Correlates of SARS-CoV-2 Breakthrough Infections in Kidney Transplant Recipients Following a Third SARS-CoV-2 mRNA Vaccine Dose
Abstract
1. Introduction
2. Materials and Methods
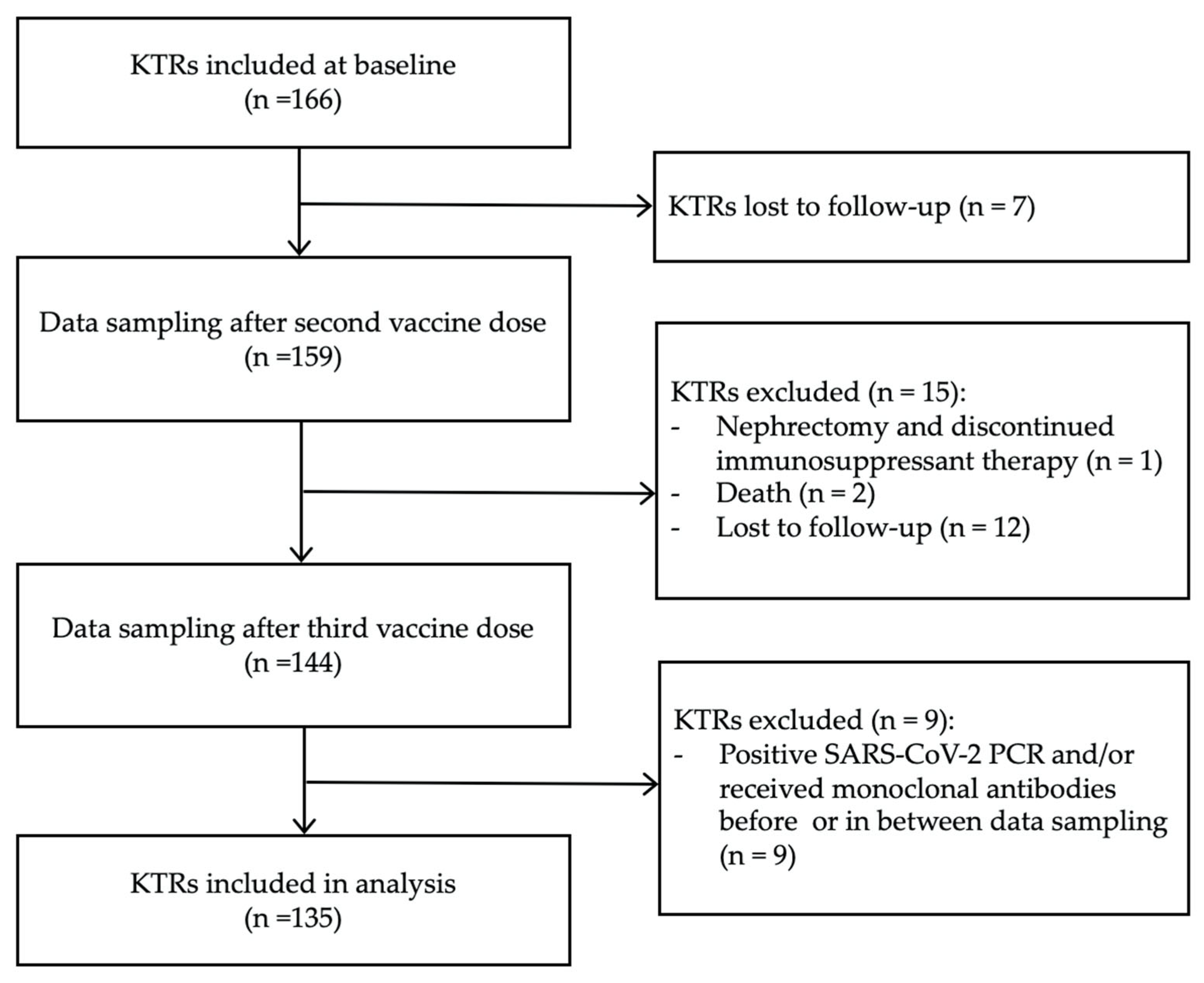
2.1. Participants
2.2. SARS-CoV-2 Antibody Measurement
2.3. Antibody Neutralization
2.4. Statistical Analysis
2.5. Machine Learning Models
2.6. National COVID-19 Infection Rates
3. Results
3.1. Clinical and Demographic Characteristics
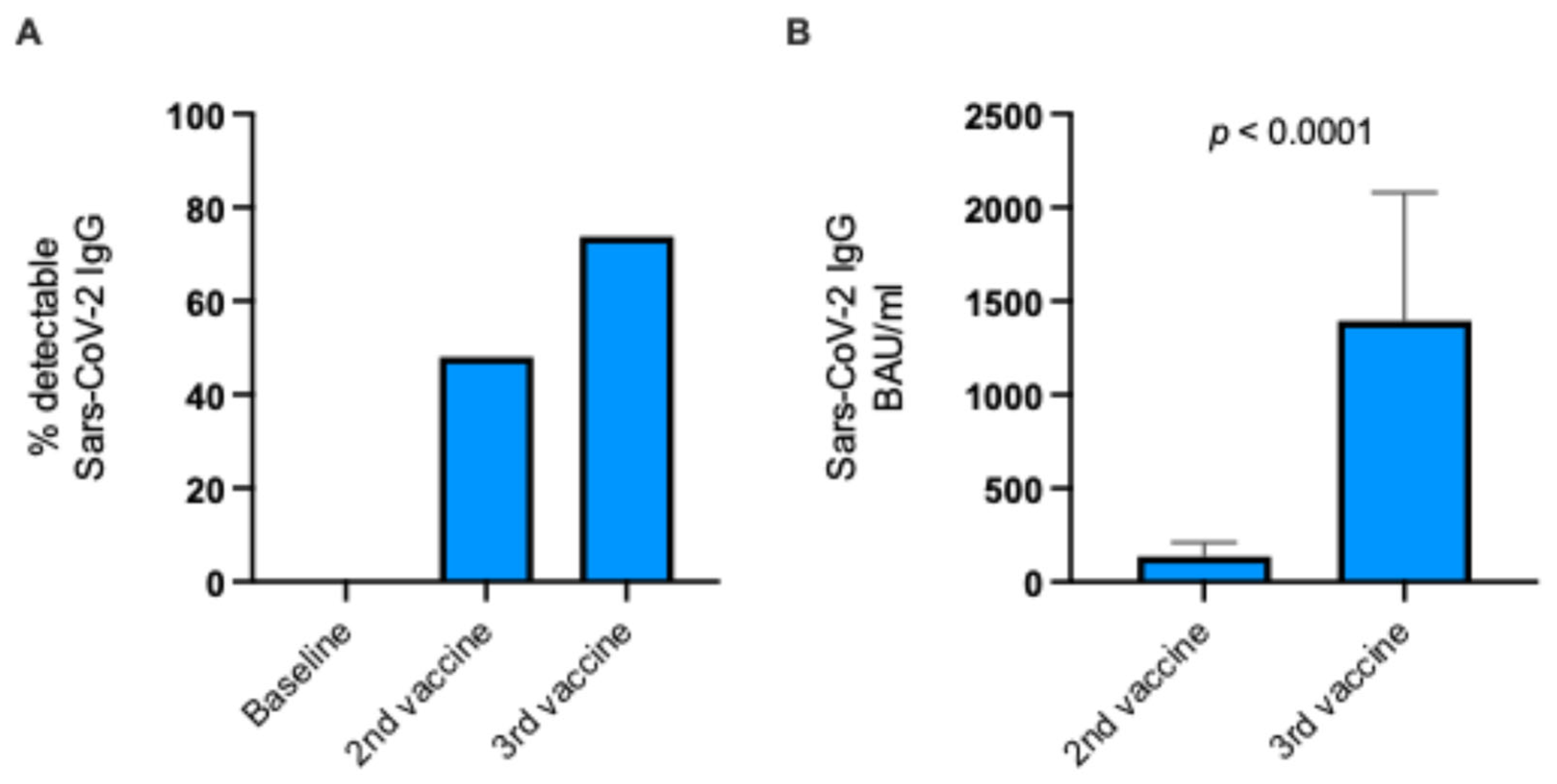
3.2. Vaccine-Induced SARS-CoV-2 Antibody Response
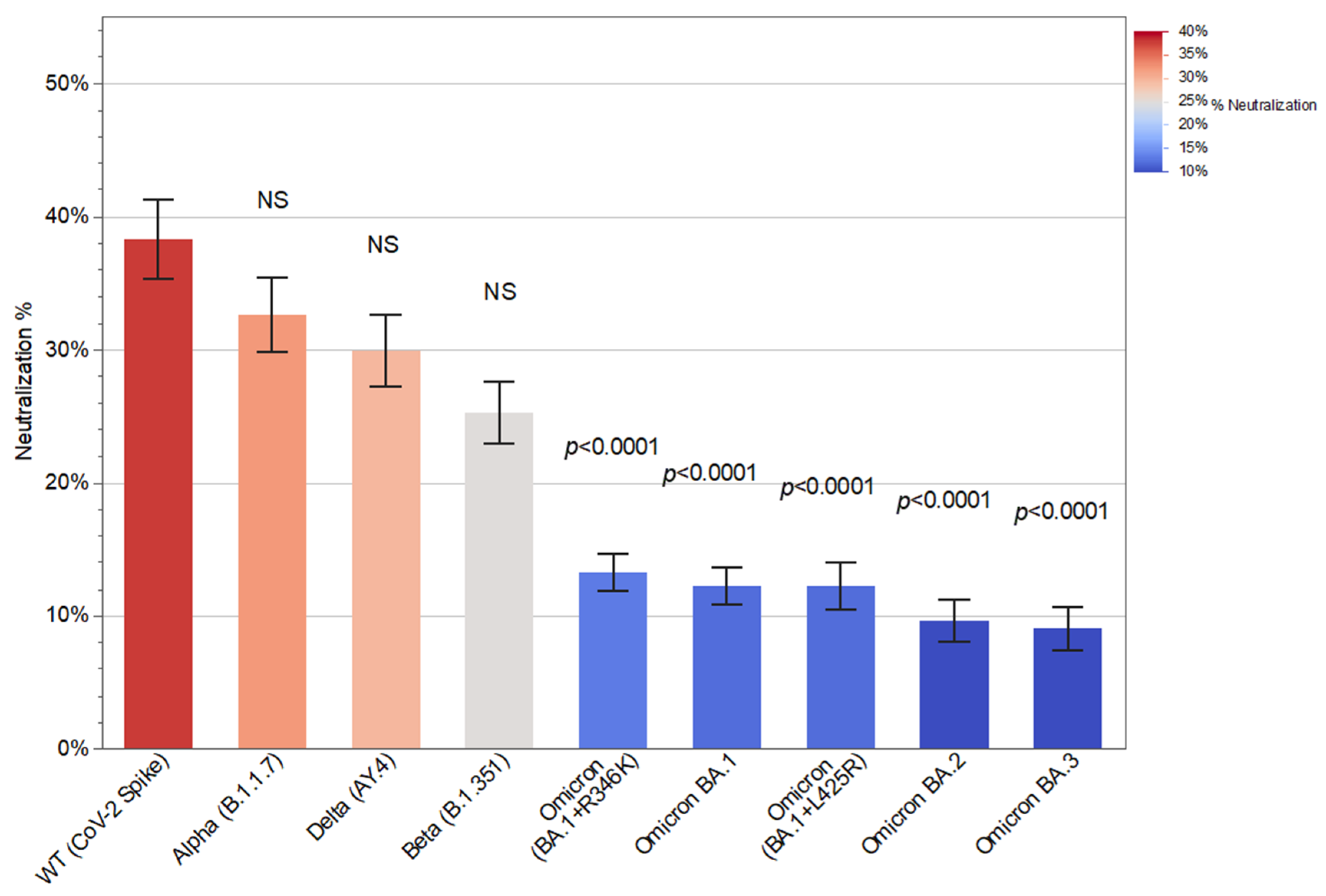
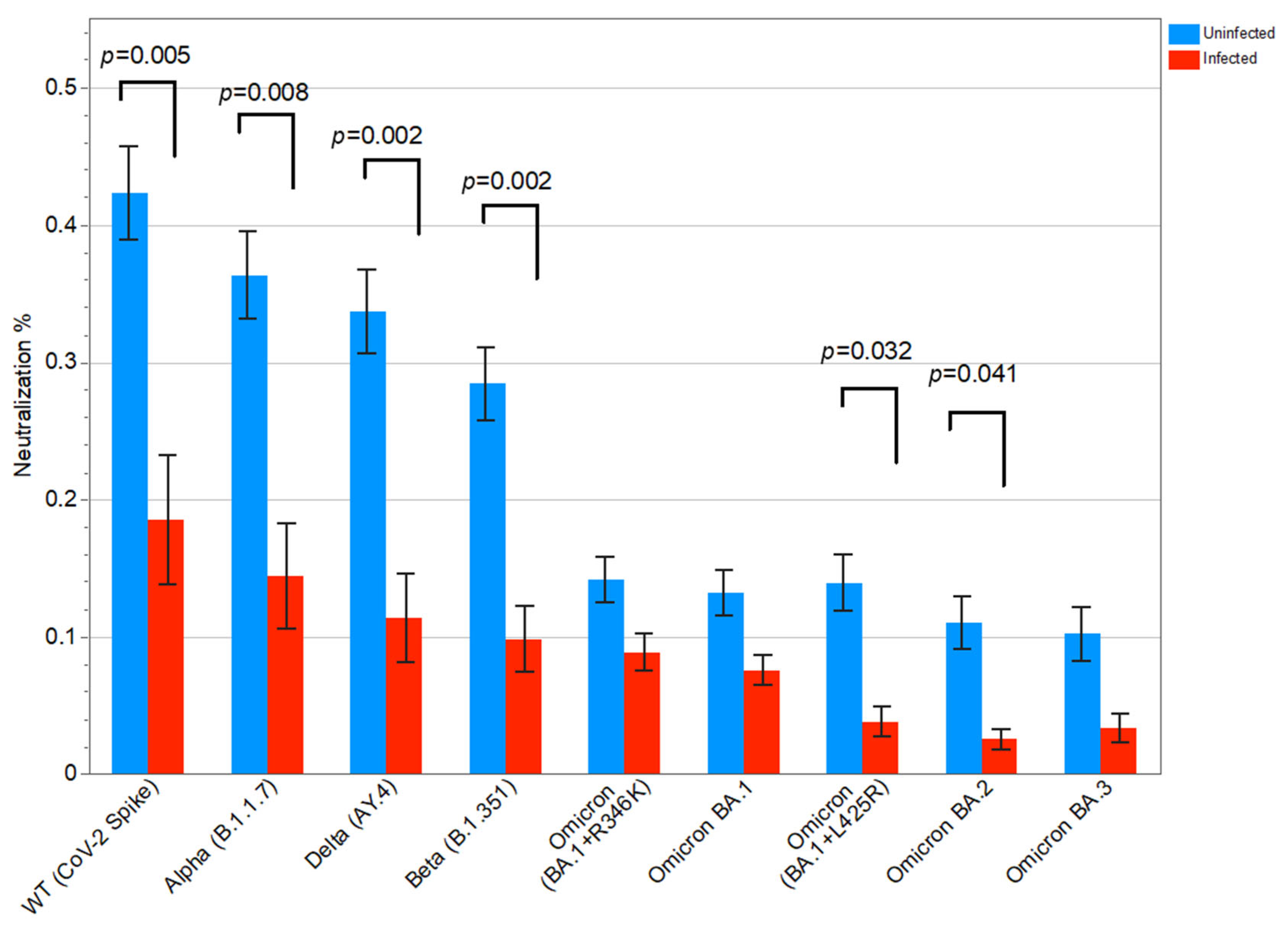
3.3. Neutralizing Antibodies to Different SARS-CoV-2 Variants and Associations with SARS-CoV-2 Breakthrough Infection
3.4. Immunosuppressive Regimen and Risk of SARS-CoV-2 Breakthrough Infection
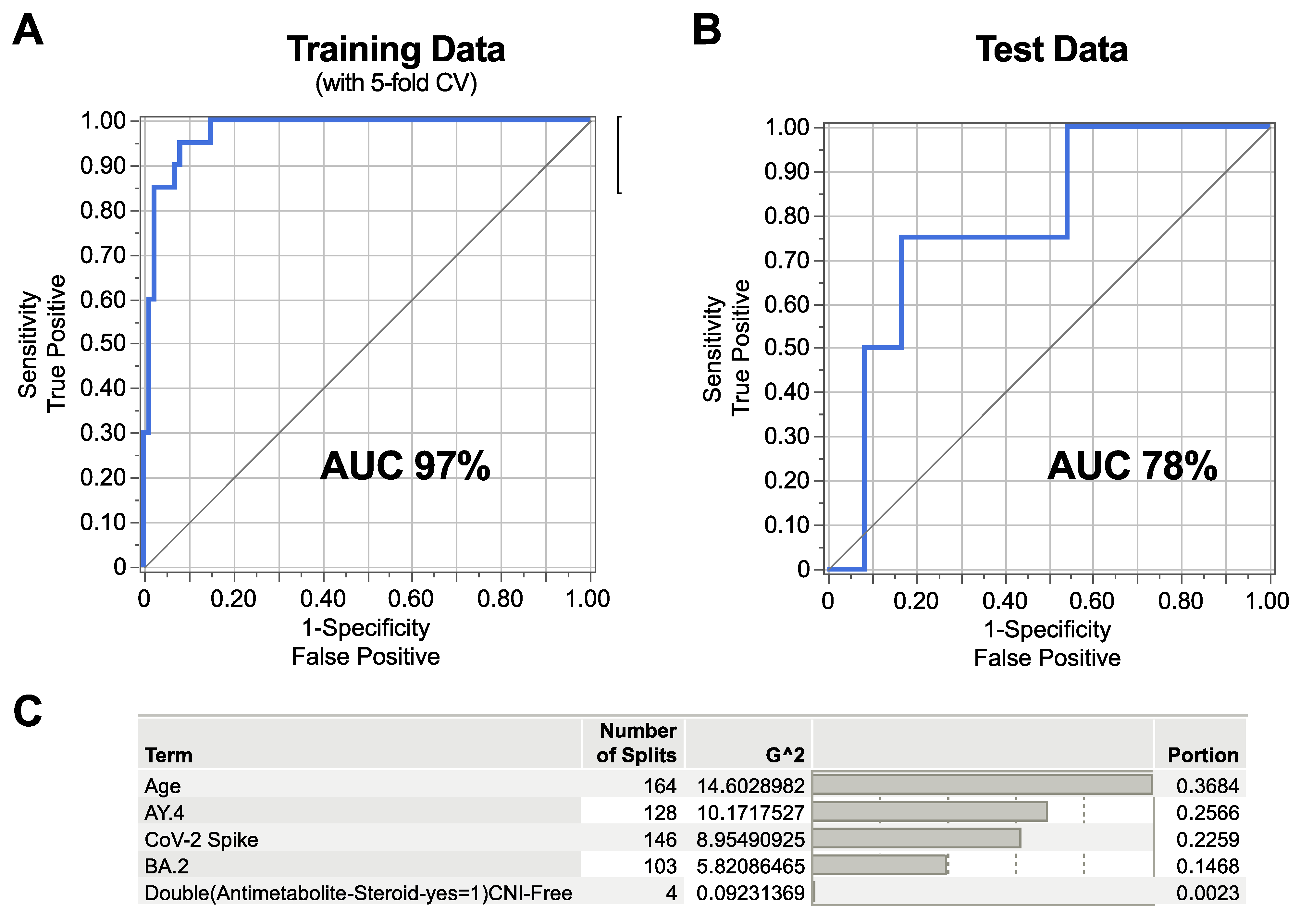
3.5. Machine Learning Model Identifying Correlates of SARS-CoV-2 Breakthrough Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef] [PubMed]
- Akyol, M.; Cevik, E.; Ucku, D.; Tanriover, C.; Afsar, B.; Kanbay, A.; Covic, A.; Ortiz, A.; Basile, C.; Kanbay, M. Immunogenicity of SARS-CoV-2 mRNA vaccine in dialysis and kidney transplant patients: A systematic review. Tuberk Toraks 2021, 69, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D.; Pieters, T.T.; Verhaar, M.C.; Berger, S.P.; Bakker, S.J.L.; van Zuilen, A.D.; Joles, J.A.; Vernooij, R.W.M.; van Balkom, B.W.M. A systematic review and meta-analysis of COVID-19 in kidney transplant recipients: Lessons to be learned. Am. J. Transplant. 2021, 21, 3936–3945. [Google Scholar] [CrossRef] [PubMed]
- Hasan, I.; Rashid, T.; Suliman, S.; Amer, H.; Chirila, R.M.; Mai, M.L.; Jarmi, T.; Khouzam, S.; Franco, P.M.; Heilig, C.W.; et al. Predictors of disease severity and outcome of hospitalized renal transplant recipients with COVID-19 infection: A systematic review of a globally representative sample. Rom. J. Intern. Med. 2021, 59, 10–42. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Hod, T.; Ben-David, A.; Olmer, L.; Levy, I.; Ghinea, R.; Mor, E.; Lustig, Y.; Rahav, G. Humoral Response of Renal Transplant Recipients to the BNT162b2 SARS-CoV-2 mRNA Vaccine Using Both RBD IgG and Neutralizing Antibodies. Transplantation 2021, 105, e234–e243. [Google Scholar] [CrossRef] [PubMed]
- Zong, K.; Peng, D.; Yang, H.; Huang, Z.; Luo, Y.; Wang, Y.; Xiang, S.; Li, T.; Mou, T.; Wu, Z. Risk Factors for Weak Antibody Response of SARS-CoV-2 Vaccine in Adult Solid Organ Transplant Recipients: A Systemic Review and Meta-Analysis. Front. Immunol. 2022, 13, 888385. [Google Scholar] [CrossRef] [PubMed]
- Charmetant, X.; Espi, M.; Barba, T.; Ovize, A.; Morelon, E.; Mathieu, C.; Thaunat, O. Predictive factors of a viral neutralizing humoral response after a third dose of COVID-19 mRNA vaccine. Am. J. Transplant. 2022, 22, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Staerke, N.B.; Reekie, J.; Nielsen, H.; Benfield, T.; Wiese, L.; Knudsen, L.S.; Iversen, M.B.; Iversen, K.; Fogh, K.; Bodilsen, J.; et al. Levels of SARS-CoV-2 antibodies among fully vaccinated individuals with Delta or Omicron variant breakthrough infections. Nat. Commun. 2022, 13, 4466. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, F.; Blocki, F.A.; Bunnell, T.; Chu, E.; De La, O.A.; Grenache, D.G.; Marzucchi, G.; Montomoli, E.; Okoye, L.; Pallavicini, L.; et al. Evaluation of the automated LIAISON((R)) SARS-CoV-2 TrimericS IgG assay for the detection of circulating antibodies. Clin. Chem. Lab. Med. 2021, 59, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Decru, B.; Van Elslande, J.; Steels, S.; Van Pottelbergh, G.; Godderis, L.; Van Holm, B.; Bossuyt, X.; Van Weyenbergh, J.; Maes, P.; Vermeersch, P. IgG Anti-Spike Antibodies and Surrogate Neutralizing Antibody Levels Decline Faster 3 to 10 Months After BNT162b2 Vaccination Than After SARS-CoV-2 Infection in Healthcare Workers. Front. Immunol. 2022, 13, 909910. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and Variable Selection Via the Elastic Net. J. R. Stat. Soc. Ser. B Stat. Methodol. 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Statens Serum Institut. Interactive National Dashboards Showing Total Numbers of COVID-19 Infections in Denmark Over Time. Available online: https://experience.arcgis.com/experience/220fef27d07d438889d651cc2e00076c (accessed on 15 June 2024).
- Statens Serum Institut. Interactive National Dashboards Showing Proportions of SARS-CoV-2 Variants of Concern (VOC) Dominating in Denmark Over Time. Available online: https://covid19genomics.dk/statistics (accessed on 15 June 2024).
- Grupper, A.; Rabinowich, L.; Ben-Yehoyada, M.; Katchman, E.; Baruch, R.; Freund, T.; Hagin, D.; Shlomo, S.B.; Schwartz, D.; Schwartz, I.F.; et al. Humoral Response to the Third Dose of Sars-CoV-2 Vaccine in Kidney Transplant Recipients. Transplant. Proc. 2022, 54, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Abedon, A.T.; Alejo, J.L.; Kim, J.D.; Thomas, L.; Mitchell, J.; Chiang, T.P.Y.; Avery, R.K.; Tobian, A.A.R.; Levan, M.L.; Warren, D.S.; et al. Six-month Antibody Kinetics and Durability After 3 Doses of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Transplantation 2022, 106, e281–e283. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Abravanel, F.; Marion, O.; Esposito, L.; Hebral, A.L.; Medrano, C.; Guitard, J.; Lavayssiere, L.; Cointault, O.; Nogier, M.B.; et al. Anti-SARS-CoV-2 spike protein and neutralizing antibodies at 1 and 3 months after three doses of SARS-CoV-2 vaccine in a large cohort of solid organ transplant patients. Am. J. Transplant. 2022, 22, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Abravanel, F.; Marion, O.; Couat, C.; Izopet, J.; Del Bello, A. Three Doses of an mRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2021, 385, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Karaba, A.H.; Zhu, X.; Liang, T.; Wang, K.H.; Rittenhouse, A.G.; Akinde, O.; Eby, Y.; Ruff, J.E.; Blankson, J.N.; Abedon, A.T.; et al. A third dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. Am. J. Transplant. 2022, 22, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ayada, I.; Wang, Y.; den Hoed, C.M.; Kamar, N.; Peppelenbosch, M.P.; de Vries, A.C.; Li, P.; Pan, Q. Factors Associated With COVID-19 Vaccine Response in Transplant Recipients: A Systematic Review and Meta-analysis. Transplantation 2022, 106, 2068–2075. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S. Efficacy and safety of booster vaccination against SARS-CoV-2 in dialysis and renal transplant patients: Systematic review and meta-analysis. Int. Urol. Nephrol. 2023, 55, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Efros, O.; Anteby, R.; Halfon, M.; Meisel, E.; Klang, E.; Soffer, S. Efficacy and Safety of Third Dose of the COVID-19 Vaccine among Solid Organ Transplant Recipients: A Systemic Review and Meta-Analysis. Vaccines 2022, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Benning, L.; Morath, C.; Bartenschlager, M.; Kim, H.; Reineke, M.; Beimler, J.; Buylaert, M.; Nusshag, C.; Kalble, F.; Reichel, P.; et al. Neutralizing antibody response against the B.1.617.2 (delta) and the B.1.1.529 (omicron) variants after a third mRNA SARS-CoV-2 vaccine dose in kidney transplant recipients. Am. J. Transplant. 2022, 22, 1873–1883. [Google Scholar] [CrossRef] [PubMed]
- Tharmaraj, D.; Boo, I.; O’Hara, J.; Sun, S.; Polkinghorne, K.R.; Dendle, C.; Turner, S.J.; van Zelm, M.C.; Drummer, H.E.; Khoury, G.; et al. Serological responses and clinical outcomes following a three-dose primary COVID-19 vaccine schedule in kidney transplant recipients and people on dialysis. Clin. Transl. Immunol. 2024, 13, e1523. [Google Scholar] [CrossRef] [PubMed]
- Al Jurdi, A.; Gassen, R.B.; Borges, T.J.; Lape, I.T.; Morena, L.; Efe, O.; Solhjou, Z.; El Fekih, R.; Deban, C.; Bohan, B.; et al. Suboptimal antibody response against SARS-CoV-2 Omicron variant after third dose of mRNA vaccine in kidney transplant recipients. Kidney Int. 2022, 101, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Panizo, N.; Gimenez, E.; Albert, E.; Zulaica, J.; Rodriguez-Moreno, A.; Rusu, L.; Gimenez-Civera, E.; Puchades, M.J.; D’Marco, L.; Gandia-Salmeron, L.; et al. SARS-CoV-2-Spike Antibody and T-Cell Responses Elicited by a Homologous Third mRNA COVID-19 Dose in Hemodialysis and Kidney Transplant Recipients. Microorganisms 2022, 10, 2275. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.M.; Hu, Q.; Abe, K.T.; Yau, K.; Oliver, M.J.; Levin, A.; Gingras, A.C.; Hladunewich, M.A.; Yuen, D.A. Humoral Responses in the Omicron Era Following 3-Dose SARS-CoV-2 Vaccine Series in Kidney Transplant Recipients. Transplant. Direct 2023, 9, e1401. [Google Scholar] [CrossRef] [PubMed]
- Kemlin, D.; Gemander, N.; Depickere, S.; Olislagers, V.; Georges, D.; Waegemans, A.; Pannus, P.; Lemy, A.; Goossens, M.E.; Desombere, I.; et al. Humoral and cellular immune correlates of protection against COVID-19 in kidney transplant recipients. Am. J. Transplant. 2023, 23, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Dimeglio, C.; Migueres, M.; Bouzid, N.; Chapuy-Regaud, S.; Gernigon, C.; Da-Silva, I.; Porcheron, M.; Martin-Blondel, G.; Herin, F.; Izopet, J. Antibody Titers and Protection against Omicron (BA.1 and BA.2) SARS-CoV-2 Infection. Vaccines 2022, 10, 1548. [Google Scholar] [CrossRef] [PubMed]
- Hovd, M.; Asberg, A.; Munthe, L.A.; Heldal, K.; Reisaeter, A.V.; Vaage, J.T.; Lund-Johansen, F.; Midtvedt, K. Humoral vaccine response and breakthrough infections in kidney transplant recipients during the COVID-19 pandemic: A nationwide cohort study. EClinicalMedicine 2023, 60, 102035. [Google Scholar] [CrossRef] [PubMed]
- Cassaniti, I.; Gregorini, M.; Bergami, F.; Arena, F.; Sammartino, J.C.; Percivalle, E.; Soleymaninejadian, E.; Abelli, M.; Ticozzelli, E.; Nocco, A.; et al. Effect of a Third Dose of SARS-CoV-2 mRNA BNT162b2 Vaccine on Humoral and Cellular Responses and Serum Anti-HLA Antibodies in Kidney Transplant Recipients. Vaccines 2022, 10, 921. [Google Scholar] [CrossRef] [PubMed]
- Elmahdi, R.; Ward, D.; Ernst, M.T.; Poulsen, G.; Hallas, J.; Pottegard, A.; Jess, T. Impact of immunosuppressive therapy on SARS-CoV-2 mRNA vaccine effectiveness in patients with immune-mediated inflammatory diseases: A Danish nationwide cohort study. BMJ Open 2024, 14, e077408. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | All KTRs (n = 135) | Seropositive After Third Vaccine Dose (n = 100) | Seronegative After Third Vaccine Dose (n = 35) |
|---|---|---|---|
| Female, n (%) | 55 (41%) | 38 (38%) | 17 (49,6%) |
| Age, median years (IQR) | 55 (47–67) | 54 (46–67) | 61 (51–68) |
| Vaccine type (%) | |||
| 3 × BNT162b2 mRNA | 131 (97%) | 98 (98%) | 33 (94.3%) |
| 3 × mRNA1273 | 4 (3%) | 2 (2%) | 2 (5.7%) |
| Time between transplantation and first vaccine dose, years (IQR) | 6.29 (2.85–11.98) | 7.64 (3.97–13.18) | 3.59 (0.71–6.54) |
| First transplant, n (%) | 112 (83%) | 81 (81%) | 31 (88.6%) |
| Type of immunosuppressive regimen, n (%) | |||
| Triple therapy (CNI, antimetabolite, steroids) | 64 (47%) | 38 (38%) | 26 (74%) |
| Steroid-free dual therapy * | 40 (29.6%) | 33 (33%) | 7 (20%) |
| Antimetabolite-free dual therapy ** | 14 (10.4%) | 13 (13%) | 1 (2.9%) |
| CNI-free dual therapy *** | 5 (3.7%) | 4 (4%) | 1 (2.9%) |
| Rituximab < 1 year prior to first vaccine dose | 6 (4.4%) | 2 (2%) | 4 (11.4%) |
| Immunosuppressive Regimen | All KTRs n (%) | Risk of SARS-CoV-2 Breakthrough Infection, OR | Lower 95% | Upper 95% | Fisher’s Exact Test |
|---|---|---|---|---|---|
| Triple therapy (CNI + antimetabolite + steroid) | 64 (47.4%) | 2.63 | 1.04 | 6.64 | 0.044 |
| Steroid-free dual therapy | 40 (29.6%) | 0.57 | 0.20 | 1.65 | 0.34 |
| Antimetabolite-free dual therapy | 14 (10.4%) | 0.75 | 0.16 | 3.59 | 1.00 |
| CNI-free dual therapy | 5 (3.7%) | 0.00 | N/A | N/A | 0.59 |
| Rituximab < 1 year prior to vaccination | 6 (4.4%) | 0.92 | 0.10 | 8.27 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thygesen, M.V.; Strandhave, C.; Kiib, J.M.; Berg, R.; Andersen, M.S.; Dall, E.B.; Hornstrup, B.G.; Østergaard, H.C.; Mose, F.H.; Gregersen, J.W.; et al. Correlates of SARS-CoV-2 Breakthrough Infections in Kidney Transplant Recipients Following a Third SARS-CoV-2 mRNA Vaccine Dose. Vaccines 2025, 13, 777. https://doi.org/10.3390/vaccines13080777
Thygesen MV, Strandhave C, Kiib JM, Berg R, Andersen MS, Dall EB, Hornstrup BG, Østergaard HC, Mose FH, Gregersen JW, et al. Correlates of SARS-CoV-2 Breakthrough Infections in Kidney Transplant Recipients Following a Third SARS-CoV-2 mRNA Vaccine Dose. Vaccines. 2025; 13(8):777. https://doi.org/10.3390/vaccines13080777
Chicago/Turabian StyleThygesen, Miriam Viktov, Charlotte Strandhave, Jeanette Mølgaard Kiib, Randi Berg, Malene Söth Andersen, Emma Berggren Dall, Bodil Gade Hornstrup, Hans Christian Østergaard, Frank Holden Mose, Jon Waarst Gregersen, and et al. 2025. "Correlates of SARS-CoV-2 Breakthrough Infections in Kidney Transplant Recipients Following a Third SARS-CoV-2 mRNA Vaccine Dose" Vaccines 13, no. 8: 777. https://doi.org/10.3390/vaccines13080777
APA StyleThygesen, M. V., Strandhave, C., Kiib, J. M., Berg, R., Andersen, M. S., Dall, E. B., Hornstrup, B. G., Østergaard, H. C., Mose, F. H., Gregersen, J. W., Jensen-Fangel, S., Bech, J. N., Birn, H., Thomsen, M. K., & Offersen, R. (2025). Correlates of SARS-CoV-2 Breakthrough Infections in Kidney Transplant Recipients Following a Third SARS-CoV-2 mRNA Vaccine Dose. Vaccines, 13(8), 777. https://doi.org/10.3390/vaccines13080777





