Year-Long Antibody Response to the EuCorVac-19 SARS-CoV-2 Vaccine in Healthy Filipinos
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analyses
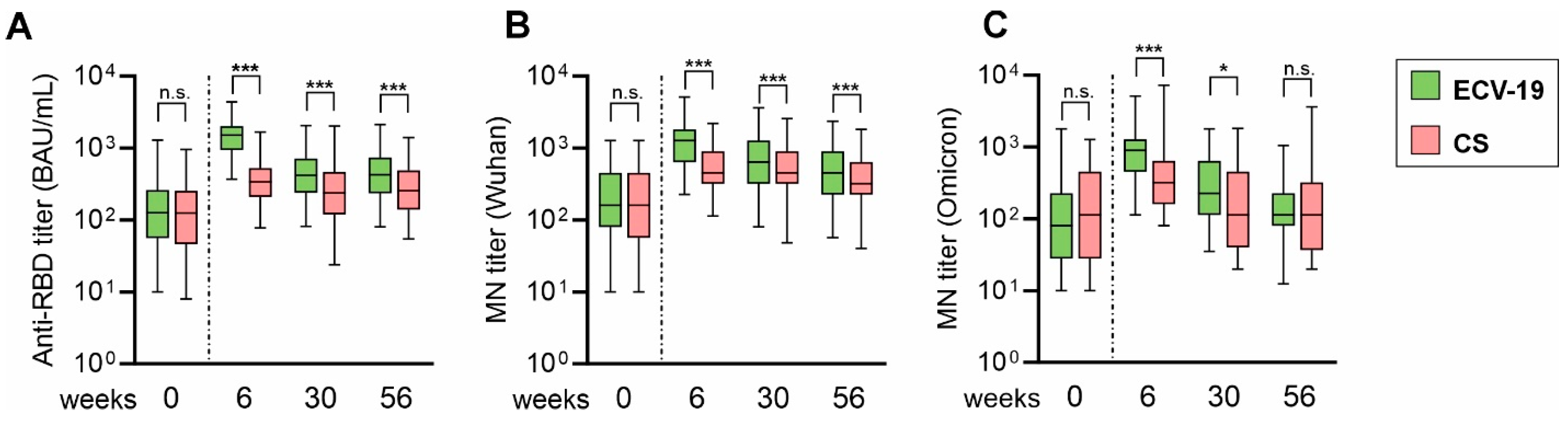
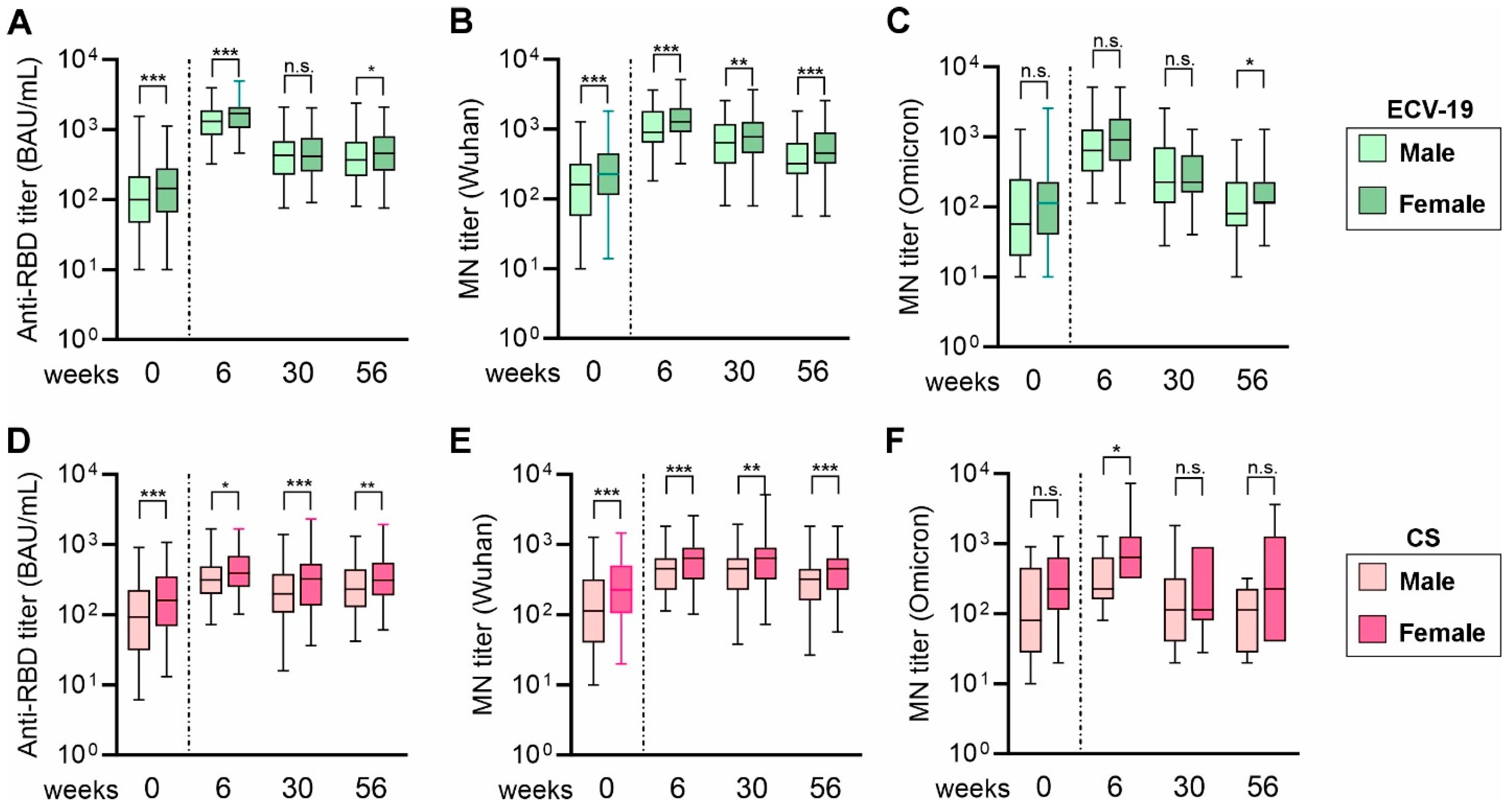
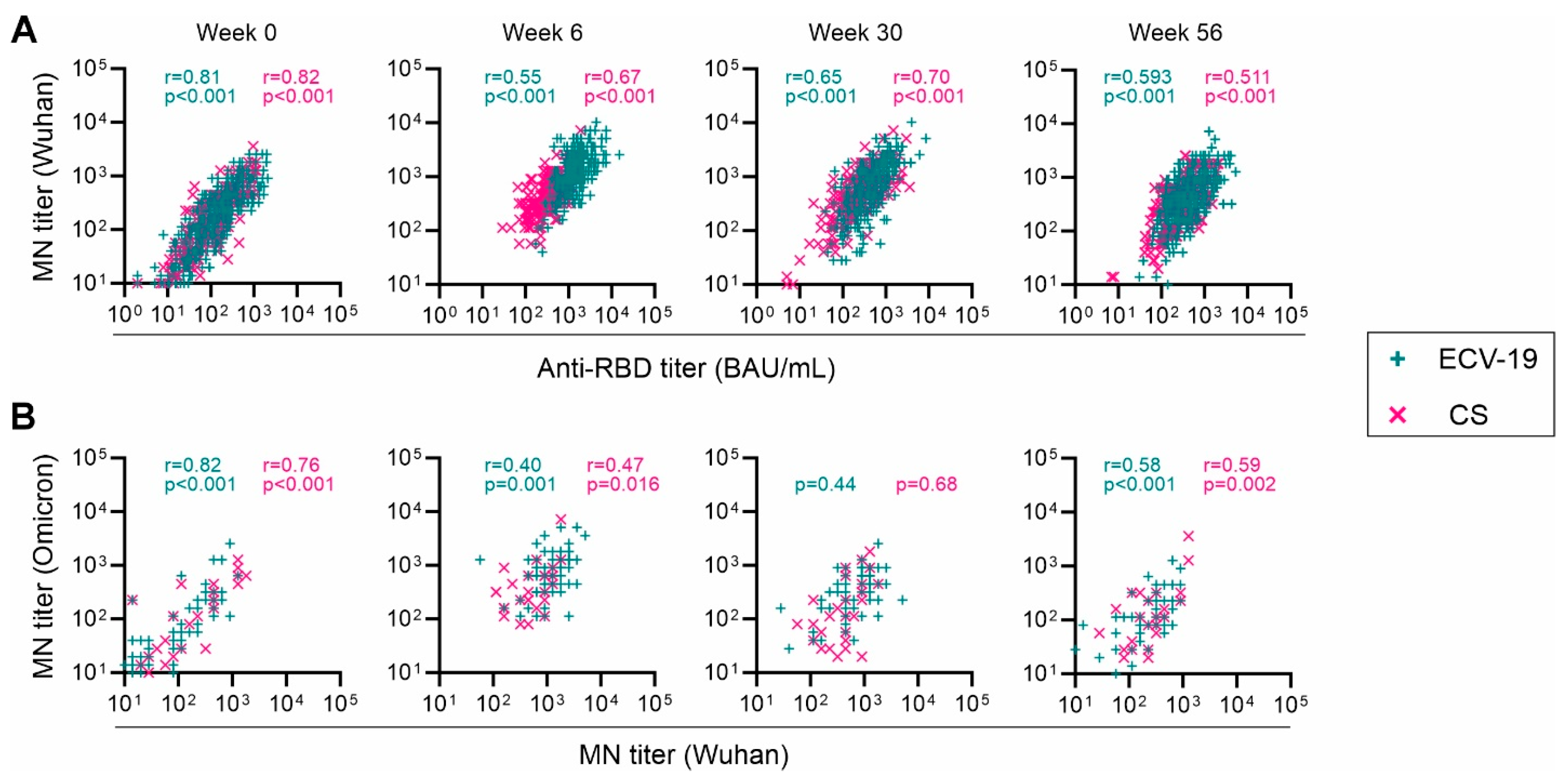
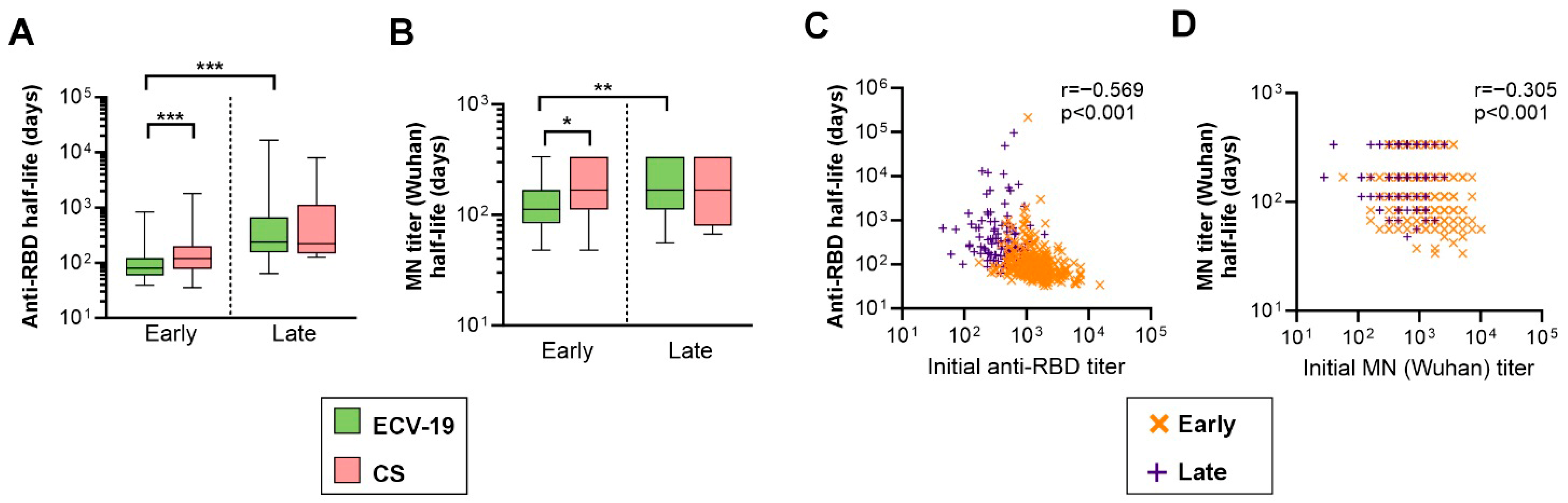
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse Event |
| AESI | Adverse Event of Special Interest |
| BAU/mL | Binding Antibody Units per milliliter |
| CPE | Cytopathic Effect |
| CS | COVISHIELDTM |
| CoPoP | Cobalt-Porphyrin Phospholipid |
| COVID-19 | Coronavirus Disease 2019 |
| EcML | E. coli-produced Monophosphoryl Lipid A |
| ECV-19 | EuCorVac-19 |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FY | Full Year |
| GCLP | Good Clinical Laboratory Practice |
| ICH | International Council for Harmonisation |
| IR | Interim Report |
| IRB | Institutional Review Board |
| ISO | International Organization for Standardization |
| IP | Immunogenicity Population (inferred) |
| MAAE | Medically Attended Adverse Event |
| MN | Microneutralization |
| NIBSC | National Institute for Biological Standards and Control |
| RBD | Receptor-Binding Domain |
| SAE | Serious Adverse Event |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| V1/V2 | Visit 1/Visit 2 |
| WHO | World Health Organization |
| Wu-MN | Wuhan Microneutralization |
References
- Vashishtha, V.M.; Kumar, P. The durability of vaccine-induced protection: An overview. Exp. Rev. Vaccines 2024, 23, 389–408. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Cancemi, P.; Mukhtar, F.; Marino, S.; Peri, E.; Di Prima, G.; De Caro, V. Efficacy and limitations of SARS-CoV-2 vaccines—A systematic review. Life Sci. 2025, 371, 123610. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Sasaki, A.; Kadowaki, T.; Mitsuhashi, T.; Takao, S.; Yorifuji, T. Longitudinal antibody dynamics after COVID-19 vaccine boosters based on prior infection status and booster doses. Sci. Rep. 2024, 14, 4564. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Hyun, H.; Heo, J.Y.; Seo, Y.B.; Noh, J.Y.; Cheong, H.J.; Kim, W.J.; Kim, H.J.; Choi, J.-Y.; Lee, Y.J.; et al. Longitudinal immune kinetics of COVID-19 booster versus primary series vaccination: Insight into the annual vaccination strategy. Heliyon 2024, 10, e27211. [Google Scholar] [CrossRef]
- Liu, H.-H.; Xie, Y.; Yang, B.-P.; Wen, H.-Y.; Yang, P.-H.; Lu, J.-E.; Liu, Y.; Chen, X.; Qu, M.-M.; Zhang, Y.; et al. Safety, immunogenicity and protective effect of sequential vaccination with inactivated and recombinant protein COVID-19 vaccine in the elderly: A prospective longitudinal study. Sig. Transduct. Target. Ther. 2024, 9, 129. [Google Scholar] [CrossRef]
- Gonzales, M.; Dans, L.F.; Tan-Lim, C.S.C.; Uy, E.; Cutiongco-Dela Paz, E.; Sulit, M.V.V.; Alejandria, M.M.; Lansang, M.A.D.; Dans, A.L.; Dator, M.A.; et al. Durability and extent of protection of SARS-CoV-2 antibodies among patients with COVID-19 in Metro Manila, Philippines. Front. Immunol. 2023, 14, 1190093. [Google Scholar] [CrossRef]
- Malijan, G.M.B.; Edwards, T.; Agrupis, K.A.; Suzuki, S.; Villanueva, A.M.G.; Sayo, A.R.; De Guzman, F.; Dimapilis, A.Q.; Solante, R.M.; Telan, E.O.; et al. SARS-CoV-2 seroprevalence and infection rate in Manila, Philippines prior to national vaccination program implementation: A repeated cross-sectional analysis. Trop. Med. Health 2022, 50, 75. [Google Scholar] [CrossRef]
- Aziz, A.B.; Sugimoto, J.D.; Hong, S.L.; You, Y.A.; Bravo, L.; Roa, C.; Borja-Tabora, C.; Montellano, M.E.B.; Carlos, J.; de Los Reyes, M.R.A.; et al. Indirect effectiveness of a novel SARS-COV-2 vaccine (SCB-2019) in unvaccinated household contacts in the Philippines: A cluster randomised analysis. J. Infect. 2024, 89, 106260. [Google Scholar] [CrossRef]
- Nagy, A.; Alhatlani, B. An overview of current COVID-19 vaccine platforms. Comp. Struct. Biotechnol. J. 2021, 19, 2508–2517. [Google Scholar] [CrossRef]
- Huang, W.-C.; Zhou, S.; He, X.; Chiem, K.; Mabrouk, M.T.; Nissly, R.H.; Bird, I.M.; Strauss, M.; Sambhara, S.; Ortega, J.; et al. SARS-CoV-2 RBD Neutralizing Antibody Induction is Enhanced by Particulate Vaccination. Adv. Mater. 2020, 32, 2005637. [Google Scholar] [CrossRef]
- Lovell, J.F.; Baik, Y.O.; Choi, S.K.; Lee, C.; Lee, J.-Y.; Miura, K.; Huang, W.-C.; Park, Y.-S.; Woo, S.-J.; Seo, S.H.; et al. Interim analysis from a phase 2 randomized trial of EuCorVac-19: A recombinant protein SARS-CoV-2 RBD nanoliposome vaccine. BMC Med. 2022, 20, 462. [Google Scholar] [CrossRef]
- Shao, S.; Geng, J.; Ah Yi, H.; Gogia, S.; Neelamegham, S.; Jacobs, A.; Lovell, J.F. Functionalization of cobalt porphyrin–phospholipid bilayers with his-tagged ligands and antigens. Nat. Chem. 2015, 7, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.F.; Miura, K.; Baik, Y.O.; Lee, C.; Choi, Y.; Her, H.; Lee, J.Y.; Ylade, M.; Lee-Llacer, R.; De Asis, N.; et al. Interim safety and immunogenicity analysis of the EuCorVac-19 COVID-19 vaccine in a Phase 3 randomized, observer-blind, immunobridging trial in the Philippines. J. Med. Virol. 2024, 96, e29927. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Mondal, M.; Zhou, D. Development of novel vaccine vectors: Chimpanzee adenoviral vectors. Hum. Vaccines Immunother. 2018, 14, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.F.; Miura, K.; Baik, Y.O.; Lee, C.; Lee, J.Y.; Park, Y.S.; Hong, I.; Lee, J.H.; Kim, T.; Seo, S.H.; et al. One-year antibody durability induced by EuCorVac-19, a liposome-displayed COVID-19 receptor binding domain subunit vaccine, in healthy Korean subjects. Int. J. Infect. Dis. 2024, 138, 73–80. [Google Scholar] [CrossRef]
- Cromer, D.; Juno, J.A.; Khoury, D.; Reynaldi, A.; Wheatley, A.K.; Kent, S.J.; Davenport, M.P. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat. Rev. Immunol. 2021, 21, 395–404. [Google Scholar] [CrossRef]
- Marchevsky, N.G.; Li, G.; Aley, P.; Costa Clemens, S.A.; Barrett, J.R.; Belij-Rammerstorfer, S.; Bibi, S.; Clutterbuck, E.; Dold, C.; Felle, S.; et al. An exploratory analysis of the response to ChAdOx1 nCoV-19 (AZD1222) vaccine in males and females. eBioMedicine 2022, 81, 104128. [Google Scholar] [CrossRef]
- Demonbreun, A.R.; Sancilio, A.; Velez, M.E.; Ryan, D.T.; Pesce, L.; Saber, R.; Vaught, L.A.; Reiser, N.L.; Hsieh, R.R.; D’Aquila, R.T.; et al. COVID-19 mRNA Vaccination Generates Greater Immunoglobulin G Levels in Women Compared to Men. J. Infect. Dis. 2021, 224, 793–797. [Google Scholar] [CrossRef]
- Fischinger, S.; Boudreau, C.M.; Butler, A.L.; Streeck, H.; Alter, G. Sex differences in vaccine-induced humoral immunity. Semin. Immunopathol. 2019, 41, 239–249. [Google Scholar] [CrossRef]
- Goldblatt, D.; Alter, G.; Crotty, S.; Plotkin, S.A. Correlates of protection against SARS-CoV-2 infection and COVID-19 disease. Immunol. Rev. 2022, 310, 6–26. [Google Scholar] [CrossRef]
- Barosa, M.; Ioannidis, J.P.A.; Prasad, V. Evidence base for yearly respiratory virus vaccines: Current status and proposed improved strategies. Eur. J. Clin. Investig. 2024, 54, e14286. [Google Scholar] [CrossRef]
- Focosi, D. From Co-Administration to Co-Formulation: The Race for New Vaccines against COVID-19 and Other Respiratory Viruses. Vaccines 2023, 11, 109. [Google Scholar] [CrossRef]
| ECV-19 | CS | |||||
|---|---|---|---|---|---|---|
| IR Set a | FY Set b | p-Value c | IR Set a | FY Set b | p-Value c | |
| Sex; Male/Female | 275/310 | 252/278 | 0.90 | 169/121 | 152/106 | 0.88 |
| Age; Median (range) | 30 (18–69) | 31 (18–69) | 0.85 | 31 (18–66) | 31 (18–66) | 0.91 |
| Anti-RBD titer; Median (IQR d) | 1509 (942–2017) | 1512 (936–2024) | 0.87 | 340 (214–525) | 339 (210–525) | 0.94 |
| MN titer (Wuhan) e; Median (IQR d) | 1280 (640–1810) | 1280 (640–1810) | 0.74 | 453 (320–905) | 453 (320–905) | 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lovell, J.F.; Miura, K.; Baik, Y.O.; Lee, C.; Choi, Y.; Lee, J.-Y.; Long, C.A.; Ylade, M.; Lee-Llacer, R.; De Asis, N.; et al. Year-Long Antibody Response to the EuCorVac-19 SARS-CoV-2 Vaccine in Healthy Filipinos. Vaccines 2025, 13, 776. https://doi.org/10.3390/vaccines13080776
Lovell JF, Miura K, Baik YO, Lee C, Choi Y, Lee J-Y, Long CA, Ylade M, Lee-Llacer R, De Asis N, et al. Year-Long Antibody Response to the EuCorVac-19 SARS-CoV-2 Vaccine in Healthy Filipinos. Vaccines. 2025; 13(8):776. https://doi.org/10.3390/vaccines13080776
Chicago/Turabian StyleLovell, Jonathan F., Kazutoyo Miura, Yeong Ok Baik, Chankyu Lee, YoungJin Choi, Jeong-Yoon Lee, Carole A. Long, Michelle Ylade, Roxas Lee-Llacer, Norman De Asis, and et al. 2025. "Year-Long Antibody Response to the EuCorVac-19 SARS-CoV-2 Vaccine in Healthy Filipinos" Vaccines 13, no. 8: 776. https://doi.org/10.3390/vaccines13080776
APA StyleLovell, J. F., Miura, K., Baik, Y. O., Lee, C., Choi, Y., Lee, J.-Y., Long, C. A., Ylade, M., Lee-Llacer, R., De Asis, N., Trinidad-Aseron, M., Manuel Ranola, J., Zoleta De Jesus, L., & Her, H. (2025). Year-Long Antibody Response to the EuCorVac-19 SARS-CoV-2 Vaccine in Healthy Filipinos. Vaccines, 13(8), 776. https://doi.org/10.3390/vaccines13080776






