The Immunity Gap Challenge: Protection against a Recent Florida Clade 2 Equine Influenza Strain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Vaccination Protocol
2.2. Viruses and Experimental Infection with EIV
2.3. Serology
2.4. Clinical Signs of Disease (Pre-Specified Analysis)
anorexia + depression)
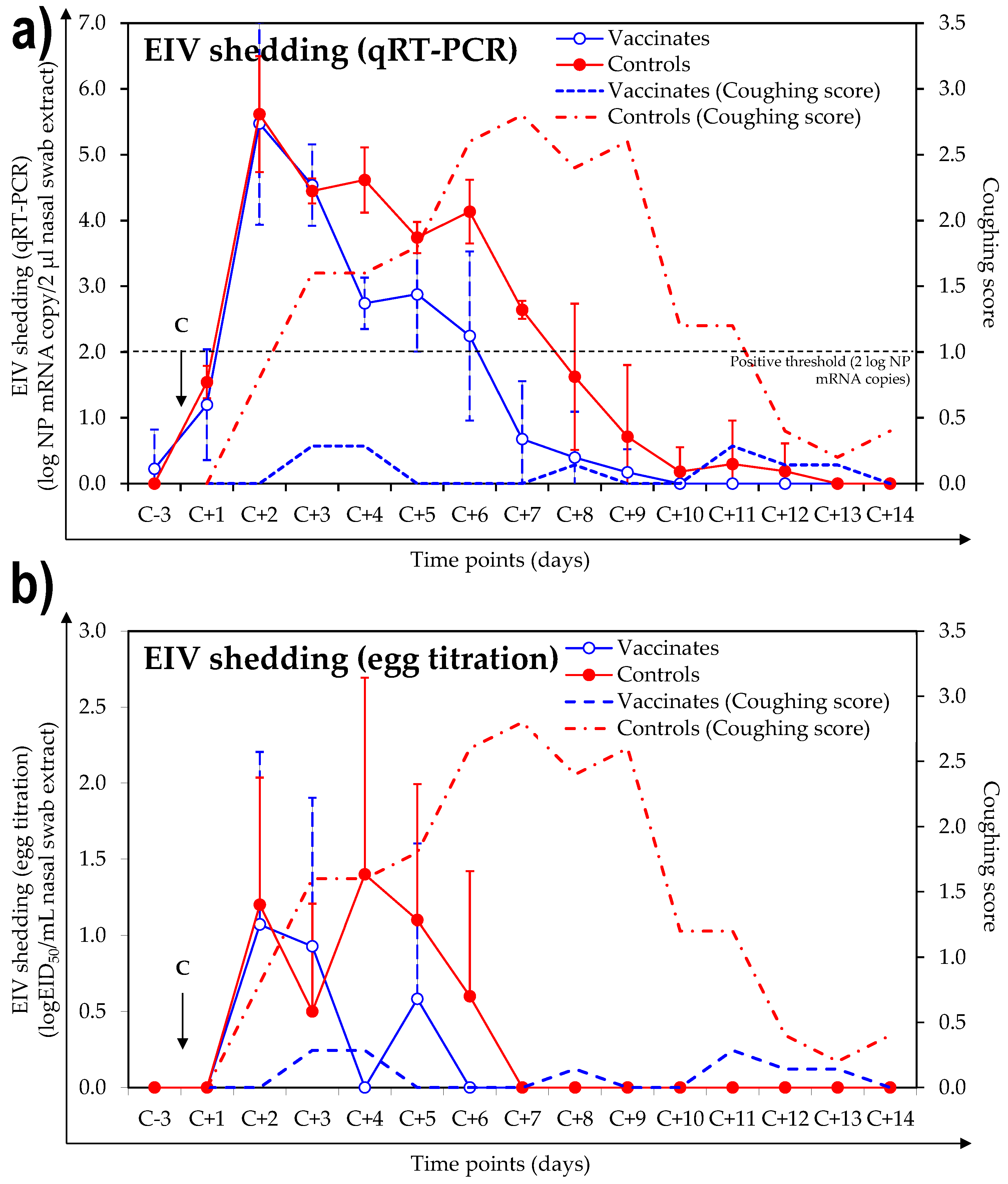
2.5. Virus Shedding
2.6. Statistical Analysis
3. Results
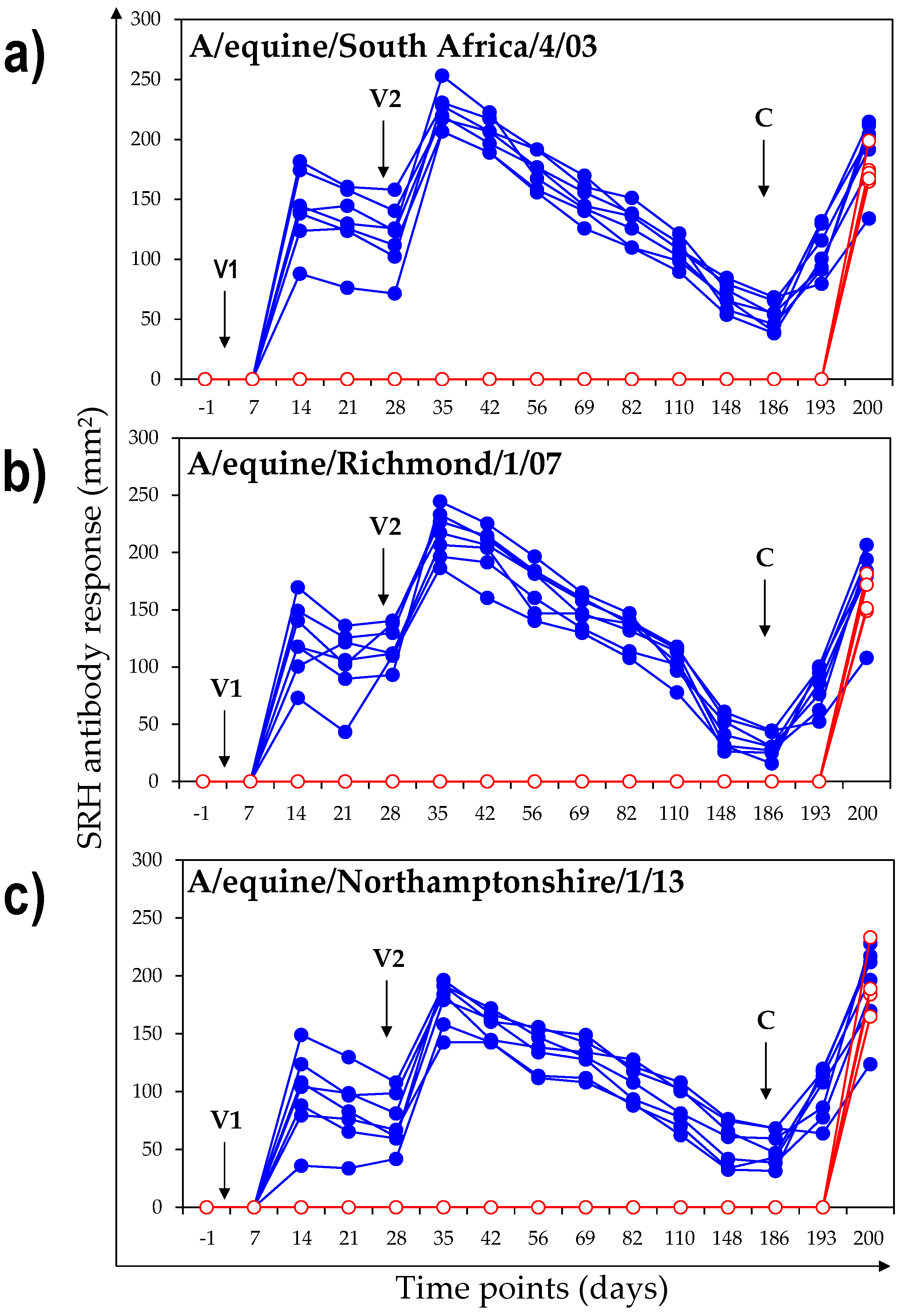
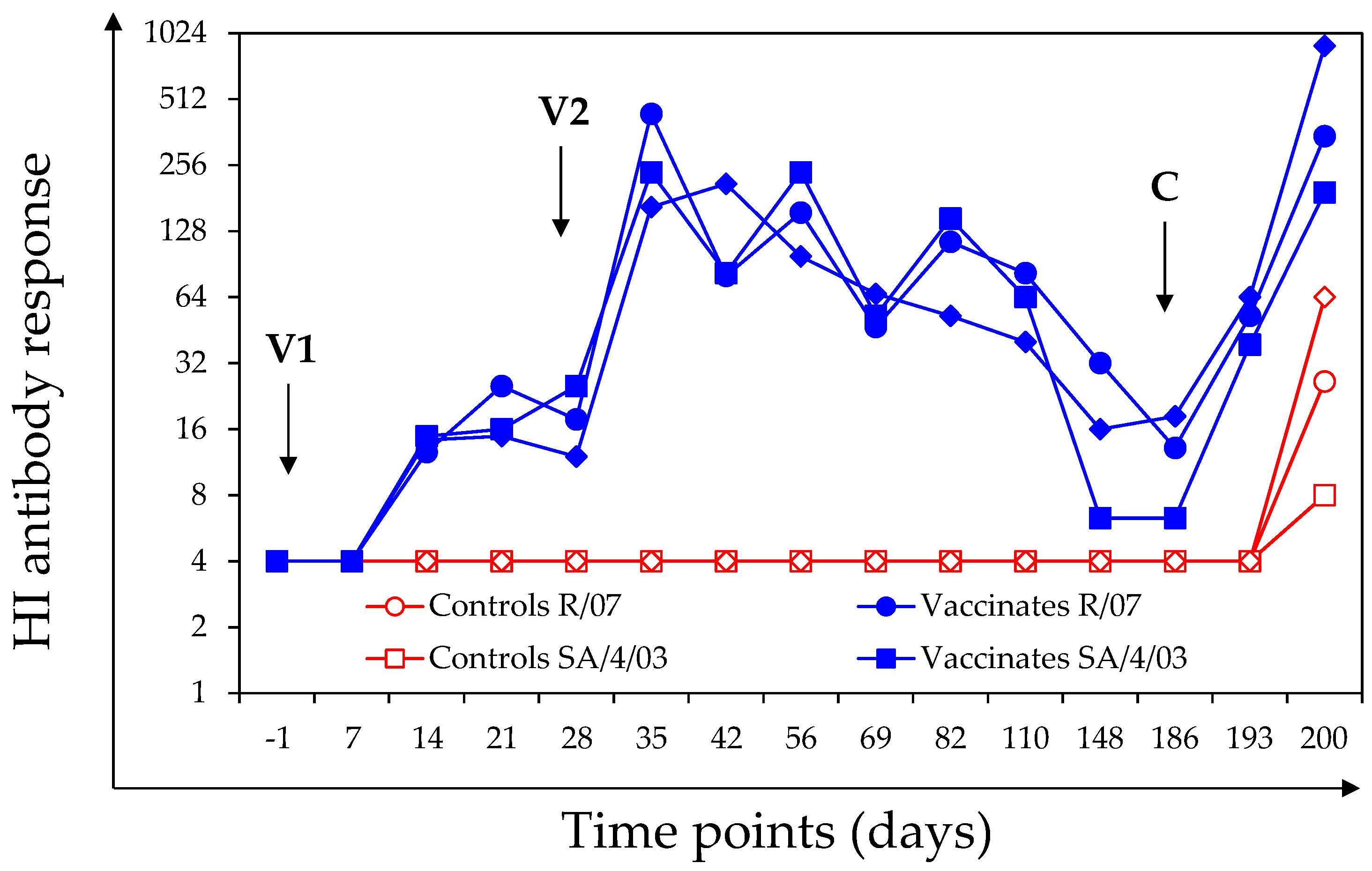
3.1. Antibody Response
3.1.1. SRH Antibody Response
3.1.2. HI Antibody Response
3.1.3. TT Antibody Response
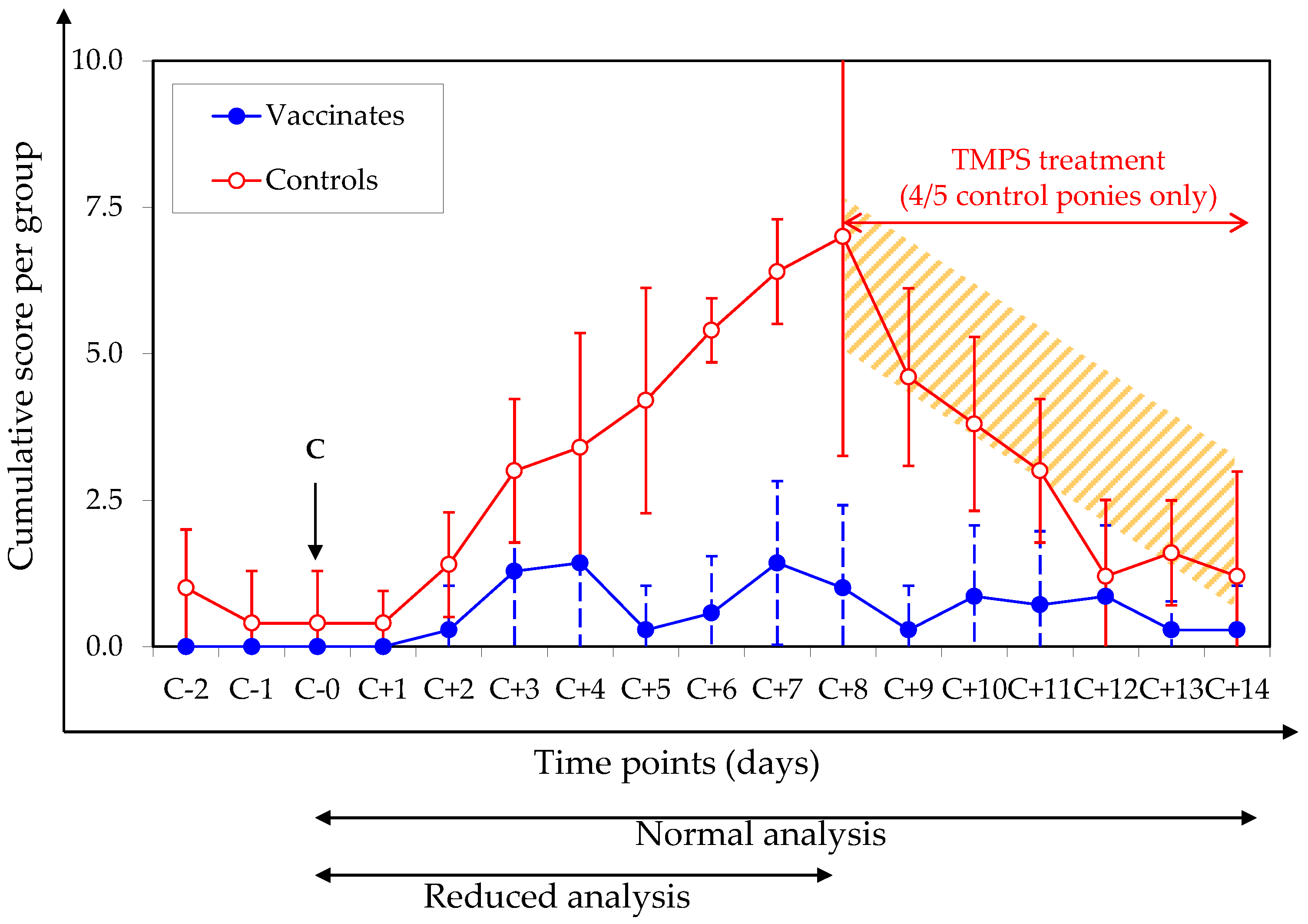
3.2. Clinical Signs of Disease after Experimental Infection with A/Equine/Northamptonshire/1/13
3.3. Virus Shedding after Experimental Infection with A/Equine/Northamptonshire/1/13
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Landolt, G.; Townsend, H.G.; Lunn, D.P. Equine influenza infection. In Equine Infectious Diseases, 2nd ed.; Sellon, D.C., Long, M.T., Eds.; Saunders Elsevier: Amsterdam, The Netherland, 2013; pp. 141–151. [Google Scholar]
- Paillot, R. A systematic review of recent advances in equine influenza vaccination. Vaccines 2014, 2, 797–831. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, A.J.; Stevens, K.B.; Bosman, P.P. The circumstances surrounding the outbreak and spread of equine influenza in South Africa. Rev. Ser. Tech. 1999, 18, 179–185. [Google Scholar] [CrossRef]
- Paillot, R.; El-Hage, C.M. The use of a recombinant canarypox-based equine influenza vaccine during the 2007 australian outbreak: A systematic review and summary. Pathogens 2016, 5, E42. [Google Scholar] [CrossRef] [PubMed]
- Gildea, S.; Arkins, S.; Cullinane, A. Management and environmental factors involved in equine influenza outbreaks in ireland 2007–2010. Equine Vet. J. 2011, 43, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Gildea, S.; Fitzpatrick, D.A.; Cullinane, A. Epidemiological and virological investigations of equine influenza outbreaks in ireland (2010–2012). Influenza Other Resp. Viruses 2013, 7 (Suppl. 4), 61–72. [Google Scholar] [CrossRef] [PubMed]
- Rash, A.; Morton, R.; Woodward, A.; Maes, O.; McCauley, J.; Bryant, N.; Elton, D. Evolution and divergence of h3n8 equine influenza viruses circulating in the united kingdom from 2013 to 2015. Pathogens 2017, 6, E6. [Google Scholar] [CrossRef] [PubMed]
- OIE. Equine influenza (Infection with Equine Influenza Virus): Chapter 2.5.7. Available online: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.05.07_EQ_INF.pdf (accessed on 1 June 2016).
- Mumford, J.A.; Wood, J. Establishing an acceptability threshold for equine influenza vaccines. Dev. Biol. Stand 1992, 79, 137–146. [Google Scholar] [PubMed]
- Mumford, J.A.; Jessett, D.M.; Rollinson, E.A.; Hannant, D.; Draper, M.E. Duration of protective efficacy of equine influenza immunostimulating complex/tetanus vaccines. Vet. Rec. 1994, 134, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Gildea, S.; Arkins, S.; Walsh, C.; Cullinane, A. A comparison of antibody responses to commercial equine influenza vaccines following annual booster vaccination of national hunt horses—A randomised blind study. Vaccine 2011, 29, 3917–3922. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Prowse, L.; Montesso, F.; Huang, C.M.; Barnes, H.; Escala, J. Whole inactivated equine influenza vaccine: Efficacy against a representative clade 2 equine influenza virus, ifngamma synthesis and duration of humoral immunity. Vet. Microbiol. 2013, 162, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.L.; Mumford, J.A.; Mair, T.S.; Slater, J. Boosting in equine influenza vaccination schedules: Timing and time for a re-evaluation of requirements of national and international authorities. Vet. J. 2007, 174, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Prowse, L.; Montesso, F.; Stewart, B.; Jordon, L.; Newton, J.R.; Gilkerson, J.R. Duration of equine influenza virus shedding and infectivity in immunised horses after experimental infection with eiv a/eq2/richmond/1/07. Vet. Microbiol. 2013, 166, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Murcia, P.R.; Baillie, G.J.; Stack, J.C.; Jervis, C.; Elton, D.; Mumford, J.A.; Daly, J.; Kellam, P.; Grenfell, B.T.; Holmes, E.C.; et al. Evolution of equine influenza virus in vaccinated horses. J. Virol. 2013, 87, 4768–4771. [Google Scholar] [CrossRef] [PubMed]
- Callinan, I. Equine Influenza, the August 2007 Outbreak in Australia. Available online: http://apo.org.au/files/Resource/commonwealthofaustralia_equineinfluenza_2008.pdf (accessed on 1 June 2016).
- Heldens, J.G.; van Loon, A.A.; van de Zande, S. Is there a benefit from an early booster vaccination in the control of equine influenza? Vet. J. 2007, 174, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Equine influenza vaccine (inactivated) monograph 0249. In European Pharmacopoeia, 8th ed.; Directorate for the Quality of Medicines & HealthCare of the Council of Europe (EDQM): Strasbourg, France, 2013; pp. 968–970. [Google Scholar]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gotzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. Consort 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Br. Med. J. 2010, 340, c869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, K.F.; Altman, D.G.; Moher, D. Consort 2010 statement: Updated guidelines for reporting parallel group randomised trials. Br. Med. J. 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Grimmett, H.; Elton, D.; Daly, J.M. Protection, systemic ifn and antibody responses induced by an iscom-based vaccine against a recent equine influenza virus in its natural host. Vet. Res. 2008, 39, 21. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percente endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Garrett, D.; Montesso, F.; Fougerolle, S.; Lopez-Alvarez, M.R.; Birand, I.; De Bock, M.; Huang, C.M.; Legrand, L.; Pronost, S.; Paillot, R. Refinement of the equine influenza model in the natural host: A meta-analysis to determine the benefits of individual nebulisation for experimental infection and vaccine evaluation in the face of decreased strain pathogenicity. Vet. Microbiol. 2017, 211, 150–159. [Google Scholar] [CrossRef] [PubMed]
- The World Organisation for Animal Health (OIE). Equine Influenza: Chapter 2.5.7. Available online: http://www.oie.int/fileadmin/Home/fr/Health_standards/tahm/2.05.07_EQ_INF.pdf (accessed on 1 June 2018).
- Paillot, R.; Fraser, S.; Prowse-Davis, L.; Rash, N.; Montesso, F.; Slootmans, N.; Thomas, A.; Besognet, B.; Meinert, T.; Ons, E.; et al. Iscom-based equine influenza vaccine: Duration of immunity and randomised clinical trials to assess an accelerated schedule of immunisation and efficacy. Trials Vaccinol. 2015, 4, 61–70. [Google Scholar] [CrossRef]
- Paillot, R.; Prowse, L.; Donald, C.; Medcalf, E.; Montesso, F.; Bryant, N.; Watson, J.; Jeggo, M.; Elton, D.; Newton, R.; et al. Efficacy of a whole inactivated ei vaccine against a recent eiv outbreak isolate and comparative detection of virus shedding. Vet. Immunol. Immunopathol. 2010, 136, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavorial Sciences; Laurence Erlbaum Associates: Mahwah, NJ, USA, 1988; p. 400. [Google Scholar]
- Sylte, M.J.; Suarez, D.L. Influenza neuraminidase as a vaccine antigen. Curr. Top. Microbiol. Immunol. 2009, 333, 227–241. [Google Scholar] [PubMed]
- Couch, R.B.; Atmar, R.L.; Franco, L.M.; Quarles, J.M.; Wells, J.; Arden, N.; Nino, D.; Belmont, J.W. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J. Infect. Dis. 2013, 207, 974–981. [Google Scholar] [CrossRef] [PubMed]
- OIE Equine influenza vaccine composition. OIE Bull. 2017, 2, 86–87.
- Slater, J.; Borchers, K.; Chambers, T.; Cullinane, A.; Duggan, V.; Elton, D.; Legrand, L.; Paillot, R.; Fortier, G. Report of the international equine influenza roundtable expert meeting at le touquet, normandy, february 2013. Equine Vet. J. 2014, 46, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Heldens, J.G.; Pouwels, H.G.; Derks, C.G.; Van de Zande, S.M.; Hoeijmakers, M.J. The first safe inactivated equine influenza vaccine formulation adjuvanted with iscom-matrix that closes the immunity gap. Vaccine 2009, 27, 5530–5537. [Google Scholar] [CrossRef] [PubMed]
- Soboll, G.; Hussey, S.B.; Minke, J.M.; Landolt, G.A.; Hunter, J.S.; Jagannatha, S.; Lunn, D.P. Onset and duration of immunity to equine influenza virus resulting from canarypox-vectored (alvac) vaccination. Vet. Immunol. Immunopathol. 2010, 135, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Minke, J.M.; Toulemonde, C.E.; Coupier, H.; Guigal, P.M.; Dinic, S.; Sindle, T.; Jessett, D.; Black, L.; Bublot, M.; Pardo, M.C.; et al. Efficacy of a canarypox-vectored recombinant vaccine expressing the hemagglutinin gene of equine influenza h3n8 virus in the protection of ponies from viral challenge. Am. J. Vet. Res. 2007, 68, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Rash, N.L.; Garrett, D.; Prowse-Davis, L.; Montesso, F.; Cullinane, A.; Lemaitre, L.; Thibault, J.C.; Wittreck, S.; Dancer, A. How to meet the last oie expert surveillance panel recommendations on equine influenza (ei) vaccine composition: A review of the process required for the recombinant canarypox-based ei vaccine. Pathogens 2016, 5, E64. [Google Scholar] [CrossRef] [PubMed]
- Heldens, J.G.; Pouwels, H.G.; Derks, C.G.; Van de Zande, S.M.; Hoeijmakers, M.J. Duration of immunity induced by an equine influenza and tetanus combination vaccine formulation adjuvanted with iscom-matrix. Vaccine 2010, 28, 6989–6996. [Google Scholar] [CrossRef] [PubMed]
- Lohrer, J.; Radvila, P. [Active tetanus prevention in the horse and the duration of immunity]. Schweiz. Arch. Tierheilk. 1970, 112, 307–314. [Google Scholar]
- Kendall, A.; Anagrius, K.; Ganheim, A.; Rosanowski, S.M.; Bergstrom, K. Duration of tetanus immunoglobulin g titres following basic immunisation of horses. Equine Vet. J. 2016, 48, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Marcillaud Pitel, C.; D’Ablon, X.; Pronost, S. Equine vaccines: How, when and why? Report of the vaccinology session, french equine veterinarians association, 2016, reims. Vaccines Basel 2017, 5, E46. [Google Scholar] [CrossRef] [PubMed]
- El-Hage, C.M.; Savage, C.J.; Minke, J.M.; Ficorilli, N.P.; Watson, J.; Gilkerson, J.R. Accelerated vaccination schedule provides protective levels of antibody and complete herd immunity to equine influenza. Equine Vet. J. 2013, 45, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, A.; Gildea, S.; Weldon, E. Comparison of primary vaccination regimes for equine influenza: Working towards an evidence-based regime. Equine Vet. J. 2014, 46, 669–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daly, J.M.; Sindle, T.; Tearle, J.; Barquero, N.; Newton, J.R.; Corning, S. Equine influenza vaccine containing older h3n8 strains offers protection against a/eq/south africa/4/03 (h3n8) strain in a short-term vaccine efficacy study. Equine Vet. J. 2007, 39, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, H.G.; Van de Zande, S.M.; Horspool, L.J.; Hoeijmakers, M.J. Efficacy of a non-updated, matrix-c-based equine influenza subunit-tetanus vaccine following florida sublineage clade 2 challenge. Vet. Rec. 2014, 174, 633. [Google Scholar] [CrossRef] [PubMed]
| Group | n | Treatment | D0 (V1) | D28 (V2) | D186 1 |
|---|---|---|---|---|---|
| Controls | 5 | Placebo | PBS | PBS | N/1/13 |
| Vaccinates | 7 | Vaccination | EI-TT vaccine | EI-TT vaccine | N/1/13 |
 | |||||
| Pony # | Group | Day-1 | Day 56 | Day 69 | Day 186 * |
|---|---|---|---|---|---|
| 1 | Vaccinates | 0.0 | 57.31 | 42.15 | 4.43 |
| 2 | Vaccinates | 0.0 | 76.13 | 38.39 | 4.75 |
| 3 | Vaccinates | 0.0 | 51.00 | 16.14 | 2.69 |
| 4 | Vaccinates | 0.0 | 60.19 | 24.34 | 2.30 |
| 5 | Vaccinates | 0.0 | 44.42 | 13.35 | 3.85 |
| 6 | Vaccinates | 0.0 | 65.32 | 28.63 | 1.36 |
| 7 | Vaccinates | 0.0 | 10.30 | 16.78 | 3.01 |
| 8 | Controls | 0.0 | 0.0 | 0.58 | 0.0 |
| 9 | Controls | 0.0 | 0.0 | 0.36 | 0.0 |
| 10 | Controls | 0.0 | 0.0 | 0.18 | 0.0 |
| 11 | Controls | 0.0 | 0.0 | 0.27 | 0.0 |
| 12 | Controls | 0.0 | 0.0 | 0.28 | 0.0 |
| average | Vaccinates | 0.0 | 52.1 | 25.7 | 3.2 |
| STDV | Vaccinates | 0.0 | 21.0 | 11.3 | 1.2 |
| average | Controls | 0.0 | 0.0 | 0.33 | 0.0 |
| STDV | Controls | 0.0 | 0.0 | 0.15 | 0.0 |
| Clinical Sign | Controls | Vaccinates | Normal A 1 | Reduced A 2 | S Power |
|---|---|---|---|---|---|
| Cumulative clinical score | 47.0 ± 9.8 | 9.6 ± 5.1 | 0.000006 * | 0.00006 * | 100% |
| Clinical severity score per day | 3.9 ± 0.5 | 2.0 ± 0.9 | 0.006 ** | 0.004 * | 99.9% |
| Disease duration (days) | 12.0 ± 2.0 | 4.1 ± 2.2 | 0.00009 * | 0.004 ** | 100% |
| Pyrexia duration (days) | 1.0 ± 0.7 | 0.1 ± 0.4 | 0.033 ** | 0.033 ** | 82.7% |
| Frequency of pyretic ponies | 4/5 (80%) | 1/7 (14.3%) | 0.032 *** | na | na |
| Cumulative nasal discharge score | 25.6 ± 5.9 | 7.9 ± 4.2 | 0.0001 * | 0.005 ** | 100% |
| Nasal discharge severity score per day | 2.5 ± 0.1 | 2.0 ± 1.0 | 0.14 * | 0.04 ** | 37% |
| Nasal discharge duration (days) | 10.2 ± 2.8 | 3.4 ± 1.9 | 0.0005 * | 0.001 * | 99.9% |
| Cumulative cough score | 19.6 ± 6.8 | 1.3 ± 3.0 | 0.005 ** | 0.004 ** | 100% |
| Cough severity score | 1.3 ± 0.5 | 0.1 ± 0.2 | 0.005 ** | 0.005 ** | 100% |
| Cough duration (days) | 8.8 ± 2.2 | 0.9 ± 1.9 | 0.004 ** | 0.00003 * | 100% |
| Cumulative ocular discharge score | 0.6 ± 0.9 | 0.4 ± 0.8 | 0.73 * | 0.45 * | 10.6% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paillot, R.; Garrett, D.; Lopez-Alvarez, M.R.; Birand, I.; Montesso, F.; Horspool, L. The Immunity Gap Challenge: Protection against a Recent Florida Clade 2 Equine Influenza Strain. Vaccines 2018, 6, 38. https://doi.org/10.3390/vaccines6030038
Paillot R, Garrett D, Lopez-Alvarez MR, Birand I, Montesso F, Horspool L. The Immunity Gap Challenge: Protection against a Recent Florida Clade 2 Equine Influenza Strain. Vaccines. 2018; 6(3):38. https://doi.org/10.3390/vaccines6030038
Chicago/Turabian StylePaillot, Romain, Dion Garrett, Maria R. Lopez-Alvarez, Ihlan Birand, Fernando Montesso, and Linda Horspool. 2018. "The Immunity Gap Challenge: Protection against a Recent Florida Clade 2 Equine Influenza Strain" Vaccines 6, no. 3: 38. https://doi.org/10.3390/vaccines6030038
APA StylePaillot, R., Garrett, D., Lopez-Alvarez, M. R., Birand, I., Montesso, F., & Horspool, L. (2018). The Immunity Gap Challenge: Protection against a Recent Florida Clade 2 Equine Influenza Strain. Vaccines, 6(3), 38. https://doi.org/10.3390/vaccines6030038






