Survey on Awareness and Attitudes Toward Maternal Immunization Against Influenza, Pertussis, Respiratory Syncytial Virus, and Group B Streptococcus Among Pregnant Women in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Statistical Analyses
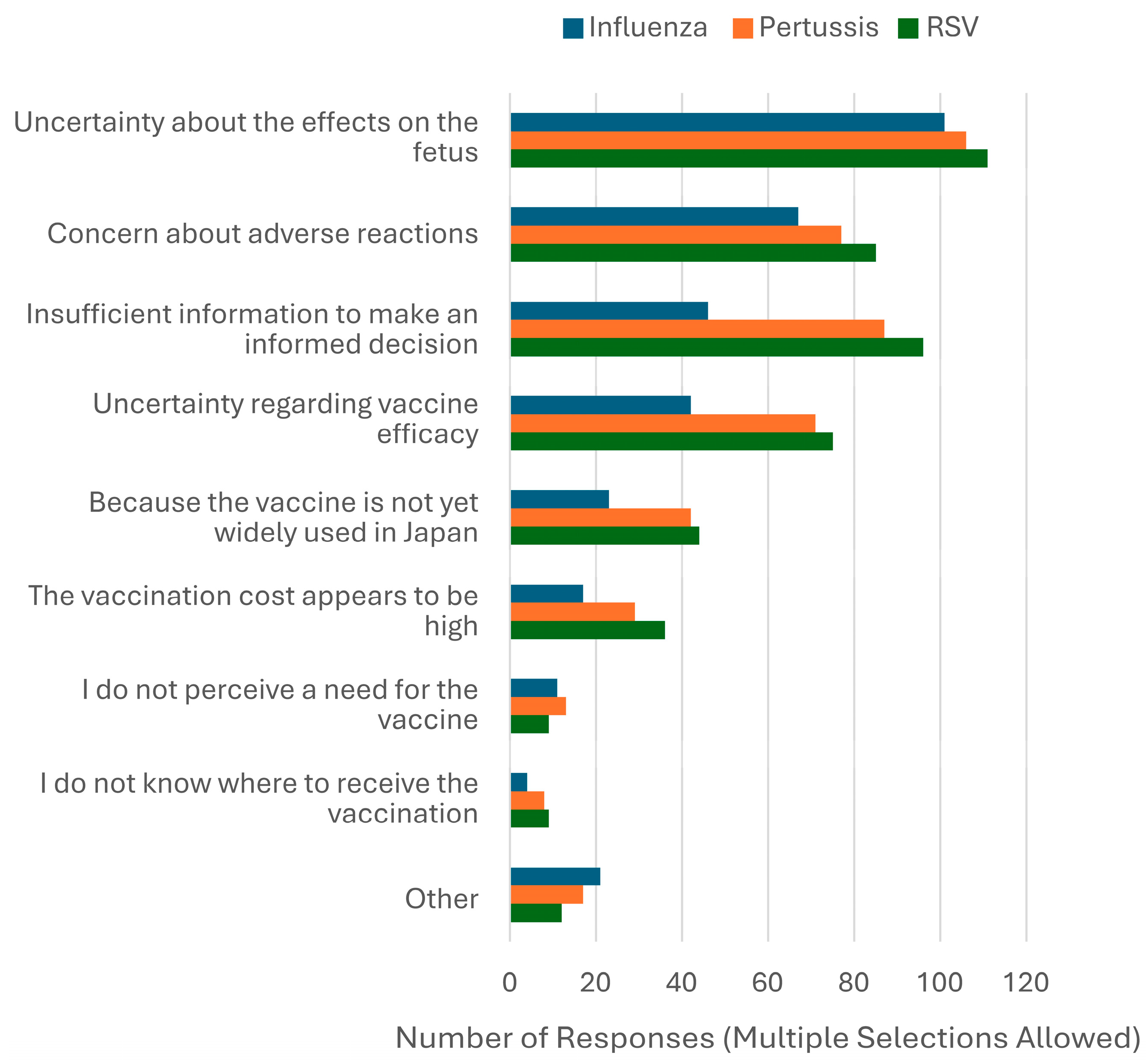
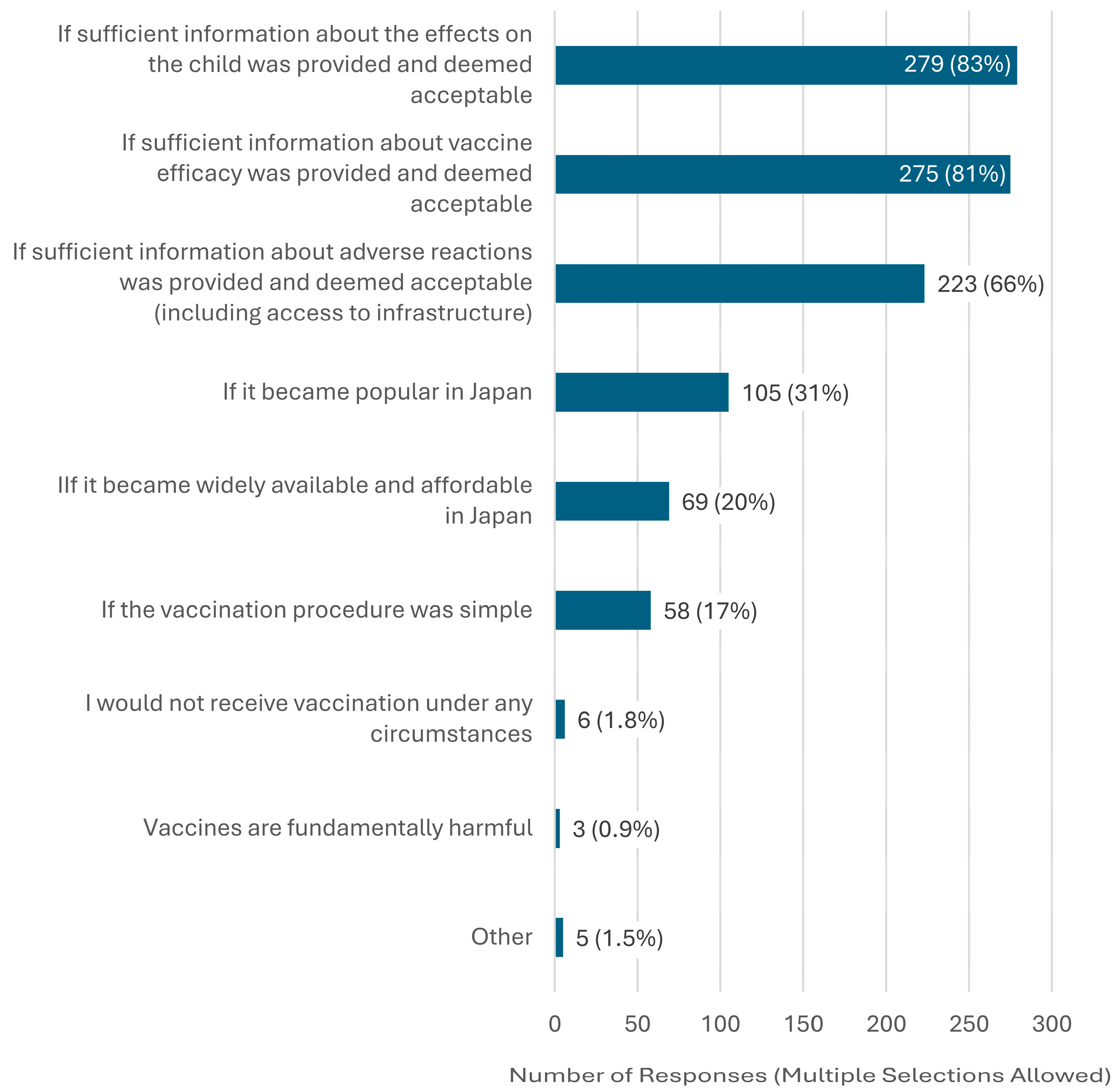
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Confidence Interval |
| DTaP | diphtheria, tetanus, and acellular pertussis (vaccine) |
| FIGO | International Federation of Gynecology and Obstetrics |
| GBS | group B streptococcus |
| ICSI | Intracytoplasmic Sperm Injection |
| IVF | In Vitro Fertilization |
| JPY | Japanese Yen |
| OR | Odds Ratio |
| DT | respiratory syncytial virus |
| SNS | Social Networking Service |
| Tdap | tetanus, diphtheria, and acellular pertussis (vaccine) |
References
- Chu, H.Y.; Englund, J.A. Maternal immunization. Clin. Infect. Dis. 2014, 59, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.L. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MWR Recomm. Rep. 2018, 67, 1. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Pertussis vaccines: WHO position paper—September 2015. Wkly Epidemiol. Rec. 2015, 90, 433–458. [Google Scholar]
- CDC. Guidelines for Vaccinating Pregnant Women. Available online: https://www.cdc.gov/vaccines-pregnancy/hcp/vaccination-guidelines/ (accessed on 15 July 2025).
- WHO. WHO Vaccine Position Papers. Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/policies/position-papers (accessed on 15 July 2025).
- Hiiragi, K.; Obata, S.; Miyagi, E.; Aoki, S. The current status, attitudes, and practices concerning maternal pertussis vaccination in obstetric delivery facilities in Kanagawa Prefecture, Japan: A questionnaire survey. Hum. Vaccin. Immunother. 2021, 17, 4235–4238. [Google Scholar] [CrossRef] [PubMed]
- Kurasawa, K. Maternal vaccination-current status, challenges, and opportunities. J. Obstet. Gynaecol. Res. 2023, 49, 493–509. [Google Scholar] [CrossRef] [PubMed]
- Kitano, T.; Onishi, T.; Takeyama, M.; Shima, M. Questionnaire survey on maternal pertussis vaccination for pregnant women and mothers in Nara Prefecture, Japan. Hum. Vaccin. Immunother. 2020, 16, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Anderson, A.S.; Absalon, J.; Radley, D.; Simon, R.; Jongihlati, B.; Strehlau, R.; van Niekerk, A.M.; Izu, A.; Naidoo, N.; et al. Potential for maternally administered vaccine for infant Group B streptococcus. N. Engl. J. Med. 2023, 389, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Blázquez-Gamero, D.; Bernstein, D.I.; Gantt, S.; Bautista, O.; Beck, K.; Conlon, A.; Rosenbloom, D.I.S.; Wang, D.; Ritter, M.; et al. Safety, efficacy, and immunogenicity of a replication-defective human cytomegalovirus vaccine, V160, in cytomegalovirus-seronegative women: A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Infect. Dis. 2023, 23, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.T.; Winje, B.A.; Stålcrantz, J.; Greve-Isdahl, M. Predictors of maternal pertussis vaccination acceptance among pregnant women in Norway. Hum. Vaccin. Immunother. 2024, 20, 2361499. [Google Scholar] [CrossRef] [PubMed]
- Li, W.F.; Huang, S.Y.; Peng, H.H.; Chang, Y.L.; Chang, S.D.; Cheng, P.J.; Taiwan Prenatal Pertussis Immunization Program (PPIP) Collaboration Group. Factors affecting pregnant women’s decisions regarding prenatal pertussis vaccination: A decision-making study in the nationwide Prenatal Pertussis Immunization Program in Taiwan. Taiwan J. Obstet. Gynecol. 2020, 59, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Dudley, M.Z.; Limaye, R.J.; Omer, S.B.; O’Leary, S.T.; Ellingson, M.K.; Spina, C.I.; Brewer, S.E.; Chamberlain, A.T.; Bednarczyk, R.A.; Malik, F.; et al. Characterizing the vaccine knowledge, attitudes, beliefs, and intentions of pregnant women in Georgia and Colorado. Hum. Vaccin. Immunother. 2020, 16, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Gavaruzzi, T.; Ceccarelli, A.; Nanni, C.; Vignali, C.; Colonnello, V.; Caserotti, M.; Riccò, M.; Gori, D. Knowledge and attitudes regarding respiratory syncytial virus (RSV) prevention: A systematic review. Vaccines 2025, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Yahata, Y.; Kamiya, H.; Griffith, M.M.; Tsuchihashi, Y.; Kawakami, S.; Nii, S.; Sunagawa, T. Knowledge, attitudes, and practices associated with pertussis vaccination during pregnancy: Japan, 2016–2017. Jpn. J. Infect. Dis. 2021, 74, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Oka, E.; Ueda, Y.; Yagi, A.; Machida, M.; Furuse, Y.; Tabuchi, T. Challenges to promoting maternal respiratory syncytial virus vaccination in Japan. Vaccine 2025, 48, 126767. [Google Scholar] [CrossRef] [PubMed]
- McAuslane, H.; Utsi, L.; Wensley, A.; Coole, L. Inequalities in maternal pertussis vaccination uptake: A cross-sectional survey of maternity units. J. Public Health 2018, 40, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Japan Society of Obstetrics and Gynecology; Japan Association of Obstetricians and Gynecologists. 2008 Guidelines for Obstetrics and Gynecology, Obstetrics Part. Available online: http://www.jaog.or.jp/wp/wp-content/uploads/2017/01/guide_2008.pdf (accessed on 28 June 2025). (In Japanese).
- Japan Society of Obstetrics and Gynecology; Japan Association of Obstetricians and Gynecologists. 2011 Guidelines for Obstetrics and Gynecology, Obstetrics Part. Available online: http://www.jaog.or.jp/sep2012/diagram/notes/guide_2011.pdf (accessed on 28 June 2025). (In Japanese).
- Shono, A.; Hoshi, S.L.; Kondo, M. Maternal influenza vaccination relates to receiving relevant information among pregnant women in Japan. Hum. Vaccin. Immunother. 2020, 16, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Japan Society of Obstetrics and Gynecology; Japan Association of Obstetricians and Gynecologists. 2023 Guidelines for Obstetrics and Gynecology, Obstetrics Part. Available online: https://www.jsog.or.jp/activity/pdf/gl_sanka_2023.pdf (accessed on 28 June 2025). (In Japanese).
- Benedetto, C.; Borella, F.; Divakar, H.; O’Riordan, S.L.; Mazzoli, M.; Hanson, M.; O’Reilly, S.; Jacobsson, B.; Conry, J.A.; McAuliffe, F.M.; et al. FIGO Preconception Checklist: Preconception care for mother and baby. Int. J. Gynaecol. Obstet. 2024, 165, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kupka, M.S.; Chambers, G.M.; Dyer, S.; Zegers-Hochschild, F.; de Mouzon, J.; Ishihara, O.; Banker, M.; Jwa, S.C.; Fu, B.; Elgindy, E.; et al. World Report. International Committee for Monitoring Assisted Reproductive Technology world report: Assisted reproductive technology, 2015 and 2016. Fertil. Steril. 2024, 122, 875–893. [Google Scholar] [CrossRef] [PubMed]
- ACOG Maternal RSV Vaccination. Available online: https://www.acog.org/clinical-information/physician-faqs/maternal-rsv-vaccination (accessed on 13 March 2025).
- Hoshi, S.L.; Seposo, X.; Okubo, I.; Kondo, M. Cost-effectiveness analysis of pertussis vaccination during pregnancy in Japan. Vaccine 2018, 36, 5133–5140. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, S.L.; Shono, A.; Seposo, X.; Okubo, I.; Kondo, M. Cost-effectiveness analysis of influenza vaccination during pregnancy in Japan. Vaccine 2020, 38, 7363–7371. [Google Scholar] [CrossRef] [PubMed]
- Gebretekle, G.B.; Yeung, M.W.; Ximenes, R.; Cernat, A.; Simmons, A.E.; Killikelly, A.; Siu, W.; Rafferty, E.; Brousseau, N.; Tunis, M.; et al. Cost-effectiveness of RSVpreF vaccine and nirsevimab for the prevention of respiratory syncytial virus disease in Canadian infants. Vaccine 2024, 42, 126164. [Google Scholar] [CrossRef] [PubMed]
- Hutton, D.W.; Prosser, L.A.; Rose, A.M.; Mercon, K.; Ortega-Sanchez, I.R.; Leidner, A.J.; McMorrow, M.L.; Fleming-Dutra, K.E.; Prill, M.M.; Pike, J.; et al. Cost-effectiveness of maternal vaccination to prevent respiratory syncytial virus illness. Pediatrics 2024, 154, e2024066481. [Google Scholar] [CrossRef] [PubMed]
- Dubé, E.; Laberge, C.; Guay, M.; Bramadat, P.; Roy, R.; Bettinger, J. Vaccine hesitancy: An overview. Hum. Vaccin. Immunother. 2013, 9, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Takei, Y.; Ishikawa, Y.; Saga, Y.; Machida, S.; Taneichi, A.; Suzuki, M. Community-based interventions to improve HPV vaccination coverage among 13- to 15-year-old females: Measures implemented by local governments in Japan. PLoS ONE 2013, 8, e84126. [Google Scholar] [CrossRef] [PubMed]
- Paterson, P.; Meurice, F.; Stanberry, L.R.; Glismann, S.; Rosenthal, S.L.; Larson, H.J. Vaccine hesitancy and healthcare providers. Vaccine 2016, 34, 6700–6706. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Tao, L.; Liu, J. Association between risk perception and influenza vaccine hesitancy for children among reproductive women in China during the COVID-19 pandemic: A national online survey. BMC Public Health 2022, 22, 385. [Google Scholar] [CrossRef] [PubMed]
- Del Riccio, M.; Guida, A.; Boudewijns, B.; Heemskerk, S.; van Summeren, J.; Schneeberger, C.; Stelma, F.; van Der Velden, K.; Timen, A.; Caini, S. A missed opportunity? Exploring changes in influenza vaccination coverage during the COVID-19 pandemic: Data from 12 countries worldwide. Influenza Other Respir. Viruses 2025, 19, e70057. [Google Scholar] [CrossRef] [PubMed]
- Cosma, C.; Radi, A.; Cattano, R.; Zanobini, P.; Bonaccorsi, G.; Lorini, C.; Del Riccio, M. Exploring chatbot contributions to enhancing vaccine literacy and uptake: A scoping review of the literature. Vaccine 2025, 44, 126559. [Google Scholar] [CrossRef] [PubMed]

| Variable | n (%) |
|---|---|
| Age (year), median (IQR) a | 33 (30–36) |
| Gestational age at response a | |
| up to 11 weeks | 145 (28) |
| 12 to 21 weeks | 117 (22) |
| 22 to 27 weeks | 82 (16) |
| 28 to 36 weeks | 136 (26) |
| 37 weeks or more | 41 (7.9) |
| Number of pregnancies, median (IQR) | 2 (1–3) |
| Number of deliveries, median (IQR) b | 0 (0–1) |
| Conception methods b | |
| Natural conception | 327 (63) |
| Timed intercourse | 51 (9.8) |
| Intrauterine insemination | 12 (2.3) |
| IVF/ICSI | 132 (25) |
| Final educational attainment b | |
| Junior high school | 14 (1.7) |
| High school | 65 (12) |
| Junior/vocational/technical college | 141 (27) |
| University | 261 (50) |
| Graduate school | 41 (7.9) |
| Experience in a healthcare occupation b | |
| None | 377 (72) |
| Hospital clerical work | 24 (4.6) |
| Medical professional (e.g., nurse, pharmacist, lab tech) | 109 (21) |
| Doctor | 12 (2.3) |
| Nationality | |
| Japan | 489 (93) |
| China | 20 (3.9) |
| South Korea | 3 (0.58) |
| Philippines | 3 (0.58) |
| Vietnam | 2 (0.38) |
| Thailand | 2 (0.38) |
| Mongolia | 2 (0.38) |
| Peru | 1 (0.19) |
| Spain | 1 (0.19) |
| Prior knowledge of maternal immunization | 86 (16) |
| Preferred Cost (JPY) | Influenza (n = 351) n (%) | Pertussis (n = 303) n (%) | RSV (n = 305) n (%) | GBS (n = 364) n (%) |
|---|---|---|---|---|
| Willing to pay more than 50,000 | 16 (4.6) | 14 (4.6) | 17 (5.6) | 13 (3.6) |
| Willing to pay up to 50,000 | 2 (0.6) | 3 (1.0) | 7 (2.3) | 4 (1.1) |
| Willing to pay up to 30,000 | 6 (1.7) | 5 (1.7) | 6 (2.0) | 4 (1.1) |
| Willing to pay up to 20,000 | 6 (1.7) | 6 (2.0) | 7 (2.3) | 9 (2.5) |
| Willing to pay up to 10,000 | 34 (9.7) | 34 (11) | 37 (12) | 38 (10) |
| Willing to pay up to 7000 | 7 (2.0) | 11 (3.6) | 10 (3.3) | 8 (2.2) |
| Willing to pay up to 5000 | 106 (30) | 90 (30) | 78 (26) | 116 (32) |
| Willing to pay up to 3000 | 160 (46) | 122 (40) | 129 (42) | 152 (42) |
| Willing to pay up to 1000 | 14 (4.0) | 18 (6.0) | 14 (4.6) | 20 (5.5) |
| Characteristic | Willing (n = 352) | Unwilling (n = 169) | OR (95% CI) |
|---|---|---|---|
| Age (year), median (IQR) a | 34 (30–37) | 33 (29–36.5) | N/A |
| Gestational age, n (%) | |||
| <22 weeks | 174 (49) | 88 (52) | Ref |
| ≥22 weeks | 178 (51) | 81 (48) | 1.12 (0.779–1.62) |
| Primiparity, n (%) | 187 (53) | 98 (58) | 0.821(0.567–1.19) |
| IVF/ICSI, n (%) b | 96 (27) | 35 (20) | 1.44 (0.928–2.24) |
| Education, n (%) b | |||
| ≤High school | 42 (12) | 37 (22) | Ref |
| ≥Junior college c | 310 (88) | 131 (78) | 2.08 (1.28–3.39) |
| Healthcare-related work (including clerical), n (%) b | 113 (32) | 31 (18) | 2.09 (1.33–3.28) |
| Information source during pregnancy, n (%) b † | |||
| Doctor or midwife | 326 (93) | 142 (84) | 2.48 (1.39–4.42) |
| Internet/SNS | 188 (54) | 90 (53) | 1.01 (0.700–1.46) |
| Friend | 55 (16) | 38 (22) | 0.641 (0.404–1.02) |
| Prior knowledge of maternal immunization, n (%) | 74 (21) | 12 (7.1) | 3.48 (1.84–6.61) |
| Prior knowledge of influenza infection, n (%) b | 343 (98) | 159 (94) | 2.70 (1.04–6.96) |
| History of influenza infection (self or child), n (%) | 295 (84) | 133 (79) | 1.40 (0.88–2.23) |
| Characteristic | Willing (n = 303) | Unwilling (n = 215) | OR (95% CI) |
|---|---|---|---|
| Age (year), median (IQR) a | 33 (30–36.5) | 34 (30–37) | N/A |
| Gestational age, n (%) a | |||
| <22 weeks | 143 (48) | 115 (53) | Ref |
| ≥22 weeks | 158 (52) | 100 (47) | 1.27 (0.895–1.80) |
| Primiparity, n (%) | 168 (55) | 116 (54) | 1.06 (0.748–1.51) |
| IVF/ICSI, n (%) b | 78 (26) | 52 (24) | 1.09 (0.728–1.64) |
| Education, n (%) b | |||
| ≤High school | 36 (12) | 42 (20) | Ref |
| ≥Junior college c | 267 (88) | 172 (80) | 1.81 (1.16–2.94) |
| Healthcare-related work (including clerical), n (%) b | 90 (30) | 52 (24) | 1.32 (0.884–1.96) |
| Information source during pregnancy, n (%) b † | |||
| Doctor or midwife | 279 (92) | 186(87) | 1.89 (1.06–3.37) |
| Internet/SNS | 160 (53) | 116 (54) | 0.961 (0.677–1.37) |
| Friend | 46 (15) | 47 (22) | 0.642 (0.409–1.01) |
| Prior knowledge of maternal immunization, n (%) | 63 (21) | 23 (11) | 2.19 (1.31–3.66) |
| Prior knowledge of pertussis infection, n (%) b | 254 (84) | 168 (79) | 1.42 (0.908–2.22) |
| History of pertussis infection (self or child), n (%) a | 9 (3.0) | 7 (3.3) | 0.916 (0.336–2.50) |
| Characteristic | Willing (n = 305) | Unwilling (n = 213) | OR (95% CI) |
|---|---|---|---|
| Age (year), median (IQR) a | 33 (30–36) | 33 (30–37) | N/A |
| Gestational age, n (%) a | |||
| <22 weeks | 150 (49) | 108 (51) | Ref |
| ≥22 weeks | 153 (51) | 105 (49) | 1.05 (0.739–1.49) |
| Primiparity, n (%) | 164 (54) | 119 (56) | 0.919 (0.646–1.31) |
| IVF/ICSI, n (%) b | 81 (27) | 49 (23) | 1.22 (0.808–1.83) |
| Education, n (%) b | |||
| ≤High school | 36 (12) | 41 (19) | Ref |
| ≥Junior college c | 269 (88) | 171 (81) | 1.79 (1.11–2.92) |
| Healthcare-related work (including clerical), n (%) b | 95 (31) | 49 (23) | 1.50 (1.01–2.25) |
| Information source during pregnancy, n (%) b † | |||
| Doctor or midwife | 279 (92) | 186 (87) | 1.62 (0.912–2.88) |
| Internet/SNS | 159 (52) | 116 (54) | 0.917 (0.645–1.30) |
| Friend | 49 (16) | 44 (21) | 0.738 (0.470–1.16) |
| Prior knowledge of maternal immunization, n (%) | 64 (21) | 22 (10) | 2.30 (1.37–3.88) |
| Prior knowledge of RSV infection, n (%) | 246 (81) | 150 (70) | 1.75 (1.16–2.64) |
| History of RSV infection (self or child), n (%) a | 72 (24) | 33 (15) | 1.70 (1.08–2.68) |
| Characteristic | Willing (n = 366) | Unwilling (n = 153) | OR (95% CI) |
|---|---|---|---|
| Age (year), median (IQR) a | 33 (30–36) | 34 (29–37) | N/A |
| Gestational age, n (%) a | |||
| <22 weeks | 171 (47) | 88 (58) | Ref |
| ≥22 weeks | 193 (53) | 65 (42) | 1.53 (1.04–2.24) |
| Primiparity, n (%) | 194 (53) | 90 (59) | 0.790 (0.539–1.16) |
| IVF/ICSI, n (%) b | 97 (27) | 33 (22) | 1.32 (0.839–2.06) |
| Education, n (%) b | |||
| ≤High school | 45 (12) | 33 (22) | Ref |
| ≥Junior college c | 321 (88) | 119 (78) | 1.98 (1.20–3.25) |
| Healthcare-related work (including clerical), n (%) b | 114 (31) | 31 (20) | 1.79 (1.14–2.81) |
| Information source during pregnancy, n (%) b † | |||
| Doctor or midwife | 335 (92) | 131(86) | 1.73 (0.961–3.12) |
| Internet/SNS | 199 (54) | 77 (51) | 1.16 (0.795–1.70) |
| Friend | 63 (17) | 30 (20) | 0.846 (0.522–1.37) |
| Prior knowledge of maternal immunization, n (%) | 70 (19) | 15 (10) | 2.18 (1.20–3.94) |
| Prior knowledge of GBS infection, n (%) b | 108 (30) | 25 (16) | 2.13 (1.31–3.45) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiiragi, K.; Obata, S.; Yamamoto, M.; Shimura, M.; Akamatsu, C.; Tochio, A.; Hagiwara, M.; Mochimaru, A.; Kiyose, A.; Tanoshima, M.; et al. Survey on Awareness and Attitudes Toward Maternal Immunization Against Influenza, Pertussis, Respiratory Syncytial Virus, and Group B Streptococcus Among Pregnant Women in Japan. Vaccines 2025, 13, 779. https://doi.org/10.3390/vaccines13080779
Hiiragi K, Obata S, Yamamoto M, Shimura M, Akamatsu C, Tochio A, Hagiwara M, Mochimaru A, Kiyose A, Tanoshima M, et al. Survey on Awareness and Attitudes Toward Maternal Immunization Against Influenza, Pertussis, Respiratory Syncytial Virus, and Group B Streptococcus Among Pregnant Women in Japan. Vaccines. 2025; 13(8):779. https://doi.org/10.3390/vaccines13080779
Chicago/Turabian StyleHiiragi, Kazuya, Soichiro Obata, Masafumi Yamamoto, Mai Shimura, Chika Akamatsu, Azusa Tochio, Mayumi Hagiwara, Aya Mochimaru, Ai Kiyose, Miki Tanoshima, and et al. 2025. "Survey on Awareness and Attitudes Toward Maternal Immunization Against Influenza, Pertussis, Respiratory Syncytial Virus, and Group B Streptococcus Among Pregnant Women in Japan" Vaccines 13, no. 8: 779. https://doi.org/10.3390/vaccines13080779
APA StyleHiiragi, K., Obata, S., Yamamoto, M., Shimura, M., Akamatsu, C., Tochio, A., Hagiwara, M., Mochimaru, A., Kiyose, A., Tanoshima, M., Miyagi, E., & Aoki, S. (2025). Survey on Awareness and Attitudes Toward Maternal Immunization Against Influenza, Pertussis, Respiratory Syncytial Virus, and Group B Streptococcus Among Pregnant Women in Japan. Vaccines, 13(8), 779. https://doi.org/10.3390/vaccines13080779







