Biodegradable Polymeric Nanoparticles-Based Vaccine Adjuvants for Lymph Nodes Targeting
Abstract
:1. Why do We Need Adjuvants?
2. Biodegradable Nanoparticles for Vaccine Delivery
2.1. Various Polymers for Vaccine Application
2.2. Polymers and Antigen/Immunopotentiator Association
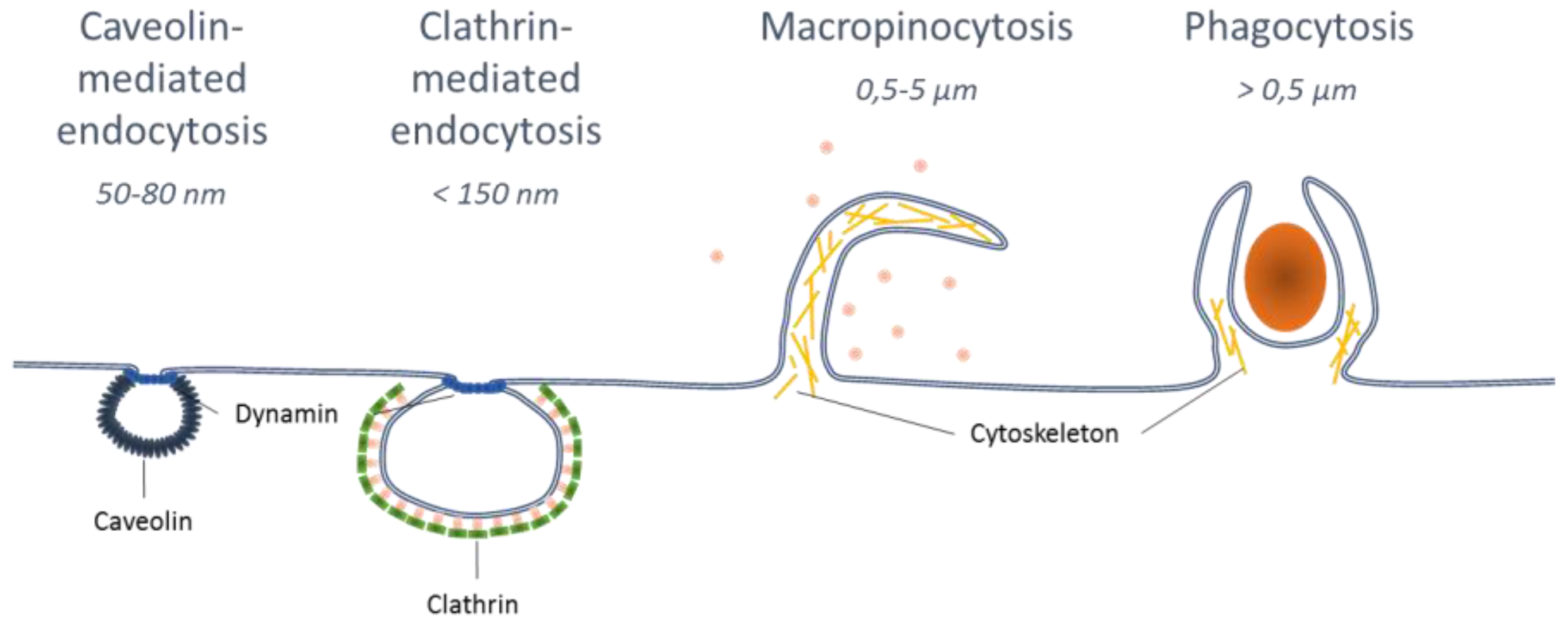
3. Influence of Particle Characteristics on APCs Uptake and Targeting to Lymph Nodes
3.1. Effect of Nanoparticle Size for APC Uptake and LN Targeting
3.2. Influence of Particle Shape for Cellular Uptake
3.3. Influence of Surface Characteristics of Polymeric NPs.
4. Immune Responses and Functionalized Nanoparticles
5. Nanodelivery of Immunopotentiators
5.1. Immunopotentiators as Powerful Vaccine Adjuvants
5.2. Improving Nanovectors Efficacy with Molecular Adjuvants
5.3. Delivery of the Immunostimulant Molecules and Antigens to a Target Compartment
5.4. The Nanodelivery of Immunomodulators Decreases Their Toxicity
5.5. Co-Delivery or Co-Administration with Antigens?
6. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Moss, B. Smallpox vaccines : Targets of protective immunity. Immunol. Rev. 2012, 239, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Dubensky, T.W.J.; Reed, S.G. Seminars in Immunology Adjuvants for cancer vaccines. Semin. Immunol. 2010, 22, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Mamo, T.; Poland, G.A. Nanovaccinology: The next generation of vaccines meets 21st century materials science and engineering. Vaccine 2012, 30, 6609–6611. [Google Scholar] [CrossRef] [PubMed]
- Astete, C.E.; Sabliov, C.M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 2006, 17, 247–289. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.B.; Geary, S.M.; Salem, A.K. Biodegradable particles as vaccine delivery systems: Size matters. AAPS J. 2013, 15, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.; Middelberg, A.P.J. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Italiani, P. From Antigen Delivery System to Adjuvanticy: The Board Application of Nanoparticles in Vaccinology. Vaccines 2015, 3, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Bolhassani, A.; Javanzad, S.; Saleh, T.; Hashemi, M.; Aghasadeghi, M.R.; Sadat, S.M. Polymeric nanoparticles Potent vectors for vaccine delivery targeting cancer and infectious diseases. Hum. Vaccines Immunother. 2014, 10, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic. Bioeng. Transl. Med. 2016, 55, 10–29. [Google Scholar] [CrossRef]
- Moon, J.J.; Suh, H.; Polhemus, M.E.; Ockenhouse, C.F.; Yadava, A.; Irvine, D.J. Antigen-displaying lipid-enveloped PLGA nanoparticles as delivery agents for a Plasmodium vivax malaria vaccine. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westwood, A.; Elvin, S.J.; Healey, G.D.; Williamson, E.D.; Eyles, J.E. Immunological responses after immunisation of mice with microparticles containing antigen and single stranded RNA (polyuridylic acid). Vaccine 2006, 24, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Kanchan, V.; Panda, A.K. Interactions of antigen-loaded polylactide particles with macrophages and their correlation with the immune response. Biomaterials 2007, 28, 5344–5357. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Singh, S.R.; Yilma, A.N.; Agee, R.D.; Taha, M.; Dennis, V.A. Poly(lactic acid)-poly(ethylene glycol) nanoparticles provide sustained delivery of a Chlamydia trachomatis recombinant MOMP peptide and potentiate systemic adaptive immune responses in mice. Nanomed. Nanotech. Biol. Med. 2014, 10, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Fredriksen, B.N.; Grip, J. PLGA/PLA micro- and nanoparticle formulations serve as antigen depots and induce elevated humoral responses after immunization of Atlantic salmon (Salmo salar L.). Vaccine 2012, 30, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Primard, C.; Rochereau, N.; Luciani, E.; Genin, C.; Delair, T.; Paul, S.; Verrier, B. Traffic of poly(lactic acid) nanoparticulate vaccine vehicle from intestinal mucus to sub-epithelial immune competent cells. Biomaterials 2010, 31, 6060–6068. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Valenta, D.T.; Altman, Y.; Harvey, S.; Xie, H.; Mitragotri, S.; Smith, J.W. Polymer particle shape independently influences binding and internalization by macrophages. J. Control. Release 2010, 147, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Pavot, V.; Rochereau, N.; Primard, C.; Genin, C.; Perouzel, E.; Lioux, T.; Paul, S.; Verrier, B. Encapsulation of Nod1 and Nod2 receptor ligands into poly(lactic acid) nanoparticles potentiates their immune properties. J. Control. Release 2013, 167, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ε-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Videira, M.; Gaspar, R.; Préat, V.; Florindo, H.F. Immune system targeting by biodegradable nanoparticles for cancer vaccines. J. Control. Release 2013, 168, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Baba, M.; Akashi, M. Biodegradable Nanoparticles as Vaccine Adjuvants and Delivery Systems: Regulation of Immune Responses by Nanoparticle-Based Vaccine. Adv. Polym. Sci. 2012, 247, 31–64. [Google Scholar]
- Roth-Walter, F.; Schöll, I.; Untersmayr, E.; Ellinger, A.; Boltz-Nitulescu, G.; Scheiner, O.; Gabor, F.; Jensen-Jarolim, E. Mucosal targeting of allergen-loaded microspheres by Aleuria aurantia lectin. Vaccine 2005, 23, 2703–2710. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Zupancic, E.; Peres, C.; Silva, L.C.; Gaspar, R.; Préat, V.; Florindo, H.F. Immunomodulation against Microbial Pathogens Through Dendritic Cells. In Microbial Pathogens and Strategies for Combating Them: Science Technolgy and Education; Méndez-Vilas, A., Ed.; Badajoz, Spain, 2013; pp. 1686–1705. [Google Scholar]
- Jain, S.; O’Hagan, D.T.; Singh, M. The long-term potential of biodegradable poly(lactideco-glycolide) microparticles as the next-generation vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.F.B. Immunobiology of Dendritic Cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.T.; van der Vlies, A.J.; Simeoni, E.; Angeli, V.; Randolph, G.J.; O′Neil, C.P.; Lee, L.K.; Swartz, M.A.; Hubbell, J.A. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007, 25, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Morachis, J.M.; Mahmoud, E.A.; Almutairi, A. Physical and chemical strategies for therapeutic delivery by using polymeric nanoparticles. Pharmacol. Rev. 2012, 64, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.G.; Wei, W.; Lv, P.P.; Yue, H.; Wang, L.Y.; Su, Z.G.; Ma, G.H. Surface Charge Affects Cellular Uptake and Intracellular Trafficking of Chitosan-Based Nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.A.; Kogure, K.; Akita, H.; Harashima, H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol. Rev. 2006, 58, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Scholzen, A.; Minigo, G.; David, C.; Apostolopoulos, V.; Mottram, P.L. Pathogen recognition and development of particulate vaccines: Does size matter? Methods 2006, 40, 1–9. [Google Scholar]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.T.; Rehor, A.; Schmoekel, H.G.; Hubbell, J.A.; Swartz, M.A. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control. Release 2006, 112, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.L.; Rosalia, R.A.; Varypataki, E.; Sibuea, S.; Ossendorp, F.; Jiskoot, W. Poly-(lactic-co-glycolic-acid)-based particulate vaccines: Particle uptake by dendritic cells is a key parameter for immune activation. Vaccine 2015, 33, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Leleux, J.; Roy, K. Micro and nanoparticle-based delivery systems for vaccine immunotherapy: An immunological and materials perspective. Adv. Healthc. Mater. 2013, 2, 72–94. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.B.; Geary, S.M.; Salem, A.K. Biodegradable particles as vaccine antigen delivery systems for stimulating cellular immune responses. Hum. Vaccin. Immunother. 2013, 912, 2584–2590. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.A.; Green, J.J. Shaping the future of nanomedicine: Anisotropy in polymeric nanoparticle design. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.A.N.; Dalhaimer, P.; Cai, S.; Tsai, R.; Minko, T.; Discher, D.E. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007, 2, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Doshi, N.; Prabhakarpandian, B.; Rea-Ramsey, A.; Pant, K.; Sundaram, S.; Mitragotri, S. Flow and adhesion of drug carriers in blood vessels depend on their shape: A study using model synthetic microvascular networks. J. Control. Release 2010, 146, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Thielbeer, F.; Duffin, R.; Johansson, E.M.V.; Megson, I.L.; MacNee, W.; Bradley, M.; Donaldson, K. Surface functionalization affects the zeta potential, coronal stability and membranolytic activity of polymeric nanoparticles. Nanotoxicology 2014, 8, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Zhu, J.; Yang, X.; Xu, H. Surface modification of poly(d,l-lactic-co-glycolic acid) nanoparticles with protamine enhanced cross-presentation of encapsulated ovalbumin by bone marrow-derived dendritic cells. J. Biomed. Mater. Res. A 2011, 96, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Petrizzo, A.; Conte, C.; Tagliamonte, M.; Napolitano, M.; Bifulco, K.; Carriero, V.; De Stradis, A.; Tornesello, M.L.; Buonaguro, F.M.; Quaglia, F.; et al. Functional characterization of biodegradable nanoparticles as antigen delivery system. J. Exp. Clin. Cancer Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Alloatti, A.; Kotsias, F.; Magalhaes, J.G.; Amigorena, S. Dendritic cell maturation and cross-presentation : Timing matters ! Immunol. Rev. 2016, 272, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Lamalle-Bernard, D.; Munier, S.; Compagnon, C.; Charles, M.H.; Kalyanaraman, V.S.; Delair, T.; Verrier, B.; Ataman-Önal, Y. Coadsorption of HIV-1 p24 and gp120 proteins to surfactant-free anionic PLA nanoparticles preserves antigenicity and immunogenicity. J. Control. Release 2006, 115, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Saluja, S.S.; Hanlon, D.J.; Sharp, F.A.; Hong, E.; Khalil, D.; Robinson, E.; Tigelaar, R.; Fahmy, T.M.; Edelson, R.L. Targeting human dendritic cells via DEC-205 using PLGA nanoparticles leads to enhanced cross-presentation of a melanoma-associated antigen. Int. J. Nanomed. 2014, 9, 5231–5246. [Google Scholar] [PubMed]
- Demento, S.L.; Cui, W.; Criscione, J.M.; Stern, E.; Tulipan, J.; Kaech, S.M.; Fahmy, T.M. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials 2012, 33, 4957–4964. [Google Scholar] [CrossRef] [PubMed]
- Hirosue, S.; Kourtis, I.C.; van der Vlies, A.J.; Hubbell, J.A.; Swartz, M.A. Antigen delivery to dendritic cells by poly(propylene sulfide) nanoparticles with disulfide conjugated peptides: Cross-presentation and T cell activation. Vaccine 2010, 28, 7897–7906. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.A.; Thomas, S.N.; Swartz, M.A. Materials engineering for immunomodulation. Nat. Rev. 2009, 462, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.A.; Kafatos, F.C.; Janeway, C.A.; Ezekowitz, R.A.B. Phylogenetic Perspectives in Innate Immunity. Science 1999, 284, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Gutjahr, A.; Tiraby, G.; Perouzel, E.; Verrier, B.; Paul, S. Triggering Intracellular Receptors for Vaccine Adjuvantation. Trends Immunol. 2016, 37, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappa B by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G.; Hsu, F.C.; Carter, D.; Orr, M.T. The science of vaccine adjuvants: Advances in TLR4 ligand adjuvants. Curr. Opin. Immunol. 2016, 41, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Diebold, S.S. Recognition of viral single-stranded RNA by Toll-like receptors. Adv. Drug Deliv. Rev. 2008, 60, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Pohar, J.; Krajnik, A.K.; Jerala, R.; Bencina, M. Minimal Sequence Requirements for Minimal Sequence Requirements for Oligodeoxyribonucleotides Activating Human TLR9. J Immunol. 2015, 194, 3901–3908. [Google Scholar] [CrossRef] [PubMed]
- Bentala, H.; Verweij, W.R.; Huizinga-Van der Vlag, A.; van Loenen-Weemaes, A.M.; Meijer, D.K.F.; Poelstra, K. Removal of phosphate from lipid A as a strategy to detoxify lipopolysaccharide. Shock 2002, 18, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Margaroni, M.; Agallou, M.; Kontonikola, K.; Karidi, K.; Kammona, O.; Kiparissides, C.; Gaitanaki, C.; Karagouni, E. PLGA nanoparticles modified with a TNFa mimicking peptide, soluble Leishmania antigens and MPLA induce T cell priming in vitro via dendritic cell functional differentiation. Eur. J. Pharm. Biopharm. 2016, 105, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Wischke, C.; Mathew, S.; Roch, T.; Frentsch, M.; Lendlein, A. Potential of NOD receptor ligands as immunomodulators in particulate vaccine carriers. J. Control Release 2012, 164, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, S.; Molavi, O.; Ma, Z.; Haddadi, A.; Alshamsan, A.; Gobti, Z.; Elhasi, S.; Samuel, J.; Lavasanifar, A. Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine 2008, 26, 5046–5057. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Reichardt, W.; Koerner, J.; Groettrup, M. Coencapsulation of tumor lysate and CpG-ODN in PLGA-microspheres enables successful immunotherapy of prostate carcinoma in TRAMP mice. J. Control. Release 2012, 162, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, S.; Fransen, M.F.; Kleinovink, J.W.; Christensen, J.R.; Amidi, M.; Hennink, W.E.; Ossendorp, F. Polymeric nanoparticles for co-delivery of synthetic long peptide antigen and poly IC as therapeutic cancer vaccine formulation. J. Control. Release 2015, 203, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Tacken, P.J.; Zeelenberg, I.S.; Cruz, L.J.; Van Hout-Kuijer, M.A.; Van De Glind, G.; Fokkink, R.G.; Lambeck, A.J.A.; Figdor, C.G. Targeted delivery of TLR ligands to human and mouse dendritic cells strongly enhances adjuvanticity. Blood 2011, 118, 6836–6844. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, M.V.; Sznol, M.; Zhao, B.; Wang, D.; Carvajal, R.D.; Keohan, M.L.; Chuang, E.; Sanborn, R.E.; Lutzky, J.; Powderly, J.; et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci. Transl. Med. 2014, 6, 232–251. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.J.; Rosalia, R.A.; Kleinovink, J.W.; Rueda, F.; Löwik, C.W.; Ossendorp, F. Targeting nanoparticles to CD40, DEC-205 or CD11c molecules on dendritic cells for efficient CD8+ T cell response: A comparative study. J. Control. Release 2014, 192, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Siefert, A.L.; Caplan, M.J.; Fahmy, T.M. Artificial bacterial biomimetic nanoparticles synergize pathogen- associated molecular patterns for vaccine efficacy. Biomaterials 2016, 97, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Kasturi, S.P.; Skountzou, I.; Albrecht, R.A.; Koutsonanos, D.; Hua, T.; Nakaya, H.I.; Ravindran, R.; Stewart, S.; Alam, M.; Kwissa, M.; et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011, 470, 543–547. [Google Scholar] [CrossRef] [PubMed]
- De Titta, A.; Ballester, M.; Julier, Z.; Nembrini, C.; Jeanbart, L.; Van Der Vlies, A.J.; Swartz, M.A.; Hubbell, J.A. Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc. Natl. Acad. Sci. USA 2013, 110, 19902–19907. [Google Scholar] [CrossRef] [PubMed]
- Lynn, G.M.; Laga, R.; Darrah, P.A.; Ishizuka, A.S.; Balaci, A.J.; Dulcey, A.E.; Pechar, M.; Pola, R.; Gerner, M.Y.; Yamamoto, A.; et al. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat. Biotechnol. 2015, 33, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Ilyinskii, P.O.; Roy, C.J.; O′Neil, C.P.; Browning, E.A.; Pittet, L.A.; Altreuter, D.H.; Alexis, F.; Tonti, E.; Shi, J.; Basto, P.A.; et al. Adjuvant-carrying synthetic vaccine particles augment the immune response to encapsulated antigen and exhibit strong local immune activation without inducing systemic cytokine release. Vaccine 2014, 32, 2882–2895. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.C.; Crespo, M.P.; Abraham, W.; Moynihan, K.D.; Szeto, G.L.; Chen, S.H.; Melo, M.B.; Mueller, S.; Irvine, D.J. Nanoparticulate STING agonists are potent lymph node—targeted vaccine adjuvants. J. Clin. Invest. 2015, 125, 2532–2546. [Google Scholar] [CrossRef] [PubMed]
- Heit, A.; Schmitz, F.; Haas, T.; Busch, D.H.; Wagner, H. Antigen co-encapsulated with adjuvants efficiently drive protective T cell immunity. Eur. J. Immunol. 2007, 37, 2063–2074. [Google Scholar] [CrossRef] [PubMed]
- Shima, F.; Uto, T.; Akagi, T.; Akashi, M. Synergistic stimulation of antigen presenting cells via tlr by combining CpG ODN and Poly(g-glutamic acid)-based nanoparticles as vaccine adjuvants. Bioconjug. Chem. 2013, 24, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Bruno, C.; Agnolon, V.; Berti, F.; Bufali, S.; O′hagan, D.T.; Baudner, B.C. The preparation and characterization of PLG nanoparticles with an entrapped synthetic TLR7 agonist and their preclinical evaluation as adjuvant for an adsorbed DTaP vaccine. Eur. J. Pharm. Biopharm. 2016, 105, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Demento, S.L.; Bonafé, N.; Cui, W.; Kaech, S.M.; Caplan, M.J.; Fikrig, E.; Ledizet, M.; Fahmy, T.M. TLR9-Targeted Biodegradable Nanoparticles as Immunization Vectors Protect against West Nile Encephalitis. J. Immunol. 2010, 185, 2989–2997. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, S.; Elamanchili, P.; Alshamsan, A.; Satou, T.; Samuel, J. Enhanced antigen-specific primary CD4+ and CD8+ responses by codelivery of ovalbumin and toll-like receptor ligand monophosphoryl lipid A in poly(d,l-lactic-co-glycolic acid) nanoparticles. Clin. Exp. Rheumatol. 2015, 33, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Lee, Y.R.; Lee, Y.H.; Kim, K.H.; Im, S.A. Induction of Potent Antigen-specific Cytotoxic T Cell Response by PLGA-nanoparticles Containing Antigen and TLR Agonist. Immune. Netw. 2013, 13, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Zupancic, E.; Vandermeulen, G.; Oliveira, V.G.; Salgado, A.; Videira, M.; Gaspar, M.; Graca, L.; Préat, V.; Florindo, H.F. In vivo delivery of peptides and Toll-like receptor ligands by mannose-functionalized polymeric nanoparticles induces prophylactic and therapeutic anti-tumor immune responses in a melanoma model. J. Control. Release 2015, 198, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Schlosser, E.; Mueller, M.; Csaba, N.; Merkle, H.P.; Groettrup, M.; Gander, B. Concomitant delivery of a CTL-restricted peptide antigen and CpG ODN by PLGA microparticles induces cellular immune response. J. Drug Target. 2009, 17, 652–661. [Google Scholar] [CrossRef] [PubMed]

| Association | Type of Interaction | Polymers Involved |
|---|---|---|
 Encapsulation | / | PLA, PLGA, PCL |
 Adsorption | Electrostatic or hydrophobic | PLA, PLGA, PCL |
 Conjugation | Chemical cross-linking | PLA, PLGA |
| Particles Characteristics (Polymer-Size) | Model (Antigen-Model) | Target Receptor | Co-Administration or Co-Delivery | Immune Response | Ref. |
|---|---|---|---|---|---|
| γ-PGA-Phe-200 nm | OVA-mouse | TLR9 | Co-delivery | -CD8+ T cells response | [73] |
| HPMA-NIPAM-1 µm | OVA-mouse | TLR7 | Co-administration | -CD8+ T cells response CD4+ T cells response -Antibody | [69] |
| Mannose-functionalized aliphatic polyester-150 nm | OVA-mouse | TLR3 + TLR9 | Co-delivery | -CD8+ T cells response | [78] |
| PLA-200 nm | HIV-1 p24-mouse | NOD1 or NOD2 | Co-administration | -Antibody | [18] |
| PLGA-200 nm | OVA-mouse | TLR3 + TLR7 | Co-delivery | -CD8+ T cells response -CD4+ T cells response -Antibody | [65] |
| PLGA-200 nm | OVA-mouse | TLR4 + TLR9 | Co-delivery | -CD8+ T cells response | [66] |
| PLGA-200 nm | OVA-mouse | TLR3 + TLR7 | Co-delivery | -CD8+ T cells response | [63] |
| PLGA-250 nm | rWNVE-mouse | TLR9 | Co-delivery | -Antibody -Protection to West Nile Encephalitis | [75] |
| PLGA-300 nm | OVA-mouse | TLR4 + TLR7 | Co-delivery | -Antibody | [67] |
| PLGA-350 nm | OVA-mouse | TLR3 | Co-administration | -CD8+ T cells response | [33] |
| PLGA-350 nm | DTaP-mouse | TLR7 | Co-delivery | -Antibody | [74] |
| PLGA-400 nm | OVA-mouse | TLR4 | Co-delivery | -CD8+ T cells response -CD4+ T cells response | [76] |
| PLGA-400 nm | Melanoma antigen-mouse | TLR4 | Co-delivery | -Anti-tumor effect -CD8+ T cells response | [60] |
| PLGA-1 µm | OVA-mouse | TLR3 or TLR9 | Co-delivery | -CD8+ T cells response -Antitumor activity | [77] |
| PLGA-1–10 µm | Tumor lysate-mouse | TLR9 | Co-delivery | -CD8+ T cells response -Antitumor activity | [61] |
| PLGA-1–30 µm | OVA-mouse | TLR7 + TLR9 | Co-delivery | -CD8+ T cells response -CD4+ T cells response | [72] |
| pLHMGA-450 nm | HPV synthetic long peptide-mouse | TLR3 | Co-delivery | -CD8+ T cells response | [62] |
| PPS-30 nm | OVA-mouse | TLR9 | Co-delivery | -Cross-presentation -CD8+ T cells response | [68] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutjahr, A.; Phelip, C.; Coolen, A.-L.; Monge, C.; Boisgard, A.-S.; Paul, S.; Verrier, B. Biodegradable Polymeric Nanoparticles-Based Vaccine Adjuvants for Lymph Nodes Targeting. Vaccines 2016, 4, 34. https://doi.org/10.3390/vaccines4040034
Gutjahr A, Phelip C, Coolen A-L, Monge C, Boisgard A-S, Paul S, Verrier B. Biodegradable Polymeric Nanoparticles-Based Vaccine Adjuvants for Lymph Nodes Targeting. Vaccines. 2016; 4(4):34. https://doi.org/10.3390/vaccines4040034
Chicago/Turabian StyleGutjahr, Alice, Capucine Phelip, Anne-Line Coolen, Claire Monge, Anne-Sophie Boisgard, Stéphane Paul, and Bernard Verrier. 2016. "Biodegradable Polymeric Nanoparticles-Based Vaccine Adjuvants for Lymph Nodes Targeting" Vaccines 4, no. 4: 34. https://doi.org/10.3390/vaccines4040034
APA StyleGutjahr, A., Phelip, C., Coolen, A.-L., Monge, C., Boisgard, A.-S., Paul, S., & Verrier, B. (2016). Biodegradable Polymeric Nanoparticles-Based Vaccine Adjuvants for Lymph Nodes Targeting. Vaccines, 4(4), 34. https://doi.org/10.3390/vaccines4040034






