SARS-CoV-2 RBD Scaffolded by AP205 or TIP60 Nanoparticles and Delivered as mRNA Elicits Robust Neutralizing Antibody Responses
Abstract
1. Introduction
2. Methods
2.1. Structural Modeling and Analysis
2.2. Gibson Cloning and Assembly
- RBD-AP205 F: 5′—GTCTCCTCCGATACAACTTGATGATAAGTCTAGAGGGC—3′
- RBD-AP205 R: 5′—GGCTGCATGGGCTTATTGCCCCCTCCACCAGAACC—3′
- RBD-TIP60 F: 5′—CAGACGCCTTGAGGAGGAATGATGATAAGTCTAGAGGG—3′
- RBD-TIP60 R: 5′—CATTATTTTTATATTTTTGCCCCCTCCACCAGAACC—3′
2.3. Transient Transfection
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Gel Electrophoresis, Coomassie Staining, and Western Blot
2.6. In Vitro Transcription (IVT)
2.7. Formulation of mRNA Lipid Nanoparticles (LNPs)
2.8. In Vivo Methods
2.9. SARS-CoV-2 Pseudovirus Neutralization Assay
2.10. Graphing and Statistical Tests
3. Results
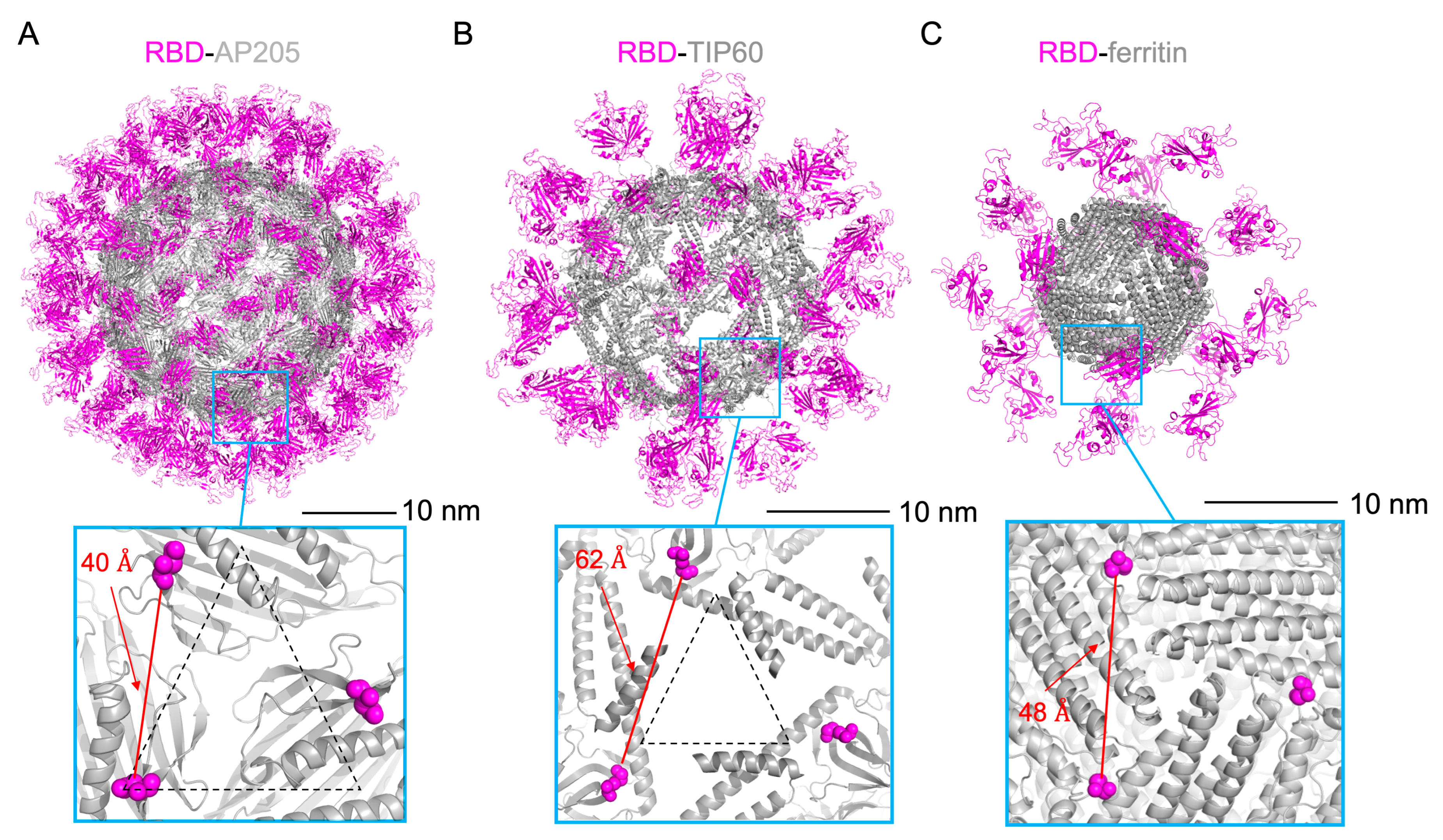
3.1. Design of Scaffolded RBD Constructs
3.2. Characterization of Scaffolded RBD Constructs
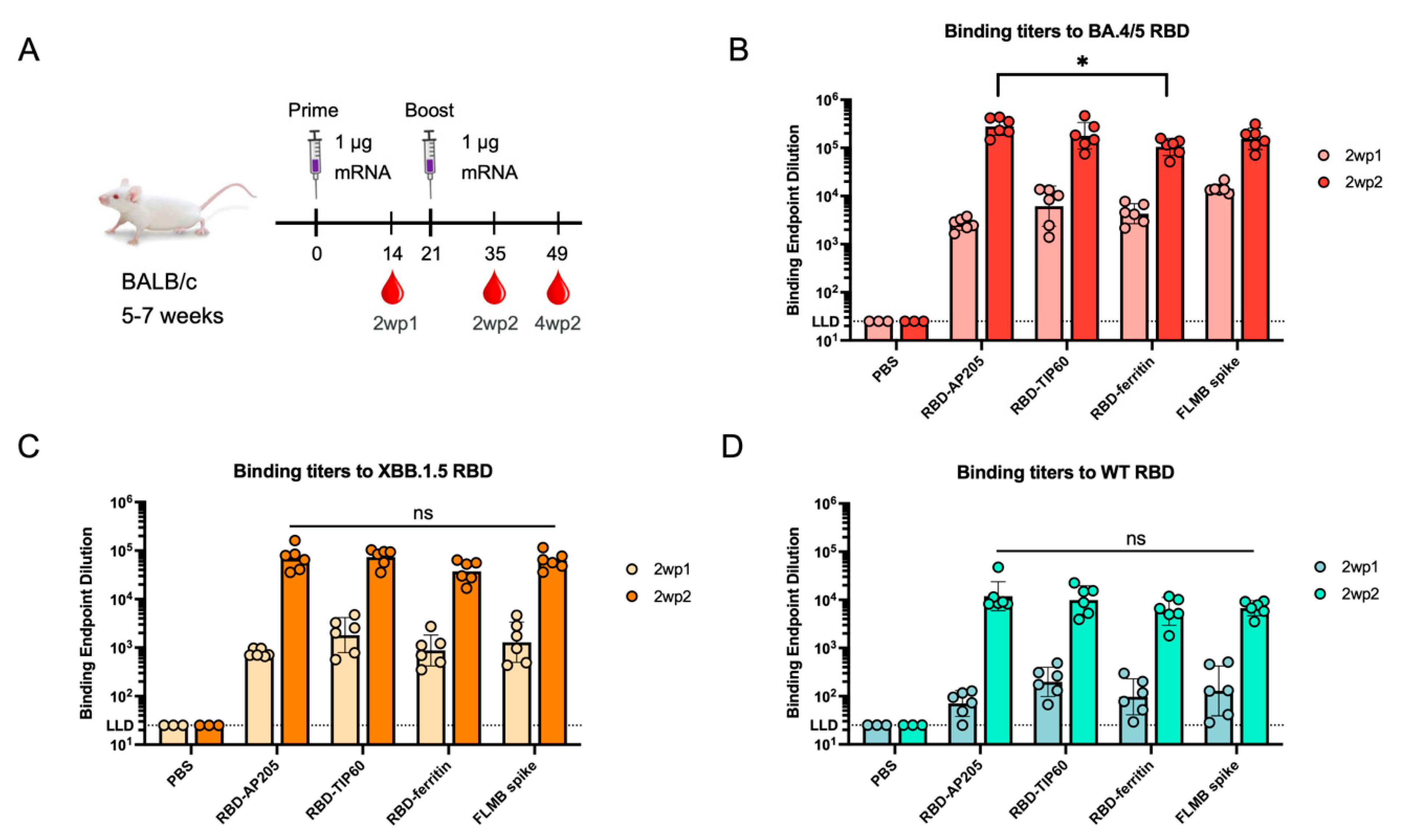
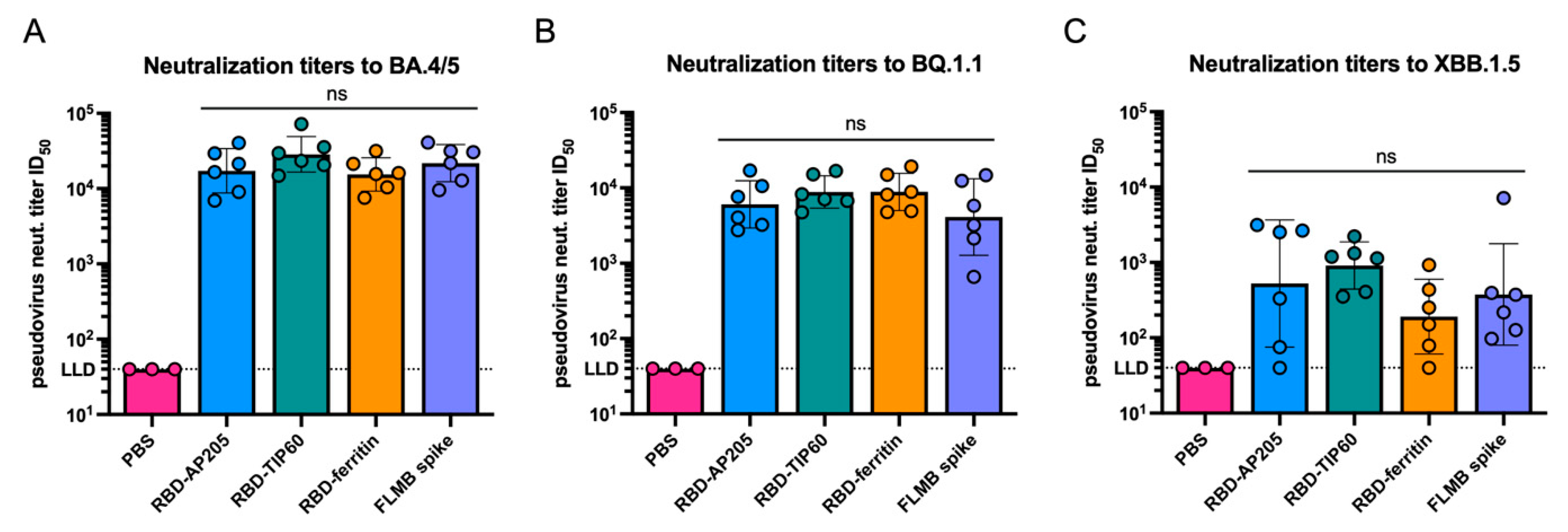
3.3. Immunogenicity of Scaffolded RBD Constructs
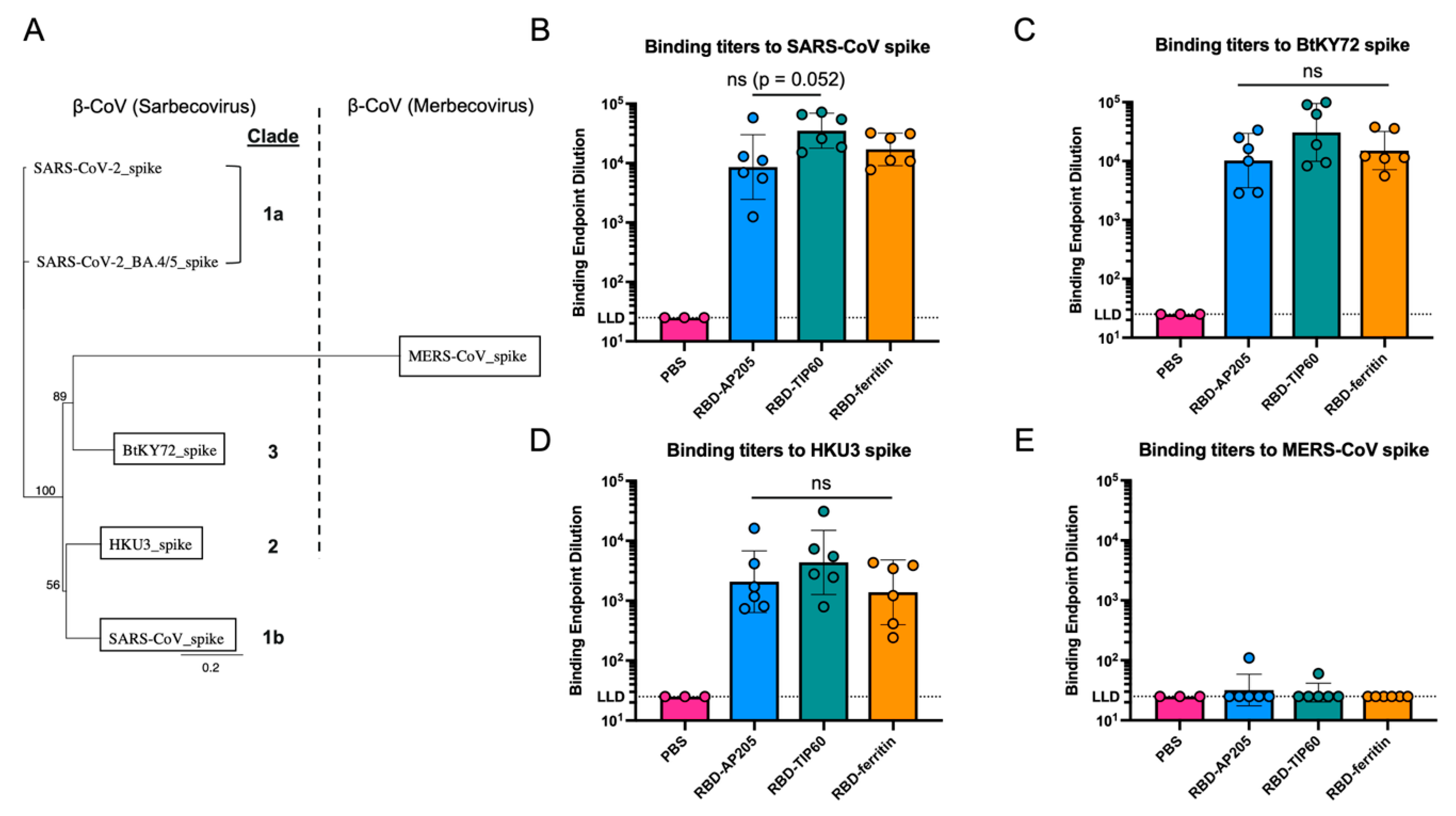
3.4. Comparison of Antibody Reactivity Breadth to Other Coronaviruses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acuti Martellucci, C.; Flacco, M.E.; Cappadona, R.; Bravi, F.; Mantovani, L.; Manzoli, L. SARS-CoV-2 pandemic: An overview. Adv. Biol. Regul. 2020, 77, 100736. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Bangaru, S.; Ozorowski, G.; Turner, H.L.; Antanasijevic, A.; Huang, D.; Wang, X.; Torres, J.L.; Diedrich, J.K.; Tian, J.H.; Portnoff, A.D.; et al. Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate. Science 2020, 370, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Sun, Q. Antibodies and Vaccines Target RBD of SARS-CoV-2. Front. Mol. Biosci. 2021, 8, 671633. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, X.; Zhou, H.; Zhu, H.; Jiang, S.; Wang, P. Broadly neutralizing antibodies to SARS-CoV-2 and other human coronaviruses. Nat. Rev. Immunol. 2023, 23, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Zost, S.J.; Gilchuk, P.; Case, J.B.; Binshtein, E.; Chen, R.E.; Nkolola, J.P.; Schafer, A.; Reidy, J.X.; Trivette, A.; Nargi, R.S.; et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 2020, 584, 443–449. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wu, L.; Xu, Z.; Wang, X.; Xie, Y.; Chai, Y.; Zheng, A.; Zhou, J.; Qiao, S.; Huang, M.; et al. An updated atlas of antibody evasion by SARS-CoV-2 Omicron sub-variants including BQ.1.1 and XBB. Cell Rep. Med. 2023, 4, 100991. [Google Scholar] [CrossRef] [PubMed]
- Qu, P.; Evans, J.P.; Zheng, Y.M.; Carlin, C.; Saif, L.J.; Oltz, E.M.; Xu, K.; Gumina, R.J.; Liu, S.L. Evasion of neutralizing antibody responses by the SARS-CoV-2 BA.2.75 variant. Cell Host Microbe 2022, 30, 1518–1526.e4. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 2023, 186, 279–286.e8. [Google Scholar] [CrossRef] [PubMed]
- Yajima, H.; Anraku, Y.; Kaku, Y.; Kimura, K.T.; Plianchaisuk, A.; Okumura, K.; Nakada-Nakura, Y.; Atarashi, Y.; Hemmi, T.; Kuroda, D.; et al. Structural basis for receptor-binding domain mobility of the spike in SARS-CoV-2 BA.2.86 and JN.1. Nat. Commun. 2024, 15, 8574. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Park, Y.J.; Beltramello, M.; Walls, A.C.; Tortorici, M.A.; Bianchi, S.; Jaconi, S.; Culap, K.; Zatta, F.; De Marco, A.; et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 2020, 583, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Wu, N.C.; Zhu, X.; Lee, C.D.; So, R.T.Y.; Lv, H.; Mok, C.K.P.; Wilson, I.A. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science 2020, 368, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, L.; Park, Y.J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell 2020, 183, 1024–1042.e21. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Shi, J.; Verma, A.K.; Guan, X.; Perlman, S.; Du, L. mRNA vaccines elicit potent neutralization against multiple SARS-CoV-2 omicron subvariants and other variants of concern. iScience 2022, 25, 105690. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zheng, J.; Zhang, X.; Tai, W.; Odle, A.E.; Perlman, S.; Du, L. RBD-mRNA vaccine induces broadly neutralizing antibodies against Omicron and multiple other variants and protects mice from SARS-CoV-2 challenge. Transl. Res. 2022, 248, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Uraki, R.; Imai, M.; Ito, M.; Yamayoshi, S.; Kiso, M.; Jounai, N.; Miyaji, K.; Iwatsuki-Horimoto, K.; Takeshita, F.; Kawaoka, Y. An mRNA vaccine encoding the SARS-CoV-2 receptor-binding domain protects mice from various Omicron variants. NPJ Vaccines 2024, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Wang, Y.; Zhang, S.; Sun, J.; Sun, W.; Li, J.; Liu, Y.; Li, M.; Cheng, L.; Jiang, Y.; et al. RBD trimer mRNA vaccine elicits broad and protective immune responses against SARS-CoV-2 variants. iScience 2022, 25, 104043. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, W.; Chen, Z.; Lu, S.; Yang, F.; Bi, Z.; Bao, L.; Mo, F.; Li, X.; Huang, Y.; et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 2020, 586, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, Y.; Dai, L.; Wang, J.; He, P.; Li, C.; Fang, X.; Wang, C.; Zhao, X.; Huang, E.; et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: Two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021, 21, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Dickey, T.H.; Ma, R.; Orr-Gonzalez, S.; Ouahes, T.; Patel, P.; McAleese, H.; Butler, B.; Eudy, E.; Eaton, B.; Murphy, M.; et al. Design of a stabilized RBD enables potently neutralizing SARS-CoV-2 single-component nanoparticle vaccines. Cell Rep. 2023, 42, 112266. [Google Scholar] [CrossRef] [PubMed]
- Myeni, S.K.; Leijs, A.A.; Bredenbeek, P.J.; Morales, S.T.; Linger, M.E.; Fougeroux, C.; van Zanen-Gerhardt, S.; Zander, S.A.L.; Sander, A.F.; Kikkert, M. Protection of K18-hACE2 Mice against SARS-CoV-2 Challenge by a Capsid Virus-like Particle-Based Vaccine. Vaccines 2024, 12, 766. [Google Scholar] [CrossRef] [PubMed]
- Lainscek, D.; Fink, T.; Forstneric, V.; Hafner-Bratkovic, I.; Orehek, S.; Strmsek, Z.; Mancek-Keber, M.; Pecan, P.; Esih, H.; Malensek, S.; et al. A Nanoscaffolded Spike-RBD Vaccine Provides Protection against SARS-CoV-2 with Minimal Anti-Scaffold Response. Vaccines 2021, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Cheng, K.; Zhang, L.; Wang, D.; Gao, X.; Liang, J.; Liu, G.; Ma, N.; Xu, C.; Tang, M.; et al. Rationally designed multimeric nanovaccines using icosahedral DNA origami for display of SARS-CoV-2 receptor binding domain. Nat. Commun. 2024, 15, 9581. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yuan, Y.; Liu, B.; Epstein, N.D.; Yang, Y. Protein-based nano-vaccines against SARS-CoV-2: Current design strategies and advances of candidate vaccines. Int. J. Biol. Macromol. 2023, 236, 123979. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sagaseta, J.; Malito, E.; Rappuoli, R.; Bottomley, M.J. Self-assembling protein nanoparticles in the design of vaccines. Comput. Struct. Biotechnol. J. 2016, 14, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Slifka, M.K.; Amanna, I.J. Role of Multivalency and Antigenic Threshold in Generating Protective Antibody Responses. Front. Immunol. 2019, 10, 956. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.K.; Sahoo, B.R.; Pattnaik, A.K. Protein Nanoparticles as Vaccine Platforms for Human and Zoonotic Viruses. Viruses 2024, 16, 936. [Google Scholar] [CrossRef] [PubMed]
- Backlund, C.; Jalili-Firoozinezhad, S.; Kim, B.; Irvine, D.J. Biomaterials-Mediated Engineering of the Immune System. Annu. Rev. Immunol. 2023, 41, 153–179. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Yu, W.; Shen, L.; Xiao, L.; Qi, J.; Hu, T. A SARS-CoV-2 nanoparticle vaccine based on chemical conjugation of loxoribine and SpyCatcher/SpyTag. Int. J. Biol. Macromol. 2023, 253, 127159. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Arranz, C.; Rincon, S.; Zurita, L.; Ponz, F.; Truchado, D.A. Antigen-functionalized turnip mosaic virus nanoparticles increase antibody sensing in saliva. A case study with SARS-CoV-2 RBD. Diagn. Microbiol. Infect. Dis. 2024, 109, 116298. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.A.; van Doremalen, N.; Greaney, A.J.; Andersen, H.; Sharma, A.; Starr, T.N.; Keeffe, J.R.; Fan, C.; Schulz, J.E.; Gnanapragasam, P.N.P.; et al. Mosaic RBD nanoparticles protect against challenge by diverse sarbecoviruses in animal models. Science 2022, 377, eabq0839. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.K.; Rijal, P.; Rahikainen, R.; Keeble, A.H.; Schimanski, L.; Hussain, S.; Harvey, R.; Hayes, J.W.P.; Edwards, J.C.; McLean, R.K.; et al. A COVID-19 vaccine candidate using SpyCatcher multimerization of the SARS-CoV-2 spike protein receptor-binding domain induces potent neutralising antibody responses. Nat. Commun. 2021, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Argentinian AntiCovid, C. Covalent coupling of Spike’s receptor binding domain to a multimeric carrier produces a high immune response against SARS-CoV-2. Sci. Rep. 2022, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, X.; Cui, H.; Zhang, C.; Wang, S.; Feng, N.; Zhao, Y.; Wang, T.; Yan, F.; Xia, X. Self-assembled ferritin-based nanoparticles elicit a robust broad-spectrum protective immune response against SARS-CoV-2 variants. Int. J. Biol. Macromol. 2024, 264, 130820. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.D.; Kim, N.; Lee, Y.; Lee, E.J. Protein-Based Nanoparticle Vaccines for SARS-CoV-2. Int. J. Mol. Sci. 2021, 22, 13445. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lin, X.; Wang, Y.; Abraham, C.; Sou, C.; Ngo, T.; Zhang, Y.; Wilson, I.A.; Zhu, J. Single-component, self-assembling, protein nanoparticles presenting the receptor binding domain and stabilized spike as SARS-CoV-2 vaccine candidates. Sci. Adv. 2021, 7, eabf1591. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.R.; Minns, A.M.; Sim, D.G.; Field, C.J.; Kerr, A.E.; Heinly, T.A.; Luley, E.H.; Rossi, R.M.; Bator, C.M.; Moustafa, I.M.; et al. Intranasal SARS-CoV-2 RBD decorated nanoparticle vaccine enhances viral clearance in the Syrian hamster model. Microbiol. Spectr. 2024, 12, e0499822. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Kim, D.; Yu, K.M.; Seo, H.D.; Lee, S.A.; Casel, M.A.B.; Jang, S.G.; Kim, S.; Jung, W.; Lai, C.J.; et al. Development of Spike Receptor-Binding Domain Nanoparticles as a Vaccine Candidate against SARS-CoV-2 Infection in Ferrets. mBio 2021, 12, e00230-21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, J.; Zheng, J.; Li, S.; Rao, H.; Dai, J.; Zhang, Z.; Wang, Y.; Liu, D.; Chen, Z.; et al. Mosaic RBD Nanoparticles Elicit Protective Immunity Against Multiple Human Coronaviruses in Animal Models. Adv. Sci. 2024, 11, e2303366. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zou, F.; Yu, F.; Li, R.; Yuan, Y.; Zhang, Y.; Zhang, X.; Deng, J.; Chen, T.; Song, Z.; et al. Nanoparticle Vaccines Based on the Receptor Binding Domain (RBD) and Heptad Repeat (HR) of SARS-CoV-2 Elicit Robust Protective Immune Responses. Immunity 2020, 53, 1315–1330.e9. [Google Scholar] [CrossRef] [PubMed]
- Brandys, P.; Montagutelli, X.; Merenkova, I.; Barut, G.T.; Thiel, V.; Schork, N.J.; Trueb, B.; Conquet, L.; Deng, A.; Antanasijevic, A.; et al. A mRNA Vaccine Encoding for a RBD 60-mer Nanoparticle Elicits Neutralizing Antibodies and Protective Immunity Against the SARS-CoV-2 Delta Variant in Transgenic K18-hACE2 Mice. Front. Immunol. 2022, 13, 912898. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; He, L.; Zhang, H.; Tian, X.; Bai, Z.; Sun, L.; Yang, L.; Jia, X.; Bi, Y.; Luo, T.; et al. The self-assembled nanoparticle-based trimeric RBD mRNA vaccine elicits robust and durable protective immunity against SARS-CoV-2 in mice. Signal Transduct. Target. Ther. 2021, 6, 340. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zhang, C.; Xing, J.; Zhang, T.; Xu, Z.; Di, Y.; Yang, S.; Jiang, R.; Tang, J.; Zhuang, X.; et al. Ferritin-binding and ubiquitination-modified mRNA vaccines induce potent immune responses and protective efficacy against SARS-CoV-2. Int. Immunopharmacol. 2024, 129, 111630. [Google Scholar] [CrossRef] [PubMed]
- Sultana, F.; Ghosh, A. Exploring the evolutionary landscape and structural resonances of ferritin with insights into functional significance in plant. Biochimie 2024, 227, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.M.; Le Brun, N.E.; Moore, G.R. Ferritins: Furnishing proteins with iron. J. Biol. Inorg. Chem. 2016, 21, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Cho, S.; Kim, I.S. Ferritin—A multifaceted protein scaffold for biotherapeutics. Exp. Mol. Med. 2022, 54, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Ahmadivand, S.; Fux, R.; Palic, D. Ferritin Vaccine Platform for Animal and Zoonotic Viruses. Vaccines 2024, 12, 1112. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Abbott, R.K.; Freeman, B.L.; Haupt, S.; Groschel, B.; Silva, M.; Menis, S.; Irvine, D.J.; Schief, W.R.; Crotty, S. Multifaceted Effects of Antigen Valency on B Cell Response Composition and Differentiation In Vivo. Immunity 2020, 53, 548–563.e8. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Eliciting B cell immunity against infectious diseases using nanovaccines. Nat. Nanotechnol. 2021, 16, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.U. Minimum Sizes of Respiratory Particles Carrying SARS-CoV-2 and the Possibility of Aerosol Generation. Int. J. Environ. Res. Public. Health 2020, 17, 6960. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.; Dosey, A.; Boyoglu-Barnum, S.; Park, Y.J.; Gillespie, R.; Syeda, H.; Hutchinson, G.B.; Tsybovsky, Y.; Murphy, M.; Pettie, D.; et al. Antigen spacing on protein nanoparticles influences antibody responses to vaccination. Cell Rep. 2023, 42, 113552. [Google Scholar] [CrossRef] [PubMed]
- Veneziano, R.; Moyer, T.J.; Stone, M.B.; Wamhoff, E.C.; Read, B.J.; Mukherjee, S.; Shepherd, T.R.; Das, J.; Schief, W.R.; Irvine, D.J.; et al. Role of nanoscale antigen organization on B-cell activation probed using DNA origami. Nat. Nanotechnol. 2020, 15, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Joyce, M.G.; Chen, W.H.; Sankhala, R.S.; Hajduczki, A.; Thomas, P.V.; Choe, M.; Martinez, E.J.; Chang, W.C.; Peterson, C.E.; Morrison, E.B.; et al. SARS-CoV-2 ferritin nanoparticle vaccines elicit broad SARS coronavirus immunogenicity. Cell Rep. 2021, 37, 110143. [Google Scholar] [CrossRef] [PubMed]
- Tuekprakhon, A.; Nutalai, R.; Dijokaite-Guraliuc, A.; Zhou, D.; Ginn, H.M.; Selvaraj, M.; Liu, C.; Mentzer, A.J.; Supasa, P.; Duyvesteyn, H.M.E.; et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell 2022, 185, 2422–2433.e13. [Google Scholar] [CrossRef] [PubMed]
- Shishovs, M.; Rumnieks, J.; Diebolder, C.; Jaudzems, K.; Andreas, L.B.; Stanek, J.; Kazaks, A.; Kotelovica, S.; Akopjana, I.; Pintacuda, G.; et al. Structure of AP205 Coat Protein Reveals Circular Permutation in ssRNA Bacteriophages. J. Mol. Biol. 2016, 428, 4267–4279. [Google Scholar] [CrossRef] [PubMed]
- Obata, J.; Kawakami, N.; Tsutsumi, A.; Nasu, E.; Miyamoto, K.; Kikkawa, M.; Arai, R. Icosahedral 60-meric porous structure of designed supramolecular protein nanoparticle TIP60. Chem. Commun. 2021, 57, 10226–10229. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.J.; Shin, H.J.; Lee, J.H.; Kim, K.J.; Park, S.S.; Lee, Y.; Lee, C.; Park, S.S.; Kim, K.H. The crystal structure of ferritin from Helicobacter pylori reveals unusual conformational changes for iron uptake. J. Mol. Biol. 2009, 390, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Trikha, J.; Theil, E.C.; Allewell, N.M. High resolution crystal structures of amphibian red-cell L ferritin: Potential roles for structural plasticity and solvation in function. J. Mol. Biol. 1995, 248, 949–967. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; DiMaio, F.; Wang, R.Y.; Kim, D.; Miles, C.; Brunette, T.; Thompson, J.; Baker, D. High-resolution comparative modeling with RosettaCM. Structure 2013, 21, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.S.; Ban, Y.E.; Richter, F.; Andre, I.; Vernon, R.; Schief, W.R.; Baker, D. RosettaRemodel: A generalized framework for flexible backbone protein design. PLoS ONE 2011, 6, e24109. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.G.; Young, L.; Chuang, R.Y.; Venter, J.C.; Hutchison, C.A., 3rd; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Baldi, L.; Hacker, D.L.; Adam, M.; Wurm, F.M. Recombinant protein production by large-scale transient gene expression in mammalian cells: State of the art and future perspectives. Biotechnol. Lett. 2007, 29, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Dalton, A.C.; Barton, W.A. Over-expression of secreted proteins from mammalian cell lines. Protein Sci. 2014, 23, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, M.S.; Ahmed, M. Enzyme-Linked Immunosorbent Assay (ELISA). Methods Mol. Biol. 2022, 2508, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Kohl, T.O.; Ascoli, C.A. Immunometric Double-Antibody Sandwich Enzyme-Linked Immunosorbent Assay. Cold Spring Harb. Protoc. 2017, 2017, pdb.prot093724. [Google Scholar] [CrossRef] [PubMed]
- Engvall, E. The ELISA, enzyme-linked immunosorbent assay. Clin. Chem. 2010, 56, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Westendorf, K.; Zentelis, S.; Wang, L.; Foster, D.; Vaillancourt, P.; Wiggin, M.; Lovett, E.; van der Lee, R.; Hendle, J.; Pustilnik, A.; et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Rep. 2022, 39, 110812. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.B.; Wobig, W.J.; Petering, D.H. Native SDS-PAGE: High resolution electrophoretic separation of proteins with retention of native properties including bound metal ions. Metallomics 2014, 6, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Sapan, C.V.; Lundblad, R.L.; Price, N.C. Colorimetric protein assay techniques. Biotechnol. Appl. Biochem. 1999, 29, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Kurien, B.T.; Scofield, R.H. Western blotting. Methods 2006, 38, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; de Jong, R.N.; van den Bremer, E.T.J.; Parren, P.; Heck, A.J.R. Enhancing Accuracy in Molecular Weight Determination of Highly Heterogeneously Glycosylated Proteins by Native Tandem Mass Spectrometry. Anal. Chem. 2017, 89, 4793–4797. [Google Scholar] [CrossRef] [PubMed]
- Kariko, K. In vitro-Transcribed mRNA Therapeutics: Out of the Shadows and Into the Spotlight. Mol. Ther. 2019, 27, 691–692. [Google Scholar] [CrossRef] [PubMed]
- Kamakaka, R.T.; Kraus, W.L. In vitro transcription. Curr. Protoc. Cell Biol. 2001, 2, 11.6.1–11.6.17. [Google Scholar] [CrossRef]
- Beckert, B.; Masquida, B. Synthesis of RNA by in vitro transcription. Methods Mol. Biol. 2011, 703, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Yanez Arteta, M.; Kjellman, T.; Bartesaghi, S.; Wallin, S.; Wu, X.; Kvist, A.J.; Dabkowska, A.; Szekely, N.; Radulescu, A.; Bergenholtz, J.; et al. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc. Natl. Acad. Sci. USA 2018, 115, E3351–E3360. [Google Scholar] [CrossRef] [PubMed]
- Bahl, K.; Senn, J.J.; Yuzhakov, O.; Bulychev, A.; Brito, L.A.; Hassett, K.J.; Laska, M.E.; Smith, M.; Almarsson, O.; Thompson, J.; et al. Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol. Ther. 2017, 25, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Rajan, S.; Hayes, C.F.; Kwong, K.Y.; Goncalves, A.R.; Zemla, A.T.; Lau, E.Y.; Zhang, Y.; Cai, Y.; Goforth, J.W.; et al. Preemptive optimization of a clinical antibody for broad neutralization of SARS-CoV-2 variants and robustness against viral escape. Sci. Adv. 2025, 11, eadu0718. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Klovins, J.; Overbeek, G.P.; van den Worm, S.H.E.; Ackermann, H.W.; van Duin, J. Nucleotide sequence of a ssRNA phage from Acinetobacter: Kinship to coliphages. J. Gen. Virol. 2002, 83, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, N.; Kondo, H.; Matsuzawa, Y.; Hayasaka, K.; Nasu, E.; Sasahara, K.; Arai, R.; Miyamoto, K. Design of Hollow Protein Nanoparticles with Modifiable Interior and Exterior Surfaces. Angew. Chem. Int. Ed. Engl. 2018, 57, 12400–12404. [Google Scholar] [CrossRef] [PubMed]
- Zinkhan, S.; Ogrina, A.; Balke, I.; Resevica, G.; Zeltins, A.; de Brot, S.; Lipp, C.; Chang, X.; Zha, L.; Vogel, M.; et al. The impact of size on particle drainage dynamics and antibody response. J. Control. Release 2021, 331, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Zepeda, S.K.; Park, Y.J.; Taylor, A.L.; Quispe, J.; Stewart, C.; Leaf, E.M.; Treichel, C.; Corti, D.; King, N.P.; et al. Broad receptor tropism and immunogenicity of a clade 3 sarbecovirus. Cell Host Microbe 2023, 31, 1961–1973.e11. [Google Scholar] [CrossRef] [PubMed]
- Starr, T.N.; Zepeda, S.K.; Walls, A.C.; Greaney, A.J.; Alkhovsky, S.; Veesler, D.; Bloom, J.D. ACE2 binding is an ancestral and evolvable trait of sarbecoviruses. Nature 2022, 603, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Drosten, C.; Gunther, S.; Preiser, W.; van der Werf, S.; Brodt, H.R.; Becker, S.; Rabenau, H.; Panning, M.; Kolesnikova, L.; Fouchier, R.A.; et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; Lau, S.K.P.; Chen, Y.; Wong, E.Y.M.; Chan, K.H.; Chen, H.; Zhang, L.; Xia, N.; Yuen, K.Y. Rapid detection of MERS coronavirus-like viruses in bats: Pote1ntial for tracking MERS coronavirus transmission and animal origin. Emerg. Microbes Infect. 2018, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Tong, S. Complete Genome Sequence of a Severe Acute Respiratory Syndrome-Related Coronavirus from Kenyan Bats. Microbiol. Resour. Announc. 2019, 8, e00548-19. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.L.; Werner, A.P.; Leist, S.R.; Stevens, L.J.; Falconer, E.; Goldsmith, J.A.; Chou, C.W.; Abiona, O.M.; West, A.; Westendorf, K.; et al. Stabilized coronavirus spike stem elicits a broadly protective antibody. Cell Rep. 2021, 37, 109929. [Google Scholar] [CrossRef] [PubMed]
- Dacon, C.; Tucker, C.; Peng, L.; Lee, C.D.; Lin, T.H.; Yuan, M.; Cong, Y.; Wang, L.; Purser, L.; Williams, J.K.; et al. Broadly neutralizing antibodies target the coronavirus fusion peptide. Science 2022, 377, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.P.; Huang, Y.; Nguyen, A.W.; Hsieh, C.L.; Olaluwoye, O.S.; Kaoud, T.S.; Wilen, R.E.; Qerqez, A.N.; Park, J.G.; Khalil, A.M.; et al. Identification of a conserved S2 epitope present on spike proteins from all highly pathogenic coronaviruses. Elife 2023, 12, e83710. [Google Scholar] [CrossRef] [PubMed]
- Llanes, A.; Restrepo, C.M.; Caballero, Z.; Rajeev, S.; Kennedy, M.A.; Lleonart, R. Betacoronavirus Genomes: How Genomic Information has been Used to Deal with Past Outbreaks and the COVID-19 Pandemic. Int. J. Mol. Sci. 2020, 21, 4546. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.F.; Yuen, T.T.; Gong, H.R.; Hu, B.; Hu, J.C.; Lin, X.S.; Rong, L.; Zhou, C.L.; Chen, L.L.; Wang, X.; et al. Rational design of a booster vaccine against COVID-19 based on antigenic distance. Cell Host Microbe 2023, 31, 1301–1316.e8. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.J.; Chang, W.C.; Chen, W.H.; Hajduczki, A.; Thomas, P.V.; Jensen, J.L.; Choe, M.; Sankhala, R.S.; Peterson, C.E.; Rees, P.A.; et al. SARS-CoV-2 ferritin nanoparticle vaccines produce hyperimmune equine sera with broad sarbecovirus activity. iScience 2024, 27, 110624. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, M.; Bu, W.; Joyce, M.G.; Meng, G.; Whittle, J.R.; Baxa, U.; Yamamoto, T.; Narpala, S.; Todd, J.P.; Rao, S.S.; et al. Rational Design of an Epstein-Barr Virus Vaccine Targeting the Receptor-Binding Site. Cell 2015, 162, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, M.; Joyce, M.G.; Gillespie, R.A.; Gallagher, J.R.; Andrews, S.F.; Yassine, H.M.; Wheatley, A.K.; Fisher, B.E.; Ambrozak, D.R.; Creanga, A.; et al. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat. Immunol. 2019, 20, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chao, C.W.; Tsybovsky, Y.; Abiona, O.M.; Hutchinson, G.B.; Moliva, J.I.; Olia, A.S.; Pegu, A.; Phung, E.; Stewart-Jones, G.; et al. A Platform Incorporating Trimeric Antigens into Self-Assembling Nanoparticles Reveals SARS-CoV-2-Spike Nanoparticles to Elicit Substantially Higher Neutralizing Responses than Spike Alone. bioRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Arai, R. Design of helical linkers for fusion proteins and protein-based nanostructures. Methods Enzymol. 2021, 647, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zaro, J.L.; Shen, W.C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Ayyagari, V.S.; Venkateswarulu, T.C.; Karlapudi, A.P.; Srirama, K. Design of a multi-epitope-based vaccine targeting M-protein of SARS-CoV2: An immunoinformatics approach. J. Biomol. Struct. Dyn. 2022, 40, 2963–2977. [Google Scholar] [CrossRef] [PubMed]
- Tokatlian, T.; Read, B.J.; Jones, C.A.; Kulp, D.W.; Menis, S.; Chang, J.Y.H.; Steichen, J.M.; Kumari, S.; Allen, J.D.; Dane, E.L.; et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science 2019, 363, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Thimmiraju, S.R.; Adhikari, R.; Villar, M.J.; Lee, J.; Liu, Z.; Kundu, R.; Chen, Y.L.; Sharma, S.; Ghei, K.; Keegan, B.; et al. A Recombinant Protein XBB.1.5 RBD/Alum/CpG Vaccine Elicits High Neutralizing Antibody Titers against Omicron Subvariants of SARS-CoV-2. Vaccines 2023, 11, 1557. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.; Brunette, N.; Crawford, K.H.D.; Walls, A.C.; Pham, M.N.; Chen, C.; Herpoldt, K.L.; Fiala, B.; Murphy, M.; Pettie, D.; et al. Stabilization of the SARS-CoV-2 Spike Receptor-Binding Domain Using Deep Mutational Scanning and Structure-Based Design. Front. Immunol. 2021, 12, 710263. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, G.G.; Grigoryan, L.; Navarro, M.J.; Catanzaro, N.J.; Hubbard, M.L.; Powers, J.M.; Mattocks, M.; Treichel, C.; Walls, A.C.; Lee, J.; et al. Computationally designed mRNA-launched protein nanoparticle vaccines. bioRxiv 2024. [Google Scholar] [CrossRef]
- Yamashita, M.; Kawakami, N.; Miyamoto, K. Hydrophobization of a TIP60 Protein Nanocage for the Encapsulation of Hydrophobic Compounds. Chempluschem 2023, 88, e202200392. [Google Scholar] [CrossRef] [PubMed]
- Ohara, N.; Kawakami, N.; Arai, R.; Adachi, N.; Moriya, T.; Kawasaki, M.; Miyamoto, K. Reversible Assembly of an Artificial Protein Nanocage Using Alkaline Earth Metal Ions. J. Am. Chem. Soc. 2023, 145, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Peng, Y.; Lin, L.; Pan, X.; Fang, M.; Zhao, Y.; Bao, K.; Li, R.; Han, J.; Chen, J.; et al. A pathogen-like antigen-based vaccine confers immune protection against SARS-CoV-2 in non-human primates. Cell Rep. Med. 2021, 2, 100448. [Google Scholar] [CrossRef] [PubMed]
- Fougeroux, C.; Goksoyr, L.; Idorn, M.; Soroka, V.; Myeni, S.K.; Dagil, R.; Janitzek, C.M.; Sogaard, M.; Aves, K.L.; Horsted, E.W.; et al. Capsid-like particles decorated with the SARS-CoV-2 receptor-binding domain elicit strong virus neutralization activity. Nat. Commun. 2021, 12, 324. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, A.; Koopman, G.; Mooij, P.; Verschoor, E.J.; Verstrepen, B.E.; Bogers, W.; Idorn, M.; Paludan, S.R.; Vang, S.; Nielsen, M.A.; et al. A Capsid Virus-Like Particle-Based SARS-CoV-2 Vaccine Induces High Levels of Antibodies and Protects Rhesus Macaques. Front. Immunol. 2022, 13, 857440. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.J.; Sander, A.F.; Ariaans, M.; Fougeroux, C.; Heinzel, C.; Fendel, R.; Esen, M.; Kremsner, P.G.; Ter Heine, R.; Wertheim, H.F.; et al. First-in-human use of a modular capsid virus-like vaccine platform: An open-label, non-randomised, phase 1 clinical trial of the SARS-CoV-2 vaccine ABNCoV2. Lancet Microbe 2023, 4, e140–e148. [Google Scholar] [CrossRef] [PubMed]
- Hatlem, D.; Trunk, T.; Linke, D.; Leo, J.C. Catching a SPY: Using the SpyCatcher-SpyTag and Related Systems for Labeling and Localizing Bacterial Proteins. Int. J. Mol. Sci. 2019, 20, 2129. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chang, X.; Rothen, D.; Derveni, M.; Krenger, P.; Roongta, S.; Wright, E.; Vogel, M.; Tars, K.; Mohsen, M.O.; et al. AP205 VLPs Based on Dimerized Capsid Proteins Accommodate RBM Domain of SARS-CoV-2 and Serve as an Attractive Vaccine Candidate. Vaccines 2021, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Dalvie, N.C.; Rodriguez-Aponte, S.A.; Hartwell, B.L.; Tostanoski, L.H.; Biedermann, A.M.; Crowell, L.E.; Kaur, K.; Kumru, O.S.; Carter, L.; Yu, J.; et al. Engineered SARS-CoV-2 receptor binding domain improves manufacturability in yeast and immunogenicity in mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2106845118. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Balke, I.; Zinkhan, S.; Zeltina, V.; Liu, X.; Chang, X.; Krenger, P.S.; Plattner, K.; Gharailoo, Z.; Vogt, A.S.; et al. A scalable and highly immunogenic virus-like particle-based vaccine against SARS-CoV-2. Allergy 2022, 77, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, S.; Zheng, Y.; Xie, Y.; Xu, K.; Chai, Y.; Luo, T.; Dai, L.; Gao, G.F. Mosaic RBD nanoparticle elicits immunodominant antibody responses across sarbecoviruses. Cell Rep. 2024, 43, 114235. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.S.; Traver, M.; Barefoot, N.; Stephens, T.; Alabanza, C.; Manzella-Lapeira, J.; Zou, G.; Wolff, J.; Li, Y.; Resto, M.; et al. Mosaic quadrivalent influenza vaccine single nanoparticle characterization. Sci. Rep. 2024, 14, 4534. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.A.; Chen, X.; Mohapatra, A.; Nguyen, H.T.V.; Schimanski, L.; Tan, T.K.; Rijal, P.; Vester, S.K.; Hills, R.A.; Howarth, M.; et al. Structural basis for a conserved neutralization epitope on the receptor-binding domain of SARS-CoV-2. Nat. Commun. 2023, 14, 311. [Google Scholar] [CrossRef] [PubMed]
- Jette, C.A.; Cohen, A.A.; Gnanapragasam, P.N.P.; Muecksch, F.; Lee, Y.E.; Huey-Tubman, K.E.; Schmidt, F.; Hatziioannou, T.; Bieniasz, P.D.; Nussenzweig, M.C.; et al. Broad cross-reactivity across sarbecoviruses exhibited by a subset of COVID-19 donor-derived neutralizing antibodies. Cell Rep. 2021, 36, 109760. [Google Scholar] [CrossRef] [PubMed]
- Cankat, S.; Demael, M.U.; Swadling, L. In search of a pan-coronavirus vaccine: Next-generation vaccine design and immune mechanisms. Cell Mol. Immunol. 2024, 21, 103–118. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guest, J.D.; Zhang, Y.; Flores, D.; Atkins, E.; Ren, K.; Cai, Y.; Rosenthal, K.; Wang, Z.; Kim, K.; Chen, C.; et al. SARS-CoV-2 RBD Scaffolded by AP205 or TIP60 Nanoparticles and Delivered as mRNA Elicits Robust Neutralizing Antibody Responses. Vaccines 2025, 13, 778. https://doi.org/10.3390/vaccines13080778
Guest JD, Zhang Y, Flores D, Atkins E, Ren K, Cai Y, Rosenthal K, Wang Z, Kim K, Chen C, et al. SARS-CoV-2 RBD Scaffolded by AP205 or TIP60 Nanoparticles and Delivered as mRNA Elicits Robust Neutralizing Antibody Responses. Vaccines. 2025; 13(8):778. https://doi.org/10.3390/vaccines13080778
Chicago/Turabian StyleGuest, Johnathan D., Yi Zhang, Daniel Flores, Emily Atkins, Kuishu Ren, Yingyun Cai, Kim Rosenthal, Zimeng Wang, Kihwan Kim, Charles Chen, and et al. 2025. "SARS-CoV-2 RBD Scaffolded by AP205 or TIP60 Nanoparticles and Delivered as mRNA Elicits Robust Neutralizing Antibody Responses" Vaccines 13, no. 8: 778. https://doi.org/10.3390/vaccines13080778
APA StyleGuest, J. D., Zhang, Y., Flores, D., Atkins, E., Ren, K., Cai, Y., Rosenthal, K., Wang, Z., Kim, K., Chen, C., Roque, R., Cheng, B., Yanez Arteta, M., Zhou, L., Laliberte, J., & Francica, J. R. (2025). SARS-CoV-2 RBD Scaffolded by AP205 or TIP60 Nanoparticles and Delivered as mRNA Elicits Robust Neutralizing Antibody Responses. Vaccines, 13(8), 778. https://doi.org/10.3390/vaccines13080778






