Replicon RNA Viral Vectors as Vaccines
Abstract
:1. Introduction
2. Self-Replicating RNA Expression Systems
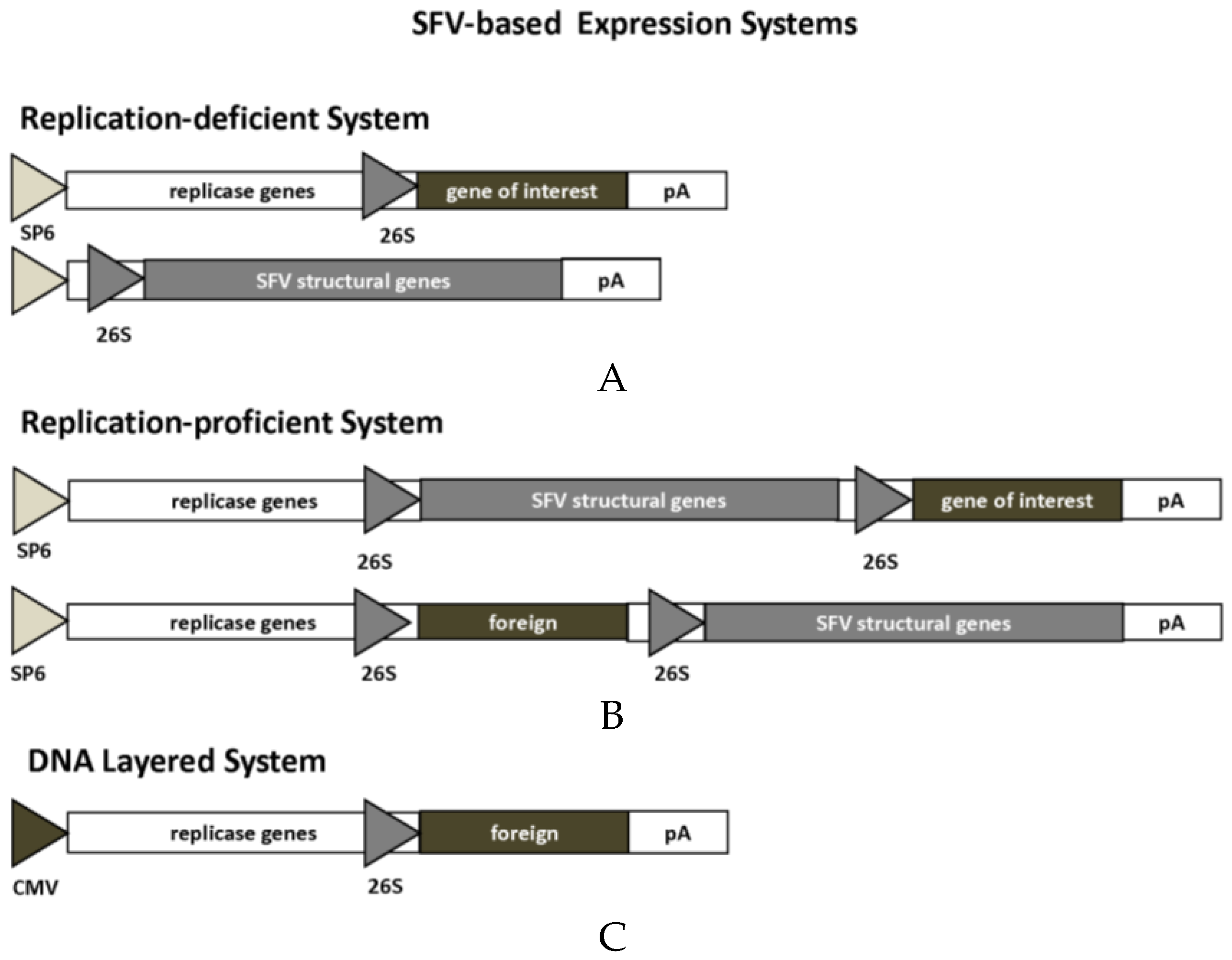
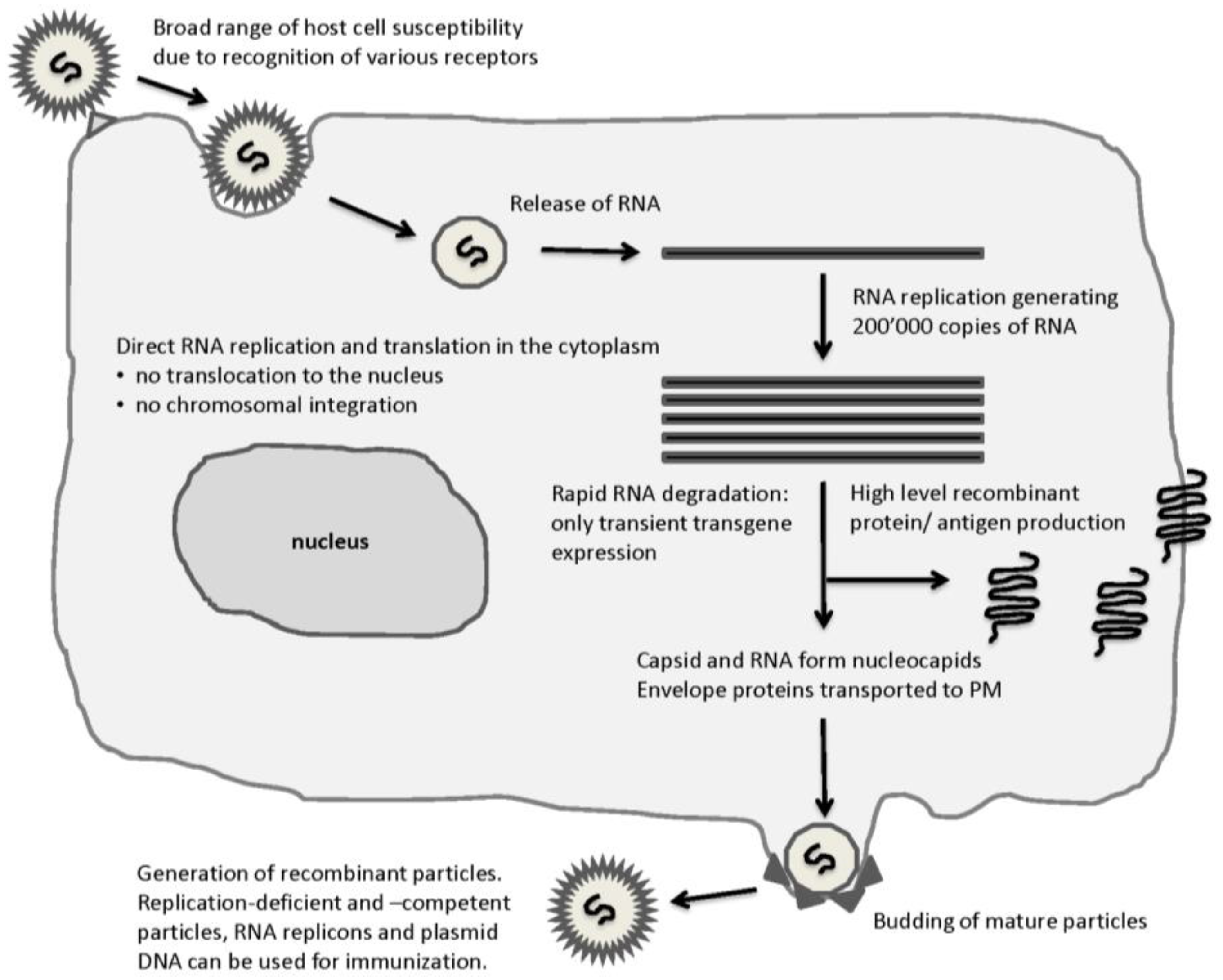
2.1. Alphaviruses
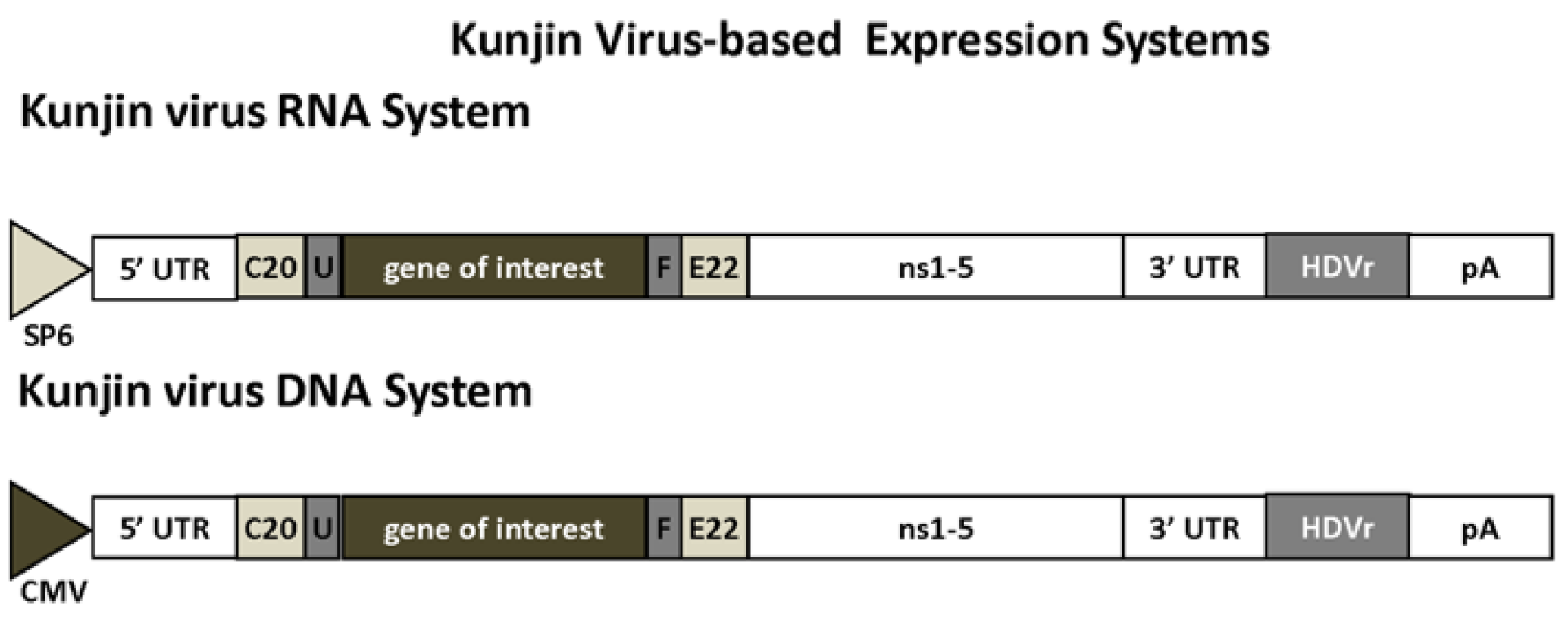
2.2. Flaviviruses
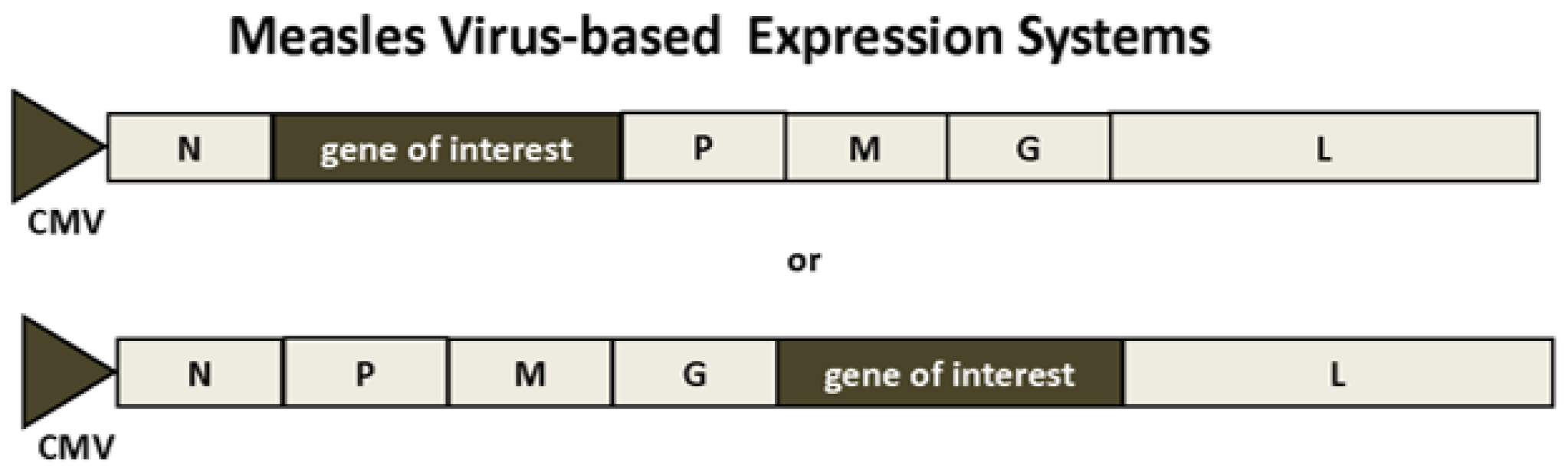
2.3. Measles Viruses
2.4. Rhabdoviruses
3. Self-Replicating RNA Virus-Based Vaccines
3.1. Vaccines against Infectious Diseases
3.2. Vaccines against Cancer
3.3. Clinical Trials
4. Conclusions
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| CEA | Carcinoembryonic antigen |
| CMV | Cytomegalovirus |
| CRPC | Castration-resistant metastatic prostate cancer |
| CRT | Calreticulin |
| CTL | Cytotoxic T-lymphocyte |
| DC | Dendritic cell |
| EGFP | Enhanced green fluorescent protein |
| EGFR | Epidermal growth factor receptor |
| GFP | Green fluorescent protein |
| GM-CSF | Granylocyte macrophage colony-stimulating factor |
| GoI | Gene of interest |
| HA | Hemagglutinin |
| HBV | Hepatitis B virus |
| HCC | Hepatocellular carcinoma |
| HPV | zhuman papilloma virus |
| IF3 | Initiation factor 3 |
| IFN | Interferon |
| IL | Interleukin |
| ISG | Interferon-stimulated gene |
| MERS-CoV | Middle East respiratory syndrome coronavirus |
| MHC | Major histocompatibility complex |
| miRNA | Micro RNA |
| MLN | Mediastinal lymph nodes |
| MV | Measles virus |
| NIS | Sodium iodide symporter |
| NP | Nucleoprotein |
| PDAC | Pancreatic ductal adenocarcinoma |
| PSMA | Prostate-specific membrane antigen |
| PSCA | Prostate stem cell antigen |
| RABV | Rabies virus; |
| RSV | Respiratory syndrome virus |
| SARS-CoV | Severe acute respiratory syndrome coronavirus |
| SFV | Semliki Forest virus |
| SEB | Staphylococcus enterotoxin B |
| SIN | Sindbis virus |
| ssRNA | Single-stranded RNA |
| STEAP | Six-transmembrane epithelial antigen of the prostate |
| TAA | Tumor-associated antigen |
| TRAMP | Transgene adenocarcinoma of the mouse prostate |
| TRP | Tyrosine-related protein |
| Tyr | Tyrosinase |
| VEE | Venezuelan equine encephalitis virus |
| VEGFR | Vascular endothelial growth factor receptor |
| VLPs | Virus-like particles |
| VSV | Vesicular stomatitis virus |
| WHV | Woodchuck hepatitis virus |
References
- Delrue, I.; Verzele, D.; Madder, A.; Nauwynck, H.J. Inactivated virus vaccines: From chemistry to prophylaxis: Merits, risks and challenges. Expert Rev. Vaccines 2012, 11, 695–719. [Google Scholar] [CrossRef]
- Deng, M.P.; Hu, Z.H.; Wan, H.L.; Deng, F. Developments of subunit and VLP vaccines against influenza A virus. Virol. Sin. 2012, 27, 145–153. [Google Scholar] [CrossRef]
- Apostolopoulos, V. Vaccine delivery methods into the future. Vaccines 2016. [Google Scholar] [CrossRef]
- Lundstrom, K. Alphavirus-based vaccines. Viruses 2014, 6, 2392–2415. [Google Scholar] [CrossRef]
- Schmidt, S.T.; Foged, C.; Korsholm, K.S.; Rades, T.; Christensen, D. Liposome-based adjuvants for subunit vaccines: Formulation strategies for subunit antigens and immunostimulators. Pharmaceutics 2016. [Google Scholar] [CrossRef]
- Cibulski, S.P.; Mourglia-Ettlin, G.; Teixeira, T.F.; Quirici, L.; Roehe, P.M.; Ferreira, F.; Silveira, F. Novel ISCOMs from Quillaja brasiliensis saponins induce mucosal and systemic antibody production, T-cell responses and improved antigen uptake. Vaccine 2016, 34, 1162–1171. [Google Scholar] [CrossRef]
- Sheng, K.C.; Kalkanidis, M.; Pouniotis, D.S.; Esparon, S.; Tang, C.K.; Apostolopoulos, V.; Pietersz, G.A. Delivery of antigen using a novel mannosylated dendrimer potentiates immunogenicity in vitro and in vivo. Eur. J. Immunol. 2008, 38, 424–436. [Google Scholar] [CrossRef]
- Lundstrom, K. Alphaviruses in gene therapy. Viruses 2015, 7, 2321–2333. [Google Scholar] [CrossRef]
- Lundstrom, K. Self-replicating RNA viral vectors in vaccine development and gene therapy. Future Virol. 2016, 11, 345–356. [Google Scholar] [CrossRef]
- Lyles, D.S.; Rupprecht, C.E. Rhabdoviridiae. In Fields’ Virology; Knipe, D.M., Howley, P.M., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2007; pp. 1364–1408. [Google Scholar]
- Rima, B.K.; Duprex, W.P. The measles virus replication cycle. Curr. Top. Microbiol. Immunol. 2009, 329, 77–102. [Google Scholar]
- Brinton, M.A. Replication cycle and molecular biology of West Nile virus. Viruses 2013, 6, 13–53. [Google Scholar] [CrossRef]
- Westaway, E.G.; Mckenzie, J.M.; Khromykh, A.A. Kunjin RNA replication and applications of Kunjin replicons. Adv. Virus Res. 2003, 59, 99–140. [Google Scholar]
- Liljestrom, P.; Garoff, H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology 1991, 9, 1356–1361. [Google Scholar] [CrossRef]
- Xiong, C.; Levis, R.; Shen, P.; Schlesinger, S.; Rice, C.M.; Huang, H.V. Sindbis virus: An efficient, broad host range vector for gene expression in animal cells. Science 1989, 243, 1188–1191. [Google Scholar] [CrossRef]
- Davis, N.L.; Willis, L.V.; Smith, J.F.; Johnston, R.E. In vitro synthesis of infectious Venezuelan equine encephalitis virus RNA from a cDNA clone: Analysis of a viable deletion mutant. Virology 1989, 171, 189–204. [Google Scholar] [CrossRef]
- Strauss, J.H.; Strauss, E.G. The Alphaviruses: Gene Expression, Replication and Evolution. Micobiol. Rev. 1994, 58, 491–562. [Google Scholar]
- Pijlman, G.P.; Suhrbier, A.; Khromykh, A.A. Kunjin virus replicons: An RNA-based, non-cytopathic viral vector system for protein production, vaccine and gene therapy applications. Exp. Opin. Biol. Ther. 2006, 6, 134–145. [Google Scholar] [CrossRef]
- Shi, P.Y.; Tilgner, M.; Lo, M.K. Construction and characterization of subgenomic replicons of New York strain of West Nile virus. Virology 2002, 296, 219–233. [Google Scholar] [CrossRef]
- Scholle, I.; Girard, Y.A.; Zhao, Q.; Higgs, S.; Mason, P.W. Trans-packaged West Nile virus-like particles: Infectious properties in vitro and in infected mosquito vectors. J. Virol. 2004, 78, 11605–11614. [Google Scholar] [CrossRef]
- Molenkamp, R.; Kooi, E.A.; Lucassen, M.A.; Greve, S.; Thijssen, J.C.; Spaan, W.J.; Bredenbeek, P.J. Yellow fever virus replicons as an expression system for hepatitis C virus structural proteins. J. Virol. 2003, 77, 1644–1648. [Google Scholar] [CrossRef]
- Jones, C.T.; Patkar, C.G.; Kuhn, R.J. Construction and applications of yellow fever virus replicons. Virology 2005, 331, 247–259. [Google Scholar] [CrossRef]
- Jones, M.; Davidson, A.; Hibbert, L.; Gruenwald, P.; Schlaak, J.; Ball, S.; Foster, G.R.; Jacobs, M. Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J. Virol. 2005, 79, 5414–5420. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, M.; Dayton, A.I. Development of Dengue virus Type 2 replicons capable of prolonged expression in host cells. BMC Microbiol. 2001. [Google Scholar] [CrossRef]
- Gherke, R.; Ecker, M.; Aberle, S.W.; Allison, S.L.; Heinz, F.X.; Mandi, C.W. Incorporation of tick-borne encephalitis virus replicons into virus-like particles by a packaging cell line. J. Virol. 2003, 77, 8924–8933. [Google Scholar] [CrossRef]
- Hayasaka, D.; Yoshii, K.; Ueki, T.; Iwasaki, T.; Takashima, I. Sub-genomic replicons of Tick-borne encephalitis virus. Arch.Virol. 2004, 149, 1245–1256. [Google Scholar] [CrossRef]
- Khromykh, A.A.; Varnavski, A.N.; Westaway, E.G. Encapsidation of the flavivirus kunjin replicon RNA by using a complementation system providing Kunjin virus structural proteins in trans. J. Virol. 1998, 72, 5967–5977. [Google Scholar]
- De Felipe, F. Skipping the co-expression problem: The new 2A “CHYSEL” technology. Genet. Vaccines Ther. 2004. [Google Scholar] [CrossRef] [Green Version]
- Radecke, F.; Spielhofer, P.; Schneider, H.; Kaelin, K.; Huber, M.; Dötsch, C.; Christiansen, G.; Billeter, M.A. Rescue of measles viruses from cloned DNA. EMBO J. 1995, 14, 5773–5784. [Google Scholar]
- Singh, M.; Cattaneo, R.; Billeter, M.A. A recombinant measles virus expressing hepatitis B surface antigen induces humoral responses in genetically modified mice. J. Virol. 1999, 73, 4823–4828. [Google Scholar]
- Ito, N.; Takayama-Ito, M.; Yamada, K.; Hosokawa, J.; Sugiyama, M.; Minamoto, N. Improved recovery of rabies virus from cloned cDNA using a vaccinia virus-free reverse genetics system. Microbiol. Immunol. 2003, 47, 613–617. [Google Scholar] [CrossRef]
- Harty, R.N.; Brown, M.E.; Hayes, F.P.; Wright, N.T.; Schnell, M.J. Vaccinia virus-free recovery of vesicular stomatitis virus. J. Mol. Microbiol. Biotechnol. 2001, 3, 513–517. [Google Scholar]
- An, H.; Kim, G.N.; Kang, C.Y. Genetically modified VSV(NJ) vector is capable of accommodating a large foreign gene insert and allows high level gene expression. Virus Res. 2013, 171, 168–177. [Google Scholar] [CrossRef]
- Dorange, F.; Piver, E.; Bru, T.; Collin, C.; Roingeard, P. Vesicular stomatitis virus glycoprotein: A transducing coat for SFV-based RNA vectors. J. Gene Med. 2004, 6, 1014–1022. [Google Scholar] [CrossRef]
- Malone, J.G.; Berglund, P.J.; Liljestrom, P.; Rhodes, G.; Malone, R.W. Mucosal immune responses associated with polynucleotide vaccination. Behring Inst. Mitt. 1997, 98, 63–72. [Google Scholar]
- Schultz-Cherry, S.; Dybing, J.K.; Davis, N.L.; Williamson, C.; Suarez, D.L.; Johnston, R.; Perdue, M.L. Influenza virus (A/HK/156/97) hemagglutinin expressed by an alphavirus replicon system protects against lethal infection with Hong Kong-origin H5N1 viruses. Virology 2000, 278, 55–59. [Google Scholar] [CrossRef]
- Bosworth, B.; Erdman, M.M.; Stine, D.L.; Harris, I.; Irwin, C.; Jens, M.; Loynachan, A.; Kamrud, K.; Harris, D.L. Replicon particle vaccine protects swine against influenza. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, e99–e103. [Google Scholar] [CrossRef]
- Vander Veen, R.L.; Loynachan, A.T.; Mogler, M.A.; Russell, B.J.; Harris, D.L.; Kamrud, K. Safety, immunogenicity and efficacy of an alphavirus replicon-based swine influenza virus hemagglutinin vaccine. Vaccine 2012, 30, 1944–1950. [Google Scholar] [CrossRef]
- Sweft-Tapia, C.; Bogaert, L.; de Jong, P.; van Hoek, V.; Schouten, T.; Damen, I.; Spek, D.; Wanningen, P.; Radosevic, K.; Zahn, R.; et al. Recombinant measles virus incorporating heterlogous viral membrane proteins for use as vaccines. J. Gen. Virol. 2016, 97, 2117–2128. [Google Scholar] [CrossRef]
- Ryder, A.B.; Nachbaqauer, R.; Buonocore, L.; Palese, P.; Krammer, F.; Rose, J.K. Vaccination with Vesicular Stomatitis virus-vectored chimeric hemagglutinins protects mice against divergent influenza virus challenge strains. J. Virol. 2015, 90, 2544–2550. [Google Scholar] [CrossRef]
- Harvey, T.J.; Anraku, I.; Linedale, R.; Harrich, D.; Mackenzie, J.; Suhrbier, A.; Khromykh, A.A. Kunjin virus replicon vectors for human immunodefiency virus vaccine development. J. Virol. 2003, 77, 7796–7803. [Google Scholar] [CrossRef]
- Brand, D.; Lemiale, F.; Turbica, I.; Buzelay, L.; Brunet, S.; Barin, F. Comparative analysis of humoral immune responses to HIV type 1 envelope glycoproteins in mice immunized with a DNA vaccine, recombinant Semliki Forest virus RNA, or recombinant Semliki Forest virus particles. AIDS Res. Hum. Retrovir. 1998, 14, 1369–1377. [Google Scholar] [CrossRef]
- Giraud, A.; Ataman-Onal, Y.; Battail, N.; Piga, N.; Brand, D.; Mandrand, B.; Vernier, B. Generation of monoclonal antibodies to native human immunodeficiency virus type 1 envelope glycoprotein by immunization of mice with naked RNA. J. Virol. Methods 1999, 79, 75–84. [Google Scholar] [CrossRef]
- Knudsen, M.L.; Ljungberg, K.; Tatoud, R.; Weber, J.; Esteban, M.; Liljestrom, P. Alphavirus replicon DNA expressing HIV antigens is an excellent prime for boosting with recombinant modified vaccinia Ankara (MVA) or with HIV gp140 protein antigen. PLoS ONE 2015, 10, e0117042. [Google Scholar] [CrossRef]
- Anraku, I.; Mokhonov, V.V.; Rattanasena, P.; Mokhonova, E.I.; Leung, J.; Pijlman, G.; Cara, A.; Schroeder, W.A.; Khromykh, A.A.; Suhrbier, A. Kunjin replicon-based simian immunodeficiency virus gag vaccines. Vaccine 2008, 26, 3268–3276. [Google Scholar] [CrossRef]
- Schell, J.B.; Bahl, K.; Folta-Stogniew, E.; Rose, N.; Buonocore, L.; Marx, P.A.; Gambhira, R.A.; Rose, J.K. Antigenic requirement for Gag in a vaccine that protects against high-dose mucosal challenge with simian immunodeficiency virus. Virology 2015, 476, 405–412. [Google Scholar] [CrossRef]
- Van Rompay, K.K.; Abel, K.; Earl, P.; Kozlowski, P.A.; Easlick, J.; Moore, J.; Buonocore-Buzzelli, L.; Schmidt, K.A.; Wilson, R.L.; Simon, I.; et al. Immunogenicity of viral vector, prime-boost SIV vaccine regimens in infant rhesus macaques: Attenuated vesicular stomatitis virus (VSV) and modified vaccinia Ankara (MVA) recombinant SIV vaccines compared to live-attenuated SIV. Vaccine 2010, 28, 1481–1492. [Google Scholar] [CrossRef]
- McKenna, P.M.; Koser, M.L.; Carlson, K.R.; Montefiori, D.C.; Letvin, N.L.; Papaneri, A.B.; Pomerantz, R.J.; Dietzschold, B.; Silvera, P.; McGettigan, J.P.; et al. Highly attenuated rabies virus-based vaccine vectors expressing simian-human immunodeficiency virus89.6P Env and simian immunodeficiency virus mac239 Gag are safe in rhesus macaques and protect from an AIDS-like disease. J. Infect. Dis. 2007, 195, 980–988. [Google Scholar] [CrossRef]
- Reynard, O.; Mokhonov, V.; Mokhonova, E.; Leung, J.; Page, A.; Mateo, M.; Pyankova, O.; Georges-Courbot, M.C.; Raoul, H.; Khromykh, A.A.; et al. Kunjin virus replicon-based vaccines expressing Ebola virus glycoprotein GP protect the guinea pig against lethal Ebola virus infection. J. Infect. Dis. 2011, 204, S1060–S1065. [Google Scholar] [CrossRef]
- Pyankov, O.V.; Bodnev, S.A.; Pyankova, O.G.; Solodkyi, V.V.; Pyankov, S.A.; Setoh, Y.X.; Volchkova, V.A.; Suhrbier, A.; Volchkov, V.E.; Agafonov, A.A.; et al. A Kunjin replicon virus-like vaccine provides protection against Ebola virus infection in nonhuman primates. J. Infect.Dis. 2015, 212, S368–S371. [Google Scholar] [CrossRef]
- Marzi, A.; Robertson, S.J.; Haddock, E.; Feldmann, F.; Hanley, P.W.; Scott, D.P.; Strong, J.E.; Kobinger, G.; Best, S.M.; Feldmann, H. EBOLA VACCINE. VSV-EBOV rapidly protects macaques against infection with the 2014/2015 Ebola virus outbreak strain. Science 2015, 349, 739–742. [Google Scholar] [CrossRef]
- Geisbert, T.W.; Feldmann, H. Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg infections. J. Infect. Dis. 2011, 204, S1075–S1081. [Google Scholar] [CrossRef]
- Pushko, P.; Bray, M.; Ludwig, G.V.; Parker, M.; Schmaljohn, A.; Sanchez, A.; Jahrling, P.B.; Smith, J.F. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine 2000, 19, 142–153. [Google Scholar] [CrossRef]
- Wilson, J.A.; Hart, M.K. Protection from Ebola virus mediated by cytotoxic T-lymphocytes specific for the viral nucleoprotein. J. Virol. 2001, 75, 2660–2664. [Google Scholar] [CrossRef]
- Safronetz, D.; Mire, C.; Rosenke, K.; Feldmann, F.; Haddock, E.; Geisbert, T.; Feldmann, H. A recombinant Vesicular stomatitis virus-based Lassa fever vaccine protects guinea pigs and macaques against challenge with geographically and genetically distinct Lassa viruses. PLoS Negl. Trop. Dis. 2015, 9, e0003736. [Google Scholar] [CrossRef]
- Pushko, P.; Geisbert, J.; Parker, M.; Jahrling, P.; Smith, J. Individual and bivalent vaccines based on alphavirus replicons protect guinea pigs against infection with Lassa and Ebola viruses. J. Virol. 2001, 75, 11677–11685. [Google Scholar] [CrossRef]
- Sheahan, T.; Whitmore, A.; Long, K.; Ferris, M.; Rockx, B.; Funkhouser, D.; Donaldson, E.; Gralinski, L.; Collier, M.; Heise, M.; et al. Successful vaccination strategies that protect aged mice from lethal challenge from influenza virus and heterologous severe acute respiratory syndrome coronavirus. J. Virol. 2011, 85, 217–230. [Google Scholar] [CrossRef]
- Malczyk, A.H.; Kupke, A.; Prüfer, S.; Scheuplein, V.A.; Hutzler, S.; Kreuz, D.; Beissert, T.; Bauer, S.; Hubich-Rau, S.; Tondera, C.; et al. A Highly immunogenic and protective middle east respiratory syndrome coronavirus vaccine based on a recombinant measles virus vaccine platform. J. Virol. 2015, 89, 11654–11667. [Google Scholar] [CrossRef]
- Geall, A.J.; Verma, A.; Otten, G.R.; Shaw, C.A.; Hekele, A.; Banerjee, K.; Cu, Y.; Beard, C.W.; Brito, L.A.; Krucker, T.; et al. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl. Acad. Sci. USA 2012, 109, 14604–14609. [Google Scholar] [CrossRef]
- Bates, J.T.; Pickens, J.A.; Schuster, J.E.; Johnson, M.; Tollefson, S.J.; Williams, J.V.; Davis, N.L.; Johnston, R.E.; Schultz-Darken, N.; Slaughter, J.C.; et al. Immunogenicity and efficacy of alphavirus-derived replicon vaccines for respiratory syncytial virus and human metapneumovirus in nonhuman primates. Vaccine 2016, 34, 950–956. [Google Scholar] [CrossRef]
- Harahap-Carrillo, I.S.; Ceballos-Olvera, I.; Valle, J.R. Immunogenic subviral particles displaying domain III of Dengue 2 envelope protein vectored by measles virus. Vaccines 2015, 3, 503–518. [Google Scholar] [CrossRef]
- Hu, H.M.; Chen, H.W.; Hsiao, Y.; Wu, S.H.; Chung, H.H.; Hsie, C.H.; Chong, P.; Leng, C.H.; Pan, C.H. The successful induction of T-cell and antibody responses by a recombinant measles virus-vectored tetravalent dengue vaccine provides partial protection against dengue-2 infection. Hum. Vaccines Immunother. 2016. [Google Scholar] [CrossRef]
- White, L.J.; Sariol, C.A.; Mattocks, M.D.; Wahala, M.P.B.W.; Yingsiwaphat, V.; Collier, M.L.; Whitley, J.; Mikkelsen, R.; Rodriguez, I.V.; Martinez, M.I.; et al. An alphavirus vector-based tetravalent dengue vaccine induces a rapid and protective immune response in macaques that differs qualitatively from immunity induced by live virus infection. J. Virol. 2013, 87, 3409–3424. [Google Scholar] [CrossRef]
- Khalil, S.M.; Tonkin, D.R.; Mattocks, M.D.; Snead, A.T.; Johnston, R.E.; White, L.J. A tetravalent alphavirus-vector based dengue vaccine provides effective immunity in an early life mouse model. Vaccine 2014, 32, 4068–4074. [Google Scholar] [CrossRef]
- Reynolds, T.D.; Buonocore, L.; Rose, N.F.; Rose, J.K.; Robek, M.D. Virus-like vesicle-based therapeutic vaccine vectors for chronic hepatis B virus infection. J. Virol. 2015, 89, 10407–10415. [Google Scholar] [CrossRef]
- Del Valle, J.R.; Devaux, P.; Hodge, G.; Wegner, N.J.; McChesney, M.B.; Catteneo, R. A vectored measles virus induces hepatitis B surface antigen antibodies while protecting macaques against virus challenge. J. Virol. 2007, 81, 10597–10605. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Reap, E.A.; Katen, K.; Watson, A.; Smith, K.; Norberg, P.; Olmsted, R.A.; Hoeper, A.; Morris, J.; Negri, S.; et al. Randomized, double-blind, Phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine 2009, 28, 484–493. [Google Scholar] [CrossRef]
- Andersson, C.; Vasconcelos, N.M.; Sievertzon, M.; Haddad, D.; Liljeqvist, S. Comparative immunization study using RNA and DNA constructs encoding a part of the Plasmodium falciparum antigen Pf332. Scand. J. Immunol. 2001, 54, 117–124. [Google Scholar] [CrossRef]
- Kirman, J.R.; Turon, T.; Su, H.; Li, A.; Kraus, C.; et al. Enhanced immunogenicity to Mycobacterium tuberculosis by vaccination with an alphavirus plasmid replicon expressing antigen 85A. Infect. Immun. 2003, 71, 575–579. [Google Scholar] [CrossRef]
- Li, N.; Yu, Y.Z.; Yu, W.Y.; Sun, Z.W. Enhancement of the immunogenicity of DNA replicon vaccine of Clostridium botulinum neurotoxin serotype A by GM-CSF gene adjuvant. Immunopharmacol. Immunotoxicol. 2011, 33, 211–219. [Google Scholar] [CrossRef]
- Cabrera, A.; Sáez, D.; Céspedes, S.; Andrews, E.; Oñate, A. Vaccination with recombinant Semliki Forest virus particles expressing translation initiation factor 3 of Brucella abortus induces protective immunity in BALB/c mice. Immunobiology 2009, 214, 467–474. [Google Scholar] [CrossRef]
- Thomas, J.M.; Moen, S.T.; Gnade, B.T.; Vargas-Inchaustegui, D.A.; Foltz, S.M.; Suarez, G.; Heidner, H.W.; König, R.; Chopra, A.K.; Peterson, J.W. Recombinant Sindbis virus vectors designed to express protective antigen of Bacillus anthracis protect animals from anthrax and display synergy with ciprofloxacin. Clin. Vaccine Immunol. 2009, 16, 1696–1699. [Google Scholar] [CrossRef]
- Tsuji, M.; Bergmann, C.C.; Takita-Sonoda, Y.; Murata, K.; Rodrigues, E.G.; Nussenzweig, R.S.; Zavala, F. Recombinant Sindbis viruses expressing a cytotoxic T-lymphocyte epitope of a malaria parasite or of influenza virus elicit protection against the corresponding pathogen in mice. J. Virol. 1998, 72, 6907–6910. [Google Scholar]
- Tober, R.; Banki, Z.; Egerer, L.; Muik, A.; Behmüller, S.; Kreppel, F.; Greczmiel, U.; Oxenius, A.; von Laer, D.; Kimpel, J. VSV-GP: A potent viral vaccine vector that boosts the immune response upon repeated applications. J. Virol. 2014, 88, 4897–907. [Google Scholar] [CrossRef]
- Krasemann, S.; Jürgens, T.; Bodemer, W. Generation of monoclonal antibodies against prion proteins with an unconventional nucleic acid-based immunization strategy. J. Biotechnol. 1999, 73, 119–129. [Google Scholar] [CrossRef]
- Lee, J.S.; Dyas, B.K.; Nystrom, S.S.; Lind, C.M.; Smith, J.F.; Ulrich, R.G. Immune protection against staphylococcal enterotoxin-induced toxic shock by vaccination with a Venezuelan equine encephalitis virus replicon. J. Infect. Dis. 2002, 185, 1192–1196. [Google Scholar] [CrossRef]
- Marzi, A.; Feldmann, F.; Geisbert, T.W.; Feldmann, H.; Safronetz, D. Vesicular stomatitis virus-based vaccines against Lassa and Ebola viruses. Emerg. Infect. Dis. 2015, 21, 305–307. [Google Scholar] [CrossRef]
- Tangy, F.; Naim, H.Y. Live attenuated measles vaccine as a potential multivalent pediatric vaccination vector. Viral Immunol. 2005, 18, 317–26. [Google Scholar] [CrossRef]
- Msaouel, P.; Iankov, I.D.; Dispenzieri, A.; Galanis, E. Attenuated oncolysis measles virus strains as cancer therapeutics. Curr. Pharm. Biotechnol. 2012, 13, 1732–1741. [Google Scholar] [CrossRef]
- Grote, D.; Russell, S.J.; Cornu, T.I.; Cattaneo, R.; Vile, R.; Poland, G.A.; Fielding, A.K. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood 2001, 97, 3746–3754. [Google Scholar] [CrossRef]
- Hasegawa, K.; Pham, L.; O’Connor, M.K.; Federspiel, M.J.; Russell, S.J.; Peng, K.W. Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter. Clin. Cancer Res. 2006, 12, 1868–1875. [Google Scholar] [CrossRef]
- Paraskevakou, G.; Allen, C.; Nakamura, T.; Zollman, P.; James, C.D.; Peng, K.W.; Schroeder, M.; Russll, S.J.; Galanis, E. Epidermal growth factor receptor (EGFR)-retargeted measles virus strains effectively target EGFR- or EGFRvIII expressing gliomas. Mol. Ther. 2007, 15, 677–686. [Google Scholar] [CrossRef]
- McDonald, C.J.; Erlichman, C.; Ingle, J.N.; Rosales, G.A.; Allen, C.; Greiner, S.M.; Harvey, M.E.; Zollman, P.J.; Russell, S.J.; Galanis, E. A measles virus vaccine strain derivative as a novel oncolytic agent against breast cancer. Breast Cancer Res. Treat. 2006, 99, 177–184. [Google Scholar] [CrossRef]
- Hastie, E.; Grdzelishvili, V.Z. Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer. J. Gen. Virol. 2012, 93, 2529–2545. [Google Scholar] [CrossRef]
- Murphy, A.M.; Besmer, D.M.; Moerdyk-Schauwecker, M.; Moesti, N.; Ornelles, D.A.; Mukherjee, P.; Grdzelishvili, V.Z. Vesicular stomatitis virus as an oncolytic agent against pancreatic ductal adenocarcinoma. J. Virol. 2012, 86, 3073–3087. [Google Scholar] [CrossRef]
- Felt, S.A.; Moerdyk-Schauwecker, M.J.; Grdzelishvili, V.Z. Induction of apoptosis in pancreatic cancer cells by vesicular stomatitis virus. Virology 2015, 474, 163–173. [Google Scholar] [CrossRef]
- Hoang-Le, D.; Smeenk, L.; Anraku, I.; Pijlman, G.P.; Wang, X.J.; de Vrij, J.; Liu, W.J.; Lee, T.T.; Schroeder, W.A.; Khromykh, A.A.; et al. A Kunjin replicon vector encoding granulocyte macrophage colony-stimulating factor for intra-tumoral gene therapy. Gene Ther. 2009, 16, 190–199. [Google Scholar] [CrossRef]
- Herd, K.A.; Harvey, T.; Khromykh, A.A.; Tindle, R.W. Recombinant Kunjin virus replicon vaccines induce protective T-cell immunity against human papillomavirus 16 E7-expressing tumour. Virology 2004, 319, 237–248. [Google Scholar] [CrossRef]
- Yamanaka, R.; Zullo, S.A.; Ramsey, J.; Onodera, M.; Tanaka, R.; Blaese, M.; Xanthopoulos, K.G. Induction of therapeutic antitumor antiangiogenesis by intratumoral injection of genetically engineered endostatin-producing Semliki Forest virus. Cancer Gene Ther. 2001, 8, 796–802. [Google Scholar] [CrossRef]
- Martikainen, M.; Niittykoski, M.; von und zu Frauenberg, M.; Immonen, A.; Koponen, S.; van Geenen, M.; Vähä-Koskela, M.; Ylösmäki, E.; Jääskeläinen, J.E.; Saksela, E.; et al. MicroRNA-attenuated clone of virulent Semliki Forest virus overcomes antiviral type I interferon in resistant mouse CT-2A glioma. J. Virol. 2015, 89, 10637–10647. [Google Scholar] [CrossRef]
- Yamanaka, R.; Zullo, S.A.; Tanaka, R.; Ramsey, J.; Blaese, M.; Xanthopoulos, K.G. Induction of a therapeutic antitumor immunological response by intratumoral injection of genetically engineered Semliki Forest virus to produce interleukin-12. Neurosurg. Focus 2000, 9, e7. [Google Scholar] [CrossRef]
- Yamanaka, R.; Zullo, S.A.; Ramsey, J.; Yajima, N.; Tsuchiya, N.; Tanaka, R.; Blaese, M.; Xanthopoulos, K.G. Marked enhancement of antitumor immune responses in mouse brain tumor models by genetically modified dendritic cells producing Semliki Forest virus-mediated interleukin-12. J. Neurosurg. 2002, 97, 611–618. [Google Scholar] [CrossRef]
- Roche, F.P.; Sheahan, B.J.; O’Mara, S.M.; Atkins, G.J. Semliki Forest virus-mediated gene therapy of the RG2 rat glioma. Neuropathol. Appl. Neurobiol. 2010, 36, 648–660. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.P.; Rao, X.M.; Price, J.E.; Zhous, H.S.; Lachman, L.B. Prime-boost vaccination with plasmid and adenovirus gene vaccines control HER2/neu+ metastatic breast cancer in mice. Breast Cancer Res. 2005, 7, R580–R588. [Google Scholar] [CrossRef]
- Moran, T.P.; Burgents, J.E.; Long, B.; Ferrer, I.; Jaffee, E.M.; Tisch, R.M.; Johnston, R.E.; Serody, J.S. Alphaviral vector-transduced dendritic cells are successful therapeutic vaccines against neu-overexpressing tumors in wild-type mice. Vaccine 2007, 25, 6604–6612. [Google Scholar] [CrossRef]
- Lyons, J.A.; Sheahan, B.J.; Galbraith, S.E.; Mehra, R.; atkins, G.J.; Fleeton, M.N. Inhibition of angiogenesis by a Semliki Forest virus vector expressing VEGFR-2 reduces tumour growth and metastasis in mice. Gene Ther. 2007, 14, 503–513. [Google Scholar] [CrossRef]
- Daemen, T.; Riezebos-Brilman, A.; Bungener, L.; Regts, J.; Dontje, B.; Wiltschut, J. Eradication of established HPV16-transformed tumours after immunisation with recombinant Semliki Forest virus expressing a fusion protein of E6 and E7. Vaccine 2003, 21, 1082–1088. [Google Scholar] [CrossRef]
- Van de Wall, S.; Walczak, M.; van Rooij, N.; Hoogeboom, B.N.; Meijerhof, T.; Nijman, H.W.; Daemen, T. Tattoo delivery of a Semliki Forest virus based vaccine encoding Human Papillomavirus E6 and E7. Vaccines 2015, 3, 221–238. [Google Scholar] [CrossRef]
- Velders, M.P.; McElhiney, S.; Cassetti, M.C.; Eiben, G.L.; Higgins, T.; Kovacs, G.R.; Elmishad, A.G.; Kast, W.M.; Smith, L.R. Eradication of established tumors by vaccination with Venezuelan equine encephalitis virus replicon particles delivering human papillomavirus 16 E7 RNA. Cancer Res. 2001, 61, 7861–7867. [Google Scholar]
- Ying, H.; Zaks, T.Z.; Wang, R.F.; Irvine, K.R.; Kammula, U.S.; Marincola, F.M.; Leitner, W.W.; Restifo, N.P. Cancer therapy using a self-replicating RNA vaccine. Nat. Med. 1999, 5, 823–827. [Google Scholar]
- Granot, T.; Yamanashi, Y.; Meruelo, D. Sindbis viral vectors transiently deliver tumor-associated antigens to lymph nodes and elicit diversified antitumor CD8+ T-cell immunity. Mol. Ther. 2014, 22, 112–122. [Google Scholar] [CrossRef]
- Rodriguez-Madoz, J.R.; Prieto, J.; Smerdou, C. Semliki forest virus vectors engineered to express higher IL-12 levels induce efficient elimination of murine colon adenocarcinomas. Mol. Ther. 2005, 12, 153–163. [Google Scholar] [CrossRef]
- Smyth, J.W.; Fleeton, M.N.; Sheahan, B.J.; Atkins, G.J. Treatment of rapidly growing K-BALB and CT26 mouse tumors using Semliki Forest virus recombinant particles. Gene Ther. 2005, 12, 147–159. [Google Scholar] [CrossRef]
- Chikkanna-Gowda, C.P.; McNally, S.; Sheahan, B.J.; Fleeton, M.N.; Atkins, G.J. Inhibition of murine K-BALB and CT26 tumour growth using a Semliki Forest virus vector with enhanced expression of IL-18. Oncol. Rep. 2006, 16, 713–719. [Google Scholar] [CrossRef]
- Rodriguez-Madoz, J.R.; Liu, K.H.; Quetglas, J.I.; Ruiz-Guillen, M.; Otano, I.; Crettaz, J.; Butler, S.D.; Bellezza, C.A.; Dykes, N.L.; Tennant, B.C.; et al. Semliki forest virus expressing interleukin-12 induces antiviral and antitumoral responses in woodchucks with chronic viral hepatitis and hepatocellular carcinoma. J. Virol. 2009, 83, 12266–12278. [Google Scholar] [CrossRef]
- Rodriguez-Madoz, J.R.; Zabala, M.; Alfaro, M.; Prieto, J.; Kramer, M.G.; Smerdou, C. Short-term intratumoral interleukin-12 expressed from an alphaviral vector is sufficient to induce an efficient antitumoral response against spontaneous hepatocellular carcinomas. Hum. Gene Ther. 2014, 25, 132–143. [Google Scholar] [CrossRef]
- Draghiciu, O.; Boerma, A.; Hoogeboom, B.N.; Nijman, H.W.; Daemen, T. A rationally designed combined treatment with an alphavirus-based cancer vaccine, sunitinib and low-dose tumor irradiation completely blocks tumor development. Oncoimmunology 2015, 4, e1029699. [Google Scholar] [CrossRef]
- Cheng, W.F.; Lee, C.N.; Su, Y.N.; Chai, C.Y.; Chang, M.C.; Polo, J.M.; Hung, C.F.; Wu, T.C.; Hsieh, C.Y.; Chen, C.A. Sindbis virus replicon particles encoding calreticulin linked to a tumor antigen generate long-term tumor-specific immunity. Cancer Gene. Ther. 2006, 13, 873–885. [Google Scholar] [CrossRef]
- Murphy, A.M.; Morris-Downes, M.M.; Sheahan, B.J.; Atkins, G.J. Inhibition of human lung carcinoma cell growth by apoptosis induction using Semliki Forest virus recombinant particles. Gene Ther. 2000, 7, 1477–1482. [Google Scholar] [CrossRef]
- Yin, X.; Wang, W.; Zhu, X.; Wang, Y.; Wu, S.; Wang, Z.; Wang, L.; Du, Z.; Gao, J.; Yu, J. Synergistic antitumor efficacy of combined DNA vaccines targeting tumor cells and angiogenesis. Biochem. Biophys. Res. Commun. 2015, 465, 239–244. [Google Scholar] [CrossRef]
- Avogadri, F.; Merghoub, T.; Maughan, M.F.; Hirschhorn-Cymerman, D.; Morris, J.; Ritter, E.; Olmsted, R.; Houghton, A.N.; Wolchok, J.D. Alphavirus replicon particles expressing TRP-2 provide potent therapeutic effect on melanoma through activation of humoral and cellular immunity. PLoS ONE 2010, 5, e12670. [Google Scholar] [CrossRef]
- Goldberg, S.M.; Bartido, S.M.; Gardner, J.P.; Guevara-Patino, J.A.; Montgomery, S.C.; Perales, M.A.; Maughan, M.F.; Dempsey, J.; Donovan, G.P.; Olson, W.C.; et al. Comparison of two cancer vaccines targeting tyrosinase: Plasmid DNA and recombinant alphavirus replicon particles. Clin. Cancer Res. 2005, 11, 8114–8121. [Google Scholar] [CrossRef]
- Tseng, J.C.; Levin, B.; Hurtado, A.; Yee, H.; de Castro, I.P.; Jimenez, M.; Shamamian, P.; Jin, R.; Novick, R.P.; Pellicier, A.; et al. Systemic tumor targeting and killing by Sindbis viral vectors. Nat. Biotechnol. 2004, 22, 70–77. [Google Scholar] [CrossRef]
- Klimp, A.H.; van der Vaart, E.; Lansink, P.O.; Withoff, S.; de Vries, E.G.; Scherphof, G.L.; Wilschut, J.; Daemen, T. Activation of peritoneal cells upon in vivo transfection with a recombinant alphavirus expressing GM-CSF. Gene Ther. 2001, 8, 300–307. [Google Scholar] [CrossRef]
- Durso, R.J.; Andjelic, S.; Gardner, J.P.; Margitich, D.J.; Donovan, G.P.; Arrigale, R.R.; Wang, X.; Maughan, M.F.; Talarico, T.L.; Olmsted, R.A.; et al. A novel alphavirus vaccine encoding prostate-specific membrane antigen elicits potent cellular and humoral immune responses. Clin. Cancer Res. 2007, 13, 3999–4008. [Google Scholar] [CrossRef]
- Garcia-Hernandez, M.L.; Gray, A.; Hubby, B.; Kast, W.M. In vivo effects of vaccination with six-transmembrane epithelial antigen of the prostate: A candidate antigen for treating prostate cancer. Cancer Res. 2007, 67, 1344–1351. [Google Scholar] [CrossRef]
- Garcia-Hernandez, M.L.; Gray, A.; Hubby, B.; Klinger, O.J.; Kast, W.M. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer Res. 2008, 68, 861–869. [Google Scholar] [CrossRef]
- Riabov, V.; Tretyakova, I.; Alexander, R.B.; Pushko, P.; Klyushnenkova, E.N. Anti-tumor effect of the alphavirus-based virus-like particle vector expressing prostate-specific antigen in a HLA-DR transgenic mouse model of prostate cancer. Vaccine 2015, 33, 5386–5395. [Google Scholar] [CrossRef]
- Colmenero, P.; Liljeström, P.; Jondal, M. Induction of P815 tumor immunity by recombinant Semliki Forest virus expressing the P1A gene. Gene Ther. 1999, 6, 1728–1733. [Google Scholar] [CrossRef]
- Morse, M.A.; Hobeika, A.C.; Osada, T.; Berglund, P.; Hubby, B.; Negri, S.; Niedzwiecki, D.; Devi, G.R.; Burnett, B.K.; Clay, T.M.; et al. An alphavirus vector overcomes the presence of neutralizing antibodies and elevated numbers of Tregs to induce immune responses in humans with advanced cancer. J. Clin. Investig. 2010, 120, 3234–3241. [Google Scholar] [CrossRef]
- Gomes, I.M.; Maia, C.J.; Santos, C.R. STEAP proteins: From structure to applications in cancer therapy. Mol. Cancer Res. 2012, 10, 573–587. [Google Scholar] [CrossRef]
- Bins, A.D.; Jorritsma, A.; Wolkers, M.C.; Hung, C.F.; Wu, T.C.; Schumacher, T.N.; Haanen, J.B. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nat. Med. 2005, 11, 899–904. [Google Scholar] [CrossRef]
- Vähä-Koskela, M.J.; Kallio, J.P.; Jansson, L.C.; Heikkilä, J.E.; Zakhartchenko, V.A.; Kallajoki, M.A.; Kähäri, V.M.; Hinkkanen, A.E. Oncolytic capacity of attenuated replicative Semliki Forest virus in human melanoma xenografts in severe combined immunodeficient mice. Cancer Res. 2006, 66, 7185–7194. [Google Scholar] [CrossRef]
- Chikkanna-Gowda, C.P.; Sheahan, B.J.; Fleeton, M.N.; Atkins, G.J. Regression of mouse tumours and inhibition of metastases following administration of a Semliki Forest virus vector with enhanced expression of IL-12. Gene Ther. 2005, 12, 1253–1263. [Google Scholar] [CrossRef]
- Huttner, A.; Dayer, J.A.; Yerly, S.; Combescure, C.; Auderset, F.; Desmeules, J.; Eickmann, M.; Finckh, A.; Goncalves, A.R.; Hooper, J.W.; et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: A randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect. Dis. 2015, 15, 1156–1166. [Google Scholar] [CrossRef]
- Fuchs, J.D.; Frank, I.; Elizaga, M.L.; Allen, M.; Frahm, N.; Kochar, N.; Li, S.; Edupuganti, S.; Kalams, S.A.; Tomaras, G.D.; et al. First-in-Human Evaluation of the Safety and Immunogenicity of a Recombinant Vesicular Stomatitis Virus Human Immunodeficiency Virus-1 gag Vaccine (HVTN 090). Open Forum Infect. Dis. 2015. [Google Scholar] [CrossRef]
- Lundstrom, K. Biology and application of alphaviruses in gene therapy. Gene Ther. 2005, 12, S92–S597. [Google Scholar] [CrossRef]
- Ren, H.; Boulikas, T.; Lundstrom, K.; Söling, A.; Warnke, P.C.; Rainov, N.G. Immunogene therapy of recurrent glioblastoma multiforme with a liposomally encapsulated replication incompetent Semliki Forest virus vector carrying the human interleukin-12 gene—A phase I/II protocol. J. Neurooncol. 2003, 64, 147–154. [Google Scholar] [CrossRef]
- Slovin, S.F.; Kehoe, M.; Durso, R.; Fernandez, C.; Olson, W.; Gao, J.P.; Israel, R.; Scher, H.I.; Morris, S. A phase I dose escalation trial of vaccine replicon particles (VRP) expressing prostate-specific membrane antigen (PSMA) in subjects with prostate cancer. Vaccine 2013, 31, 943–949. [Google Scholar] [CrossRef]
- Alba, R.; Bosch, A.; Chillon, M. Gutless adenovirus: Last-generation adenovirus for gene therapy. Gene Ther. 2005, 12, S18–S27. [Google Scholar] [CrossRef]
- Schiedner, G.; Morral, N.; Parks, R.S.; Wu, Y.; Koopmans, S.C.; Langston, C.; Graham, F.L.; Beuadet, A.L.; Kochanek, S. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat. Genet. 1998, 18, 180–183. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.; Tian, H.; Qi, M.; Zhai, Z.; Li, S.; Li, R.; Zhang, H.; Wang, W.; Fu, S.; et al. Biodistribution and safety assessment of bladder cancer specific oncolytic adenovirus in subcutaneous xenografts tumor model in nude mice. Curr. Gene Ther. 2012, 12, 67–76. [Google Scholar] [CrossRef]
- Andtbacka, R.H.; Agarwala, S.S.; Ollila, D.W.; Hallmeyer, S.; Milhem, M.; Amatruda, T.; Nemunaitis, J.J.; Harrington, K.J.; Chen, L.; Shilkrut, M.; et al. Cutaneous head and neck melanoma in OPTiM, a randomized phase 3 trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor for the treatment of unresected stage IIIB/IIIC/IV melanoma. Head Neck. 2016. [Google Scholar] [CrossRef]
- Zhang, L.; Tatsuya, T.; Nishiyama, Y. Oncotarget Strategies For Herpes Simplex Virus-1. Curr. Gene Ther. 2016, 16, 130–143. [Google Scholar] [CrossRef]
- Chahal, J.S.; Khan, O.F.; Cooper, C.L.; McPartlan, J.S.; Tsosie, J.K.; Tilley, L.D.; Sidik, S.M.; Lourido, S.; Langer, R.; Bavari, S.; et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. USA 2016, 113, E4133–E4142. [Google Scholar] [CrossRef]
- Leitner, W.W.; Ying, H.; Driver, D.A.; Dubensky, T.W.; Restifo, N.P. Enhancement of tumor-specific immune response with plasmid DNA replicon vectors. Cancer Res. 2000, 60, 51–55. [Google Scholar]

| Virus | Target | Vector | Immunization | Response | Reference |
|---|---|---|---|---|---|
| Influenza | NP | SFV VLPs | mouse | systemic NP immune response | [35] |
| HA | VEE VLPs | chicken | protection against influenza virus | [36] | |
| HA | VEE VLPs | swine | protection against influenza virus | [37] | |
| HA | VEE VLPs | swine | protection against influenza virus | [38] | |
| HA | rMV | mouse | neutralizing Abs | [39] | |
| cHA | VSV | mouse | protection against influenza virus | [40] | |
| HIV | Gag | Kunjin VLPs | mouse | protection against HIV | [41] |
| Env | SFV VLPs | mouse | neutralizing Abs, humoral response | [42] | |
| gp41 | SFV-VLPs | mouse | generation of mAbs | [43] | |
| Env | SFV DNA | mouse | T cell and IgG immune responses | [44] | |
| SIV | Gag-Pol | Kunjin VLPs | macaques | protection against SIV | [45] |
| Env | VSV VLPs | macaques | neutralizing Abs | [46] | |
| Gag-Env | VSV VLPs | macaques | protection against SIV | [47] | |
| Gag-Env | RABV VLPs | macaques | protection against SIV | [48] | |
| Ebola | GP | Kunjin VLPs | guinea pig | protection against Ebola | [49] |
| GP | Kunjin VLPs | primate | protection against Ebola | [50] | |
| GP | VSV VLPs | macaques | protection against Ebola | [51,52] | |
| GP, NP | VEE VLPs | mouse | protection against Ebola | [53] | |
| NP | VEE VLPs | mouse | protection against Ebola | [54] | |
| Lassa | G | VSV VLPs | guinea pig | protection against Lassa | [55] |
| G | VEE VLPs | guinea pig | protection against Lassa | [56] | |
| SARS-CoV | G | VEE VLPs | mouse | protection against SARS-CoV | [57] |
| MERS-CoV | G | MV | mouse | protection against SARS-CoV | [58] |
| RSV | F | MV | rat | protection against RSV | [39] |
| F | VEE LNPs | mouse | protection against RSV | [59] | |
| F | VEE VLPs | primate | protection against RSV | [60] | |
| MPV | F | VEE VLPs | primate | protection against MPV | [60] |
| Dengue | DV2-HBsAg | MV | mouse | neutralizing Abs | [61] |
| DV2 | MV | mouse | protection against dengue virus | [62] | |
| prME-E85 | VEE VLPs | macaques | protection against dengue virus | [63] | |
| prME-E85 | VEE VLPs | mouse | protection against dengue virus | [64] | |
| HBV | MHB | SFV-VSV G | mouse | protection against HBV | [65] |
| DV2-HBsAg | MV | mouse | protection against HBV | [62] | |
| HBsAg | MV | macaques | protection against HBV | [66] | |
| CMV | gB-pp65/IE1 | VEE VLPs | human | neutralizing Abs | [67] |
| Agent | Target | Vector | Immunization | Response | Reference |
|---|---|---|---|---|---|
| P. falciparum | Ag Pf332 | SFV VLPs/RNA | mouse | immunological memory | [68] |
| M. tuberculosis | Ag 85A | SIN DNA | mouse | protection against M. tuberculosis | [69] |
| C. botulinum | BoNTA-Hc | SFV DNA | mouse | Ab and lymphoproliferative response | [70] |
| B. abortus | IF3 | SFV VLPs | mouse | protection against Brucella | [71] |
| B. antracis | PA | SIN VLPs | mouse | protection against B. antracis | [72] |
| Malaria | CS | SIN VLPs | mouse | protection against malaria | [73] |
| L. monocytogenes | OVA | VSV-GP | mouse | protection against Listeria | [74] |
| Prion | PRNP | SFV VLPs | mouse | monoclonal Abs | [75] |
| Staphylococcus | SEB | VEE VLPs | mouse | protection against enterotoxin | [76] |
| Cancer | Target | Vector | Response | Reference |
|---|---|---|---|---|
| Brain | GFP, SLAM, EGFR | MV | replication in/lysis of cancer cells | [79] |
| Endostatin | SFV VLPs | tumor inhibition | [89] | |
| miR-124 | SFV-miR-124 | prolonged survival | [90] | |
| IL-12 | SFV-IL-12 | prolonged survival | [91,92,93] | |
| Breast | CEA | MV | tumor growth delay, better survival | [83] |
| Neu | SIN DNA | immune responses, tumor protection | [94] | |
| Neu | VEE VLPs + DCs | tumor regression by transduced DCs | [95] | |
| VEGFR-2 | SFV VLPs | tumor inhibition | [96] | |
| Cervical | HPV E6, 7 | SFV VLPs | tumor eradication | [97,98] |
| HPV E7 | VEE VLPs | eradication of existing tumors | [99] | |
| HPV E7 Epitope | Kunjin VLPs/RNA/DNA | tumor protection in mice | [88] | |
| Colon | GM-CSF | Kunjin VLPs | regression of tumors and metastasis | [87] |
| VEGFR-2 | SFV VLPs | reduced tumor and metastasis growth | [96] | |
| LacZ | SFV RNA | tumor protection in mice | [100] | |
| Lac Z | SIN VLPs | anti-tumor CD8+ T-cell immunity | [101] | |
| IL-12 | SFV VLPs | tumor elimination | [102] | |
| SFV | SFV VLPs | tumor growth inhibition | [103] | |
| IL-18 | SFV VLPs | tumor regression in mice | [104] | |
| Liver | IL-12 | SFV VLPs | anti-tumor responses in woodchucks | [105,106] |
| Lung | HPV E6/E7 | SFV + Sun + Rad | tumor-free survival | [107] |
| HPV E7-CRT | SIN VLPs | long-term anti-tumor effect | [108] | |
| EGFP | SFV VLPs | apoptosis, tumor regression in mice | [109] | |
| Melanoma | GM-CSF | Kunjin VLPs | tumor regression | [87] |
| VEGF-2-IL-12 + Sur + β-hCG | SFV VLPs | tumor inhibition | [110] | |
| TRP-2 | VEE VLPs | humoral and cellular immunity | [111] | |
| Tyr | VEE VLPs | T-cell responses, tumor protection in mice | [112] | |
| Ovarian | CEA, NIS | MV | superior dual therapy | [81] |
| IL-12 | SIN VLPs | tumor targeting, eradication | [113] | |
| IL-18 | SFV VLPs | therapeutic anti-tumor response | [91] | |
| GM-CSF | SFV VLPs | tumor growth inhibition | [114] | |
| Pancreatic | Matrix protein | VSV VLPs | killing of tumor cells in vitro and in vivo | [85] |
| Prostate | CEA | MV | replication in/lysis of cancer cells | [79] |
| PSMA | VEE VLPs | cellular and humoral immunity in mice | [115] | |
| STEAP | VEE VLPs | CD8+ T-cell response, tumor growth delay | [116] | |
| PSCA | DNA + VEE VLPs | long-term protective immune response | [117] | |
| Sarcoma | PSA | VEE VLPs | PSA-cell clearance, tumor growth delay | [118] |
| Skin | SFV | SFV VLPs | tumor growth inhibition | [103] |
| P1A | SFV VLPs | strong CTL-response, tumor protection | [119] |
| Viral Vector | Genome | Capacity | Special Features |
|---|---|---|---|
| Alphavirus | ssRNA | 6–8 kb | broad host range, high titer, cytoplasmic RNA, extreme transient expression, no chromosomal integration, choice of DNA, RNA replicon and particle delivery |
| Flavivirus | ssRNA | 5 kb | broad host range, packaging system, choice of DNA, RNA replicon and particle delivery |
| Measles virus | ssRNA | 5 kb | packaging cell line, measles virus strains for immunization, cytoplasmic RNA |
| Rhabdovirus | ssRNA | 5 kb | reverse genetics systems, broad host range cytoplasmic RNA |
| Adenovirus | dsDNA | >8 kb | broad host range, packaging cell line, nuclear translocation necessary, transient expression, potential integration |
| AAV | ssDNA | <4 kb | multiple AAV serotypes for avoiding immune responses, nuclear translocation necessary, chromosomal integration |
| Herpes simplex virus | dsDNA | 30–40 kb | large packaging capacity, nuclear translocation necessary, latent long-term transgene expression after integration |
| Lentivirus | dsRNA | 8 kb | transduction of dividing and non-dividing cells, nuclear translocation necessary, chromosomal integration |
| Retrovirus | dsRNA | 4 kb | transduction of only dividing cells, nuclear translocation necessary, chromosomal integration |
| Vaccinia | dsDNA | 25 kb | large packaging capacity, nuclear translocation necessary |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lundstrom, K. Replicon RNA Viral Vectors as Vaccines. Vaccines 2016, 4, 39. https://doi.org/10.3390/vaccines4040039
Lundstrom K. Replicon RNA Viral Vectors as Vaccines. Vaccines. 2016; 4(4):39. https://doi.org/10.3390/vaccines4040039
Chicago/Turabian StyleLundstrom, Kenneth. 2016. "Replicon RNA Viral Vectors as Vaccines" Vaccines 4, no. 4: 39. https://doi.org/10.3390/vaccines4040039
APA StyleLundstrom, K. (2016). Replicon RNA Viral Vectors as Vaccines. Vaccines, 4(4), 39. https://doi.org/10.3390/vaccines4040039





