Endometrial Stromal Sarcomas: A Revision of Their Potential as Targets for Immunotherapy
Abstract
1. Introduction
2. Clinicopathological Characteristics and Treatment of Endometrial Stromal Sarcoma
3. Molecular and Genetic Characteristics
3.1. Immunohistochemical Characteristics
3.2. Genetic Characteristics
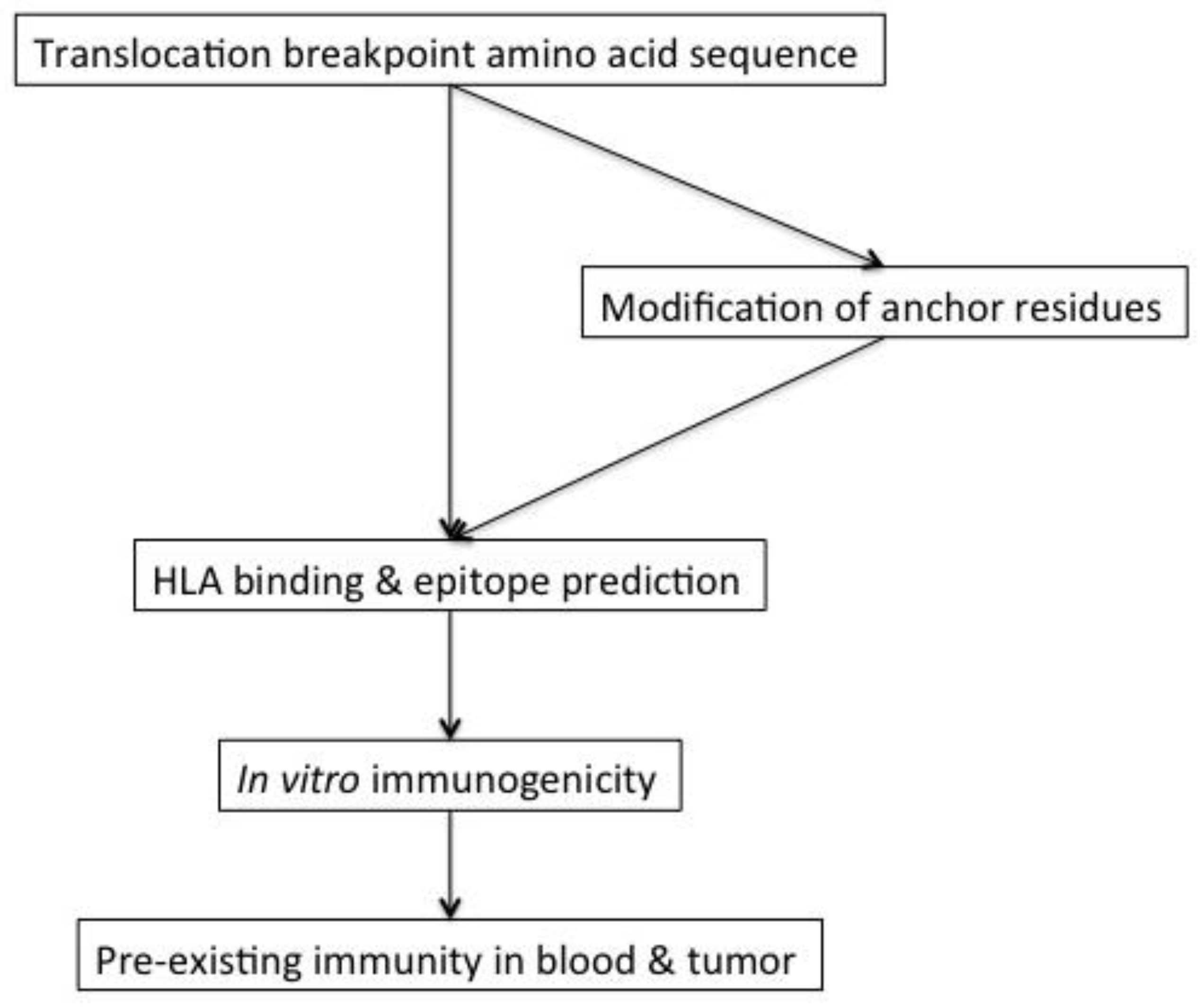
4. Immunogenicity of Translocation Fusion Proteins
5. Immunotherapeutic Studies Targeting Translocation Fusion Proteins
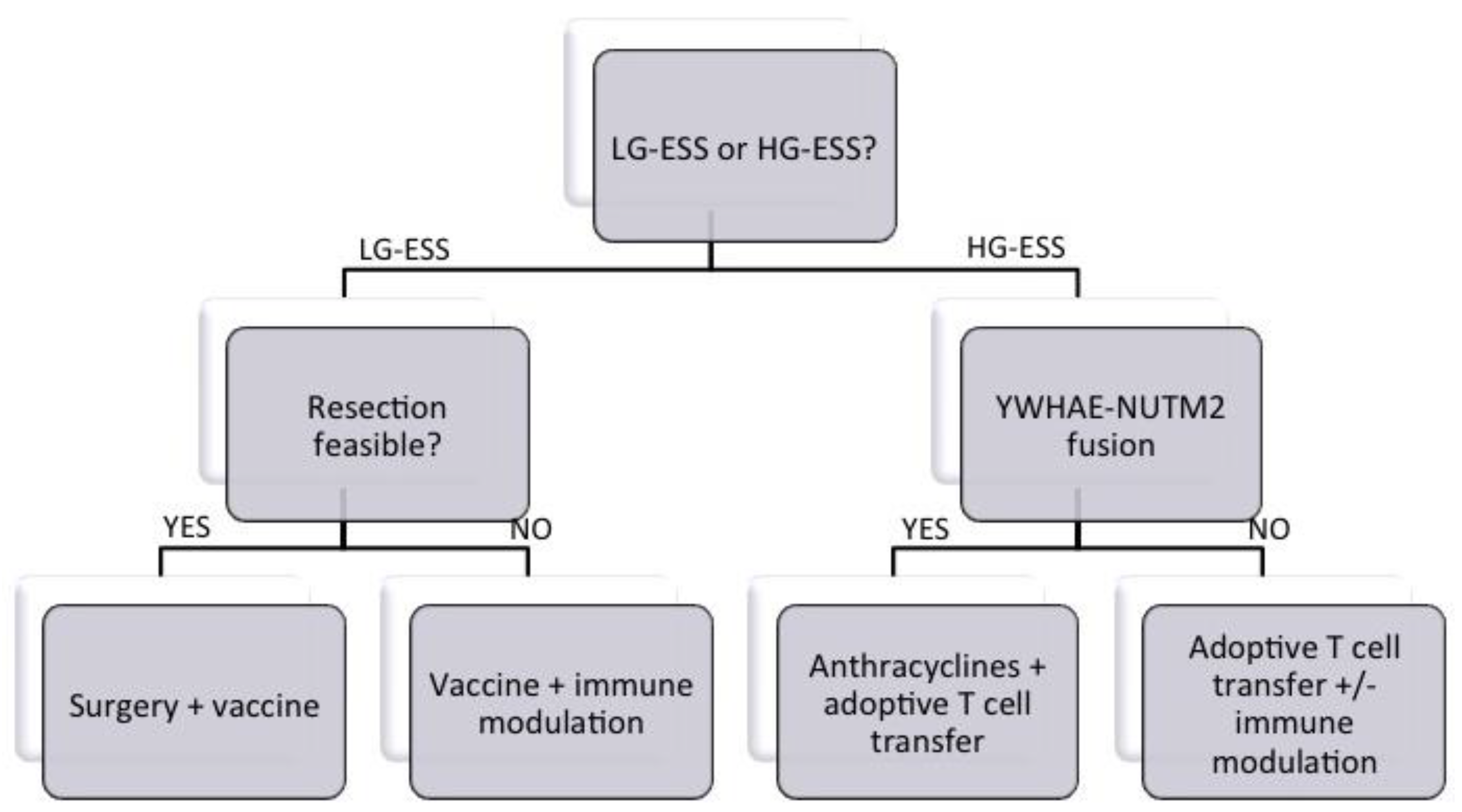
6. Roadmap to Target Translocation Fusion Proteins in ESS by Immunotherapy
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amant, F.; Floquet, A.; Friedlander, M.; Kristensen, G.; Mahner, S.; Nam, E.J.; Powell, M.A.; Ray-Coquard, I.; Siddiqui, N.; Sykes, P.; et al. Gynecologic cancer intergroup (GCIG) consensus review for endometrial stromal sarcoma. Int. J. Gynecol. Cancer 2014, 24, S67–S72. [Google Scholar] [CrossRef] [PubMed]
- Gantzer, J.; Ray-Coquard, I. Gynecological sarcomas: What‘s new in 2018, a brief review of published literature. Curr. Opin. Oncol. 2018, 30, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Anguille, S.; Van de Velde, A.L.; Smits, E.L.; Van Tendeloo, V.F.; Juliusson, G.; Cools, N.; Nijs, G.; Stein, B.; Lion, E.; Van Driessche, A.; et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood 2017, 130, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, J.; Stone, R.M.; Uhl, L.; Neuberg, D.; Joyce, R.; Levine, J.D.; Arnason, J.; McMasters, M.; Luptakova, K.; Jain, S.; et al. Individualized vaccination of AML patients in remission is associated with induction of antileukemia immunity and prolonged remissions. Sci. Transl. Med. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Chu, D.; Meyer, C.F.; Llosa, N.J.; McCarty, G.; Morris, C.D.; Levin, A.S.; Wolinsky, J.P.; Albert, C.M.; Steppan, D.A.; et al. Highly personalized detection of minimal ewing sarcoma disease burden from plasma tumor DNA. Cancer 2016, 122, 3015–3023. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Hasegawa, T. Practical use and utility of fluorescence in situ hybridization in the pathological diagnosis of soft tissue and bone tumors. J. Orthop. Sci. 2017, 22, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Loke, B.N.; Lee, V.K.M.; Sudhanshi, J.; Wong, M.K.; Kuick, C.H.; Puhaindran, M.; Chang, K.T.E. Novel exon-exon breakpoint in CIC-DUX4 fusion sarcoma identified by anchored multiplex PCR (archer fusionplex sarcoma panel). J. Clin. Pathol. 2017, 70, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Konishi, H.; Ichikawa, D.; Hamada, J.; Shoda, K.; Arita, T.; Komatsu, S.; Shiozaki, A.; Okamoto, K.; Yamazaki, S.; et al. Detection of fusion gene in cell-free DNA of a gastric synovial sarcoma. World J. Gastroenterol. 2018, 24, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, C.A.; Gattinoni, L.; Palmer, D.C.; Muranski, P.; Ji, Y.; Hinrichs, C.S.; Borman, Z.A.; Kerkar, S.P.; Scott, C.D.; Finkelstein, S.E.; et al. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin. Cancer Res. 2011, 17, 5343–5352. [Google Scholar] [CrossRef] [PubMed]
- Conklin, C.M.; Longacre, T.A. Endometrial stromal tumors: The new who classification. Adv. Anat. Pathol. 2014, 21, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Horng, H.C.; Wen, K.C.; Wang, P.H.; Chen, Y.J.; Yen, M.S.; Ng, H.T. Uterine sarcoma part II-Uterine endometrial stromal sarcoma: The tag systematic review. Taiwan J. Obstet. Gynecol. 2016, 55, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Hoang, L.; Chiang, S.; Lee, C.H. Endometrial stromal sarcomas and related neoplasms: New developments and diagnostic considerations. Pathology 2018, 50, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Seagle, B.L.; Shilpi, A.; Buchanan, S.; Goodman, C.; Shahabi, S. Low-grade and high-grade endometrial stromal sarcoma: A national cancer database study. Gynecol. Oncol. 2017, 146, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.; Soslow, R.A.; Delair, D.F.; Park, K.J.; Murali, R.; Hollmann, T.J.; Davidson, B.; Micci, F.; Panagopoulos, I.; Hoang, L.N.; et al. Zc3h7b-bcor high-grade endometrial stromal sarcomas: A report of 17 cases of a newly defined entity. Mod. Pathol. 2018, 31, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.; Lee, C.H.; Stewart, C.J.R.; Oliva, E.; Hoang, L.N.; Ali, R.H.; Hensley, M.L.; Arias-Stella, J.A., 3rd; Frosina, D.; Jungbluth, A.A.; et al. Bcor is a robust diagnostic immunohistochemical marker of genetically diverse high-grade endometrial stromal sarcoma, including tumors exhibiting variant morphology. Mod. Pathol. 2017, 30, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Koontz, J.I.; Soreng, A.L.; Nucci, M.; Kuo, F.C.; Pauwels, P.; van Den Berghe, H.; Dal Cin, P.; Fletcher, J.A.; Sklar, J. Frequent fusion of the JAZF1 and JJAZ1 genes in endometrial stromal tumors. Proc. Natl. Acad. Sci. USA 2001, 98, 6348–6353. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.R.; Harburger, D.; Koontz, J.; Dal Cin, P.; Sklar, J. Molecular analysis of the JAZF1-JJAZ1 gene fusion by RT-PCR and fluorescence in situ hybridization in endometrial stromal neoplasms. Am. J. Surg. Pathol. 2007, 31, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Oliva, E.; de Leval, L.; Soslow, R.A.; Herens, C. High frequency of JAZF1-JJAZ1 gene fusion in endometrial stromal tumors with smooth muscle differentiation by interphase fish detection. Am. J. Surg. Pathol. 2007, 31, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Micci, F.; Panagopoulos, I.; Bjerkehagen, B.; Heim, S. Consistent rearrangement of chromosomal band 6p21 with generation of fusion genes JAZF1/PHF1 and EPC1/PHF1 in endometrial stromal sarcoma. Cancer Res. 2006, 66, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, I.; Micci, F.; Thorsen, J.; Gorunova, L.; Eibak, A.M.; Bjerkehagen, B.; Davidson, B.; Heim, S. Novel fusion of MYST/Esa1-associated factor 6 and PHF1 in endometrial stromal sarcoma. PLoS ONE 2012, 7, e39354. [Google Scholar] [CrossRef] [PubMed]
- Dewaele, B.; Przybyl, J.; Quattrone, A.; Finalet Ferreiro, J.; Vanspauwen, V.; Geerdens, E.; Gianfelici, V.; Kalender, Z.; Wozniak, A.; Moerman, P.; et al. Identification of a novel, recurrent MBTD1-CXORF67 fusion in low-grade endometrial stromal sarcoma. Int. J. Cancer 2014, 134, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.J.; Ali, S.M.; Gowen, K.; Elvin, J.A.; Pejovic, T. A recurrent endometrial stromal sarcoma harbors the novel fusion JAZF1-BCORL1. Gynecol. Oncol. Rep. 2017, 20, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Tousseyn, T.; Coenegrachts, L.; Decloedt, J.; Moerman, P.; Debiec-Rychter, M. Case report of a poorly differentiated uterine tumour with t(10;17) translocation and neuroectodermal phenotype. Anticancer Res. 2011, 31, 2367–2371. [Google Scholar] [PubMed]
- Lee, C.H.; Ou, W.B.; Marino-Enriquez, A.; Zhu, M.; Mayeda, M.; Wang, Y.; Guo, X.; Brunner, A.L.; Amant, F.; French, C.A.; et al. 14-3-3 fusion oncogenes in high-grade endometrial stromal sarcoma. Proc. Natl. Acad. Sci. USA 2012, 109, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Hoang, L.N.; Aneja, A.; Conlon, N.; Delair, D.F.; Middha, S.; Benayed, R.; Hensley, M.L.; Park, K.J.; Hollmann, T.J.; Hameed, M.R.; et al. Novel high-grade endometrial stromal sarcoma: A morphologic mimicker of myxoid leiomyosarcoma. Am. J. Surg. Pathol. 2017, 41, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Marino-Enriquez, A.; Lauria, A.; Przybyl, J.; Ng, T.L.; Kowalewska, M.; Debiec-Rychter, M.; Ganesan, R.; Sumathi, V.; George, S.; McCluggage, W.G.; et al. Bcor internal tandem duplication in high-grade uterine sarcomas. Am. J. Surg. Pathol. 2018, 42, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, J.; Wang, J.; Ma, C.X.; Gao, X.; Patriub, V.; Sklar, J.L. The JAZF1-SUZ12 fusion protein disrupts PRC2 complexes and impairs chromatin repression during human endometrial stromal tumorogenesis. Oncotarget 2017, 8, 4062–4078. [Google Scholar] [CrossRef] [PubMed]
- Ried, T.; Gaiser, T. A recurrent fusion gene in high-grade endometrial stromal sarcoma: A new tool for diagnosis and therapy? Genome Med. 2012, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Hemming, M.L.; Wagner, A.J.; Nucci, M.R.; Chiang, S.; Wang, L.; Hensley, M.L.; George, S. Ywhae-rearranged high-grade endometrial stromal sarcoma: Two-center case series and response to chemotherapy. Gynecol. Oncol. 2017, 145, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.S.; van den Broeke, L.T.; Goletz, T.J.; Pendleton, C.D.; Daschbach, E.M.; Thomas, E.K.; Marincola, F.M.; Helman, L.J.; Berzofsky, J.A. Antigenicity of fusion proteins from sarcoma-associated chromosomal translocations. Cancer Res. 2001, 61, 6868–6875. [Google Scholar] [PubMed]
- Sun, J.Y.; Krouse, R.S.; Forman, S.J.; Senitzer, D.; Sniecinski, I.; Chatterjee, S.; Wong, K.K., Jr. Immunogenicity of a p210(BCR-ABL) fusion domain candidate DNA vaccine targeted to dendritic cells by a recombinant adeno-associated virus vector in vitro. Cancer Res. 2002, 62, 3175–3183. [Google Scholar] [PubMed]
- Sun, J.Y.; Senitzer, D.; Forman, S.J.; Chatterjee, S.; Wong, K.K., Jr. Identification of new MHC-restriction elements for presentation of the p210(BCR-ABL) fusion region to human cytotoxic T lymphocytes. Cancer Immunol. Immunother. 2003, 52, 761–770. [Google Scholar] [CrossRef] [PubMed]
- van den Broeke, L.T.; Pendleton, C.D.; Mackall, C.; Helman, L.J.; Berzofsky, J.A. Identification and epitope enhancement of a PAX-FKHR fusion protein breakpoint epitope in alveolar rhabdomyosarcoma cells created by a tumorigenic chromosomal translocation inducing CTL capable of lysing human tumors. Cancer Res. 2006, 66, 1818–1823. [Google Scholar] [CrossRef] [PubMed]
- Rodeberg, D.A.; Nuss, R.A.; Heppelmann, C.J.; Celis, E. Lack of effective T-lymphocyte response to the PAX3/FKHR translocation area in alveolar rhabdomyosarcoma. Cancer Immunol. Immunother. 2005, 54, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.H.; Liu, F.; Porter, R.M.; O‘Sullivan, R.P.; Merghoub, T.; Lunsford, E.P.; Robichaud, K.; Van Valen, F.; Lessnick, S.L.; Gebhardt, M.C.; et al. EWS-FLI-1-targeted cytotoxic T-cell killing of multiple tumor types belonging to the ewing sarcoma family of tumors. Clin. Cancer Res. 2012, 18, 5341–5351. [Google Scholar] [CrossRef] [PubMed]
- Popovic, J.; Li, L.P.; Kloetzel, P.M.; Leisegang, M.; Uckert, W.; Blankenstein, T. The only proposed T-cell epitope derived from the TEL-AML1 translocation is not naturally processed. Blood 2011, 118, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, S.; Wada, T.; Ida, K.; Sato, Y.; Nagoya, S.; Tsukahara, T.; Kimura, S.; Sahara, H.; Ikeda, H.; Shimozawa, K.; et al. Phase I vaccination trial of SYT-SSX junction peptide in patients with disseminated synovial sarcoma. J. Transl. Med. 2005, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, S.; Tsukahara, T.; Ida, K.; Kimura, S.; Murase, M.; Kano, M.; Emori, M.; Nagoya, S.; Kaya, M.; Torigoe, T.; et al. SYT-SSX breakpoint peptide vaccines in patients with synovial sarcoma: A study from the japanese musculoskeletal oncology group. Cancer Sci. 2012, 103, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Dagher, R.; Long, L.M.; Read, E.J.; Leitman, S.F.; Carter, C.S.; Tsokos, M.; Goletz, T.J.; Avila, N.; Berzofsky, J.A.; Helman, L.J.; et al. Pilot trial of tumor-specific peptide vaccination and continuous infusion interleukin-2 in patients with recurrent ewing sarcoma and alveolar rhabdomyosarcoma: An inter-institute nih study. Med. Pediatr. Oncol. 2002, 38, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Mackall, C.L.; Rhee, E.H.; Read, E.J.; Khuu, H.M.; Leitman, S.F.; Bernstein, D.; Tesso, M.; Long, L.M.; Grindler, D.; Merino, M.; et al. A pilot study of consolidative immunotherapy in patients with high-risk pediatric sarcomas. Clin. Cancer Res. 2008, 14, 4850–4858. [Google Scholar] [CrossRef] [PubMed]
- Pinilla-Ibarz, J.; Cathcart, K.; Korontsvit, T.; Soignet, S.; Bocchia, M.; Caggiano, J.; Lai, L.; Jimenez, J.; Kolitz, J.; Scheinberg, D.A. Vaccination of patients with chronic myelogenous leukemia with BCR-ABL oncogene breakpoint fusion peptides generates specific immune responses. Blood 2000, 95, 1781–1787. [Google Scholar] [PubMed]
- Rezvani, K.; de Lavallade, H. Vaccination strategies in lymphomas and leukaemias: Recent progress. Drugs 2011, 71, 1659–1674. [Google Scholar] [CrossRef] [PubMed]
- Comoli, P.; Basso, S.; Riva, G.; Barozzi, P.; Guido, I.; Gurrado, A.; Quartuccio, G.; Rubert, L.; Lagreca, I.; Vallerini, D.; et al. BCR-ABL-specific T-cell therapy in Ph+ ALL patients on tyrosine-kinase inhibitors. Blood 2017, 129, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Przybyl, J.; Kowalewska, M.; Quattrone, A.; Dewaele, B.; Vanspauwen, V.; Varma, S.; Vennam, S.; Newman, A.M.; Swierniak, M.; Bakula-Zalewska, E.; et al. Macrophage infiltration and genetic landscape of undifferentiated uterine sarcomas. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Vanderstraeten, A.; Luyten, C.; Verbist, G.; Tuyaerts, S.; Amant, F. Mapping the immunosuppressive environment in uterine tumors: Implications for immunotherapy. Cancer Immunol. Immunother. 2014, 63, 545–557. [Google Scholar] [CrossRef] [PubMed]
| Molecule | Low-Grade ESS (LG-ESS) | High-Grade ESS (HG-ESS) | |
|---|---|---|---|
| YWHAE-NUTM2 | ZC3H7B-BCOR | ||
| CD10 | ++ | - | +++ |
| ER PR | +++ +++ | - - | + + |
| Cyclin-D1 | - | +++ | +++ |
| cKIT | - | +++ | unknown |
| BCOR | - | +++ | ++ |
| ESS Type | Translocation | Fusion Gene | References |
|---|---|---|---|
| LG-ESS | t (7; 17) (p15; q21) | JAZF1-SUZ12 | [16,17,18] |
| t (6; 7) (p21; p15) | JAZF1-PHF1 | [17,19] | |
| t (6; 10) (p21; p11) | PHF1-EPC1 | [19] | |
| t (1; 6) (p34; p21) | PHF1-MEAF6 | [20] | |
| t (X; 17) (p11.2; q21.33) | MBTD1-CXorf67 | [21] | |
| t (7; X) (p15; q26.1) | JAZF1-BCORL1 | [22] | |
| HG-ESS | t (10; 17) (q22; p13) | YWHAE-NUTM2 | [23,24] |
| der (22) (X; 22) (p11; q13) | ZC3H7B-BCOR | [14,25] | |
| t (22; X) (q13; p11) | BCOR-ZC3H7B | [15] | |
| none | BCOR ITD | [15,26] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuyaerts, S.; Amant, F. Endometrial Stromal Sarcomas: A Revision of Their Potential as Targets for Immunotherapy. Vaccines 2018, 6, 56. https://doi.org/10.3390/vaccines6030056
Tuyaerts S, Amant F. Endometrial Stromal Sarcomas: A Revision of Their Potential as Targets for Immunotherapy. Vaccines. 2018; 6(3):56. https://doi.org/10.3390/vaccines6030056
Chicago/Turabian StyleTuyaerts, Sandra, and Frédéric Amant. 2018. "Endometrial Stromal Sarcomas: A Revision of Their Potential as Targets for Immunotherapy" Vaccines 6, no. 3: 56. https://doi.org/10.3390/vaccines6030056
APA StyleTuyaerts, S., & Amant, F. (2018). Endometrial Stromal Sarcomas: A Revision of Their Potential as Targets for Immunotherapy. Vaccines, 6(3), 56. https://doi.org/10.3390/vaccines6030056





