: http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tscoveragepcv3.html (accessed on 5 July 2018).
- World Health Organization (WHO). Reported Estimates of BCG Coverage. Available online: http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tscoveragebcg.html (accessed on 5 July 2018).
- Loke, A.Y.; Kwan, M.L.; Wong, Y.T.; Wong, A.K.Y. The uptake of human papillomavirus vaccination and its associated factors among adolescents: A systematic review. J. Prim. Care Community Health 2017, 8, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Walling, E.B.; Benzoni, N.; Dornfeld, J.; Bhandari, R.; Sisk, B.A.; Garbutt, J.; Colditz, G. Interventions to improve HPV vaccine uptake: A systematic review. Pediatrics 2016, 138. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Xu, L.; Simoens, C.; Martin-Hirsch, P.P. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst. Rev. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Fournet, N.; Mollema, L.; Ruijs, W.L.; Harmsen, I.A.; Keck, F.; Durand, J.Y.; Cunha, M.P.; Wamsiedel, M.; Reis, R.; French, J.; et al. Under-vaccinated groups in Europe and their beliefs, attitudes and reasons for non-vaccination; two systematic reviews. BMC Public Health 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, S.C.; Kegler, C. Vaccine-related internet search activity predicts H1N1 and HPV vaccine coverage: Implications for vaccine acceptance. J. Health Commun. 2015, 20, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Ryan, R.; Walsh, L.; Horey, D.; Leask, J.; Robinson, P.; Hill, S. Face-to-face interventions for informing or educating parents about early childhood vaccination. Cochrane Database Syst. Rev. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.J. Negotiating vaccine acceptance in an era of reluctance. Hum. Vaccines Immunother. 2013, 9, 1779–1781. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Rauber, D.; Betsch, C.; Lidolt, G.; Denker, M.L. Barriers of Influenza Vaccination Intention and Behavior—A Systematic Review of Influenza Vaccine Hesitancy, 2005–2016. PLoS ONE 2017, 12, e0170550. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Meldgaard, T.S.; Bertholet, S. Novel platforms for the development of a universal influenza vaccine. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Nachbagauer, R.; Krammer, F. Universal influenza virus vaccines and therapeutic antibodies. Clin. Microbiol. Infect. 2017, 23, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.S.; Webby, R.J. Traditional and new influenza vaccines. Clin. Microbiol. Rev. 2013, 26, 476–492. [Google Scholar] [CrossRef] [PubMed]
- Ladhani, S.N.; Collins, S.; Djennad, A.; Sheppard, C.L.; Borrow, R.; Fry, N.K.; Andrews, N.J.; Miller, E.; Ramsay, M.E. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: A prospective national observational cohort study. Lancet Infect. Dis. 2018, 18, 441–451. [Google Scholar] [CrossRef]

© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouvier, N.M. The Future of Influenza Vaccines: A Historical and Clinical Perspective. Vaccines 2018, 6, 58. https://doi.org/10.3390/vaccines6030058
Bouvier NM. The Future of Influenza Vaccines: A Historical and Clinical Perspective. Vaccines. 2018; 6(3):58. https://doi.org/10.3390/vaccines6030058
Chicago/Turabian StyleBouvier, Nicole M. 2018. "The Future of Influenza Vaccines: A Historical and Clinical Perspective" Vaccines 6, no. 3: 58. https://doi.org/10.3390/vaccines6030058
APA StyleBouvier, N. M. (2018). The Future of Influenza Vaccines: A Historical and Clinical Perspective. Vaccines, 6(3), 58. https://doi.org/10.3390/vaccines6030058



