The Immunogenicity of Glutaraldehyde Inactivated PTx Is Determined by the Quantity of Neutralizing Epitopes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PTd and PTd Containing Vaccine Formulation
2.2. Assay of Neutralizing Epitopes in S1 and S2/3 Subunits
2.3. Immunization
2.4. Assay of Measuring PTx-Specific Immunoglobulins (Ig), Total IgG
2.5. Ethics Statement for Animal Experiments
3. Results
3.1. Establishment of the Neutralizing Epitopes Qualification ELISA Assay
3.1.1. Working Range and Detection Limit
3.1.2. Specificity
3.1.3. Accuracy
3.1.4. Precision
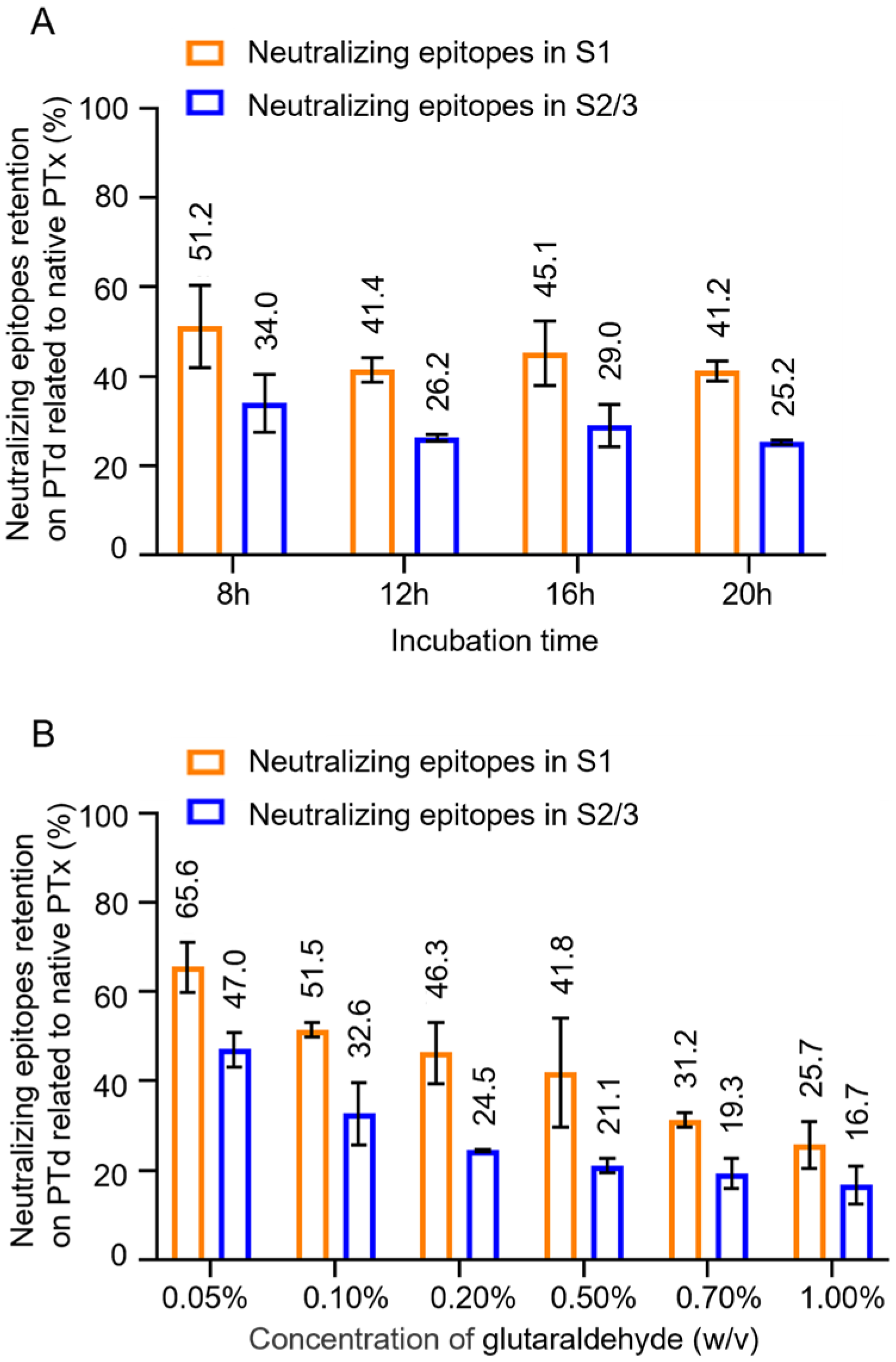
3.2. The Effect Glutaraldehyde Treatment on Antigenic Properties of PTx
3.3. Correlation Between Neutralizing Epitope Content and Immunogenicity In Vivo
3.4. Monitoring Consistency of Neutralizing Epitopes in PTd Preparations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esposito, S.; Stefanelli, P.; Fry, N.K.; Fedele, G.; He, Q.S.; Paterson, P.; Tan, T.; Knuf, M.; Rodrigo, C.; Olivier, C.W.; et al. Pertussis Prevention: Reasons for Resurgence, and Differences in the Current Acellular Pertussis Vaccines. Front. Immunol. 2019, 10, 1344. [Google Scholar] [CrossRef]
- Gregg, K.A.; Merkel, T.J. Pertussis Toxin: A Key Component in Pertussis Vaccines? Toxins 2019, 11, 557. [Google Scholar] [CrossRef]
- van den Biggelaar, A.H.J.; Poolman, J.T. Predicting future trends in the burden of pertussis in the 21st century: Implications for infant pertussis and the success of maternal immunization. Expert Rev. Vaccines 2016, 15, 69–80. [Google Scholar] [CrossRef]
- Habeeb, A.; Hiramoto, R. Reaction of proteins with glutaraldehyde. Arch. Biochem. Biophys. 1968, 126, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Richards, F.; Knowles, J.R. Glutaraldehyde as a protein cross-linking reagent. J. Mol. Biol. 1968, 37, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, J.A.; Nguyen, A.W.; Wilen, R.E.; Wijagkanalan, W.; McLellan, J.S.; Maynard, J.A. Structural Basis for Antibody Neutralization of Pertussis Toxin. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Hokyun, O.H.; Kim, B.G.; Nam, K.T.; Hong, S.H.; Ahn, D.H.; Choi, G.S.; Kim, H.; Hong, J.T.; Ahn, B.Y. Characterization of the carbohydrate binding and ADP-ribosyltransferase activi-ties of chemically detoxified pertussis toxins. Vaccine 2013, 31, 2988–2993. [Google Scholar] [CrossRef]
- Yuen, C.; Asokanathan, C.; Cook, S.; Lin, N.; Xing, D. Effect of different detoxification procedures on the residual pertussis toxin activities in vaccines. Vaccine 2016, 34, 2129–2134. [Google Scholar] [CrossRef]
- Knuutila, A.; Dalby, T.; Barkoff, A.M.; Jorgensen, C.S.; Fuursted, K.; Mertsola, J.; Markey, K.; He, Q.S. Differences in epitope-specific antibodies to pertussis toxin after infection and acellular vaccinations. Clin. Transl. Immunol. 2020, 9, e1161. [Google Scholar] [CrossRef]
- Seubert, A.; D’Oro, U.; Scarselli, M.; Pizza, M. Genetically detoxified pertussis toxin (PT-9K/129G): Implications for immunization and vaccines. Expert Rev. Vaccines 2014, 13, 1191–1204. [Google Scholar] [CrossRef]
- Ibsen, P. The effect of formaldehyde, hydrogen peroxide and genetic detoxification of pertussis toxin on epitope recognition by murine monoclonal antibodies. Vaccine 1996, 14, 359–368. [Google Scholar] [CrossRef]
- Sato, H.; Sato, Y. Relationship between structure and biological and protective activities of pertussis toxin. Dev. Biol. Stand. 1991, 73, 121–132. [Google Scholar]
- Sutherland, J.; Maynard, J.A. Characterization of a key neutralizing epitope on pertussis toxin recognized by monoclonal antibody 1B7. Biochemistry 2009, 48, 11982–11993. [Google Scholar] [CrossRef]
- Wagner, E.; Wang, X.; Bui, A.; Maynard, J.A. Synergistic Neutralization of Pertussis Toxin by a Bispecific Antibody In Vitro and In Vivo. Clin. Vaccine Immunol. 2016, 23, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Sato, Y.; Ohishi, I. Comparison of Pertussis Toxin (PT)-Neutralizing Activities and Mouse-Protective Activities of Anti-PT Mouse Monoclonal Antibodies. Infect. Immun. 1991, 59, 3832–3835. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Sato, Y. Protective Activities in Mice of Monoclonal Antibodies against Pertussis Toxin. Infect. Immun. 1990, 58, 3369–3374. [Google Scholar] [CrossRef]
- Tan, L.; Fahim, R.E.; Jackson, G.; Phillips, K.; Wah, P.; Alkema, D.; Zobrist, G.; Herbert, A.; Boux, L.; Chong, P.; et al. A novel process for preparing an acellular pertussis vaccine composed of non-pyrogenic toxoids of pertussis toxin and filamentous hemagglutinin. Mol. Immunol. 1991, 28, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Wang, X.; Zhou, Y.; Zhu, X.S.; Ma, Y.; Wei, W.M.; Zhang, Y.T. Development and Implementation of a Single Radial Diffusion Technique for Quality Control of Acellular Pertussis Vaccines. Vaccines 2025, 13, 116. [Google Scholar] [CrossRef]
- Winsnes, R.; Sesardic, D.; Daas, A.; Terao, E.; Behr-Gross, M. Collaborative study on a Guinea pig serological method for the assay of acellu-lar pertussis vaccines. Pharmeur. Bio Sci. Notes 2009, 1109, 27–40. [Google Scholar] [PubMed]
- Zhang, Y.T.; Guo, Y.C.; Dong, Y.; Liu, Y.W.; Zhao, Y.X.; Yu, S.Z.; Li, S.H.; Wu, C.Y.; Yang, B.F.; Li, W.L.; et al. Safety and immunogenicity of a combined DTacP-sIPV-Hib vaccine in animal models. Hum. Vaccines Immunother. 2005, 18, 2160158. [Google Scholar] [CrossRef]
- Schild, G.C.; Wood, J.M.; Newman, R.W. A single radial immunodiffusion technique for the assay of influenza hemagglutinin antigen: Proposals for an assay method for the haemagglutinin content of influenza vaccines. Bull. World Health Organ. 1975, 52, 223–231. [Google Scholar] [PubMed]
- Aydin, S.; Emre, E.; Ugur, K.; Aydin, M.A.; Sahin, B.; Cinar, V.; Akbulut, T. An overview of ELISA: A review and update on best laboratory practices for quantifying peptides and proteins in biological fluids. J. Int. Med. Res. 2025, 53. [Google Scholar] [CrossRef]
- Waritani, T.; Chang, J.; McKinney, B.; Terato, K. An ELISA protocol to improve the accuracy and reliability of serological antibody assays. MethodsX 2017, 4, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Alhajj, M.; Zubair, M.; Farhana, A. Enzyme Linked Immunosorbent Assay. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555922/ (accessed on 3 April 2023).
- Arciniega, J.; Shahin, R.D.; Burnette, W.N.; Bartley, T.D.; Whiteley, D.W.; Mar, V.L.; Burns, D. Contribution of the B oligomer to the protective activity of genetically attenuated pertussis toxin. Infect. Immun. 1991, 59, 3407–3410. [Google Scholar] [CrossRef]
- Sutherland, J.; Chang, C.; Yoder, S.M.; Rock, M.T.; Maynard, J.A. Antibodies Recognizing Protective Pertussis Toxin Epitopes Are Preferentially Elicited by Natural Infection versus Acellular Immunization. Clin. Vaccine Immunol. 2011, 18, 954–962. [Google Scholar] [CrossRef]
- Roberts, M.; Bacon, A.; Rappuoli, R.; Pizza, M.; Cropley, I.; Douce, G.; Dougan, G.; Marinaro, M.; McGhee, J.; Chatfield, S. A mutant pertussis toxin molecule that lacks ADP-ribosyltransferase activity, PT-9K/129G, is an effective mucosal adjuvant for intranasally delivered proteins. Infect. Immun. 1995, 63, 2100–2108. [Google Scholar] [CrossRef]
- Warfel, J.M.; Zimmerman, L.I.; Merkel, T.J. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc. Natl. Acad. Sci. USA 2014, 111, 787–792. [Google Scholar] [CrossRef]
- Warfel, J.M.; Edwards, K.M. Pertussis vaccines and the challenge of inducing durable immunity. Curr. Opin. Immunol. 2015, 35, 48–54. [Google Scholar] [CrossRef]
- Burdin, N.; Handy, L.K.; Plotkin, S.A. What Is Wrong with Pertussis Vaccine Immunity? The Problem of Waning Effectiveness of Pertussis Vaccines. Cold Spring Harb. Perspect. Biol. 2017, 9, a029454. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, B.M. Resurgence of Pertussis Linked With Switch to Acellular Vaccine. JAMA 2021, 326, 300. [Google Scholar] [CrossRef]
- Nguyen, A.W.; DiVenere, A.M.; Papin, J.F.; Connelly, S.; Kaleko, M.; Maynard, J.A. Neutralization of pertussis toxin by a single antibody prevents clinical pertussis in neonatal baboons. Sci. Adv. 2020, 6, eaay9258. [Google Scholar] [CrossRef]
- Wolf, M.A.; Boehm, D.T.; DeJong, M.A.; Wong, T.Y.; Sen-Kilic, E.; Hall, J.M.; Blackwood, C.B.; Weaver, K.L.; Kelly, C.O.; Kisamore, C.A.; et al. Intranasal Immunization with Acellular Pertussis Vaccines Results in Long-Term Immunity to Bordetella pertussis in Mice. Infect. Immun. 2021, 89, e00607–e00620. [Google Scholar] [CrossRef] [PubMed]
- Podda, A.; De Luca, E.C.; Titone, L.; Casadei, A.M.; Cascio, A.; Peppoloni, S.; Volpini, G.; Marsili, I.; Nencioni, L.; Rappuoli, R. Acellular pertussis vaccine composed of genetically inactivated pertussis toxin: Safety and immunogenicity in 12- to 24- and 2- to 4-month-old children. J. Pediatr. 1992, 120, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Sirivichayakul, C.; Chanthavanich, P.; Limkittikul, K.; Siegrist, C.A.; Wijagkanalan, W.; Chinwangso, P.; Petre, J.; Thai, P.H.; Chauhan, M.; Viviani, S. Safety and immunogenicity of a combined Tetanus, Diphtheria, recombinant acellular Pertussis vaccine (TdaP) in healthy Thai adults. Hum. Vaccines Immunother. 2017, 13, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, G.; Lattanzi, M.; Solis, C.D.; Contorni, M.; Costantini, M.; Moraschini, L.; Bardelli, M.; Bertholet, S.; Borgogni, E.; Buricchi, F.; et al. A phase I, randomized, controlled, dose-ranging study of investigational acellular pertussis (aP) and reduced tetanus-diphtheria-acellular pertussis (TdaP) booster vaccines in adults. Hum. Vaccines Immunother. 2018, 14, 45–58. [Google Scholar] [CrossRef]
- Chokephaibulkit, K.; Puthanakit, T.; Bhat, N.; Mansouri, S.; Tang, Y.; Lapphra, K.; Rungmaitree, S.; Anugulruengkitt, S.; Jantarabenjakul, W.; Andi-Lolo, I.; et al. A phase 2 randomized controlled dose-ranging trial of recombinant pertussis booster vaccines containing genetically inactivated pertussis toxin in women of childbearing age. Vaccine 2022, 40, 2352–2361. [Google Scholar] [CrossRef]
- Puthanakit, T.; Chokephaibulkit, K.; Chaithongwongwatthana, S.; Bhat, N.; Tang, Y.; Anugulruengkitt, S.; Chayachinda, C.; Anuwutnavin, S.; Lapphra, K.; Rungmaitree, S.; et al. A phase 2 randomized controlled dose-ranging trial of recombinant pertussis booster vaccines containing genetically inactivated pertussis toxin in pregnant women. Vaccine 2023, 41, 4541–4553. [Google Scholar] [CrossRef]
- Nakabembe, E.; Greenland, M.; Amaral, K.; Abu-Raya, B.; Amone, A.; Andrews, N.; Cantrell, L.; Lesne, E.; Gorringe, A.; Halkerston, R.; et al. Safety and immunogenicity of an acellular pertussis vaccine containing genetically detoxified pertussis toxin administered to pregnant women living with and without HIV and their newborns (WoMANPOWER): A randomised controlled trial in Uganda. Lancet Glob. Health 2025, 13, e81–e97. [Google Scholar] [CrossRef]
- Pitisuttithum, P.; Chokephaibulkit, K.; Sirivichayakul, C.; Sricharoenchai, S.; Dhitavat, J.; Pitisuthitham, A.; Phongsamart, W.; Boonnak, K.; Lapphra, K.; Sabmee, Y.; et al. Antibody persistence after vaccination of adolescents with monovalent and combined acellular pertussis vaccines containing genetically inactivated pertussis toxin: A phase 2/3 randomised, controlled, non-inferiority trial. Lancet Infect. Dis. 2018, 18, 1260–1268. [Google Scholar] [CrossRef]
- Ausar, S.F.; Zhu, S.; Duprez, J.; Cohen, M.; Bertrand, T.; Steier, V.; Wilson, D.J.; Li, S.; Sheung, A.; Brookes, R.H.; et al. Genetically detoxified pertussis toxin displays near identical structure to its wild-type and exhibits robust immunogenicity. Commun. Biol. 2020, 3, 427. [Google Scholar] [CrossRef]
- Nasso, M.; Fedele, G.; Spensieri, F.; Palazzo, R.; Costantino, P.; Rappuoli, R.; Ausiello, C.M. Genetically detoxified pertussis toxin induces Th1/Th17 immune response through MAPKs and IL-10-dependent mechanisms. J. Immunol. 2009, 183, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.J.; Sutton, C.E.; Higgins, S.; Allen, A.C.; Walsh, K.; Misiak, A.; Lavelle, E.C.; McLoughlin, R.M.; Mills, K.H. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: Towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog. 2013, 9, e1003264. [Google Scholar] [CrossRef] [PubMed]
- Szwejser-Zawislak, E.; Wilk, M.M.; Piszczek, P.; Krawczyk, J.; Wilczyńska, D.; Hozbor, D. Evaluation of Whole-Cell and Acellular Pertussis Vaccines in the Context of Long-Term Herd Immunity. Vaccines 2022, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Ní Chasaide, C.; Schmitt, P.; Diallo, B.K.; Borkner, L.; Leane, C.M.; Jazayeri, S.D.; Udayan, S.; O’Neill, E.; Curham, L.M.; Moran, B.; et al. Acellular Pertussis Vaccines Induce CD8+ and CD4+ Regulatory T Cells That Suppress Protective Tissue-Resident Memory CD4+ T Cells, in Part via IL-10. Eur. J. Immunol. 2025, 55, e51630. [Google Scholar] [CrossRef]
- Pitisuttithum, P.; Dhitavat, J.; Sirivichayakul, C.; Pitisuthitham, A.; Sabmee, Y.; Chinwangso, P.; Kerdsomboon, C.; Fortuna, L.; Spiegel, J.; Chauhan, M.; et al. Antibody persistence 2 and 3 years after booster vaccination of adolescents with recombinant acellular pertussis monovalent aPgen or combined TdaPgen vaccines. EClinicalMedicine 2021, 37, 100976. [Google Scholar] [CrossRef]



| Detecting Abs | Samples | Concentration (ng/mL) | OD Value | Cut-Off Value 1 |
|---|---|---|---|---|
| 1B7 | PTx | 6.25 | 0.372 | 0.347 |
| S24 | 5000 | 0.193 | ||
| Assay buffer | N/A | 0.165 | N/A | |
| 11E6 | PTx | 6.25 | 0.353 | 0.306 |
| S24 | 6.25 | 0.358 | ||
| Assay buffer | N/A | 0.146 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Cui, X.; Wu, C.; Tao, K.; Pan, S.; Wei, W. The Immunogenicity of Glutaraldehyde Inactivated PTx Is Determined by the Quantity of Neutralizing Epitopes. Vaccines 2025, 13, 817. https://doi.org/10.3390/vaccines13080817
Wang X, Cui X, Wu C, Tao K, Pan S, Wei W. The Immunogenicity of Glutaraldehyde Inactivated PTx Is Determined by the Quantity of Neutralizing Epitopes. Vaccines. 2025; 13(8):817. https://doi.org/10.3390/vaccines13080817
Chicago/Turabian StyleWang, Xi, Xinyue Cui, Chongyang Wu, Ke Tao, Shuyuan Pan, and Wenming Wei. 2025. "The Immunogenicity of Glutaraldehyde Inactivated PTx Is Determined by the Quantity of Neutralizing Epitopes" Vaccines 13, no. 8: 817. https://doi.org/10.3390/vaccines13080817
APA StyleWang, X., Cui, X., Wu, C., Tao, K., Pan, S., & Wei, W. (2025). The Immunogenicity of Glutaraldehyde Inactivated PTx Is Determined by the Quantity of Neutralizing Epitopes. Vaccines, 13(8), 817. https://doi.org/10.3390/vaccines13080817






