Circulating Antibody’s Role During Post-Exposure Prophylaxis, and Beyond for Rabies: A Review
Abstract
1. Introduction
2. Search Strategy and Selection Criteria
3. Review
3.1. Epidemiology and Global Disease Burden
3.2. Pathogenesis
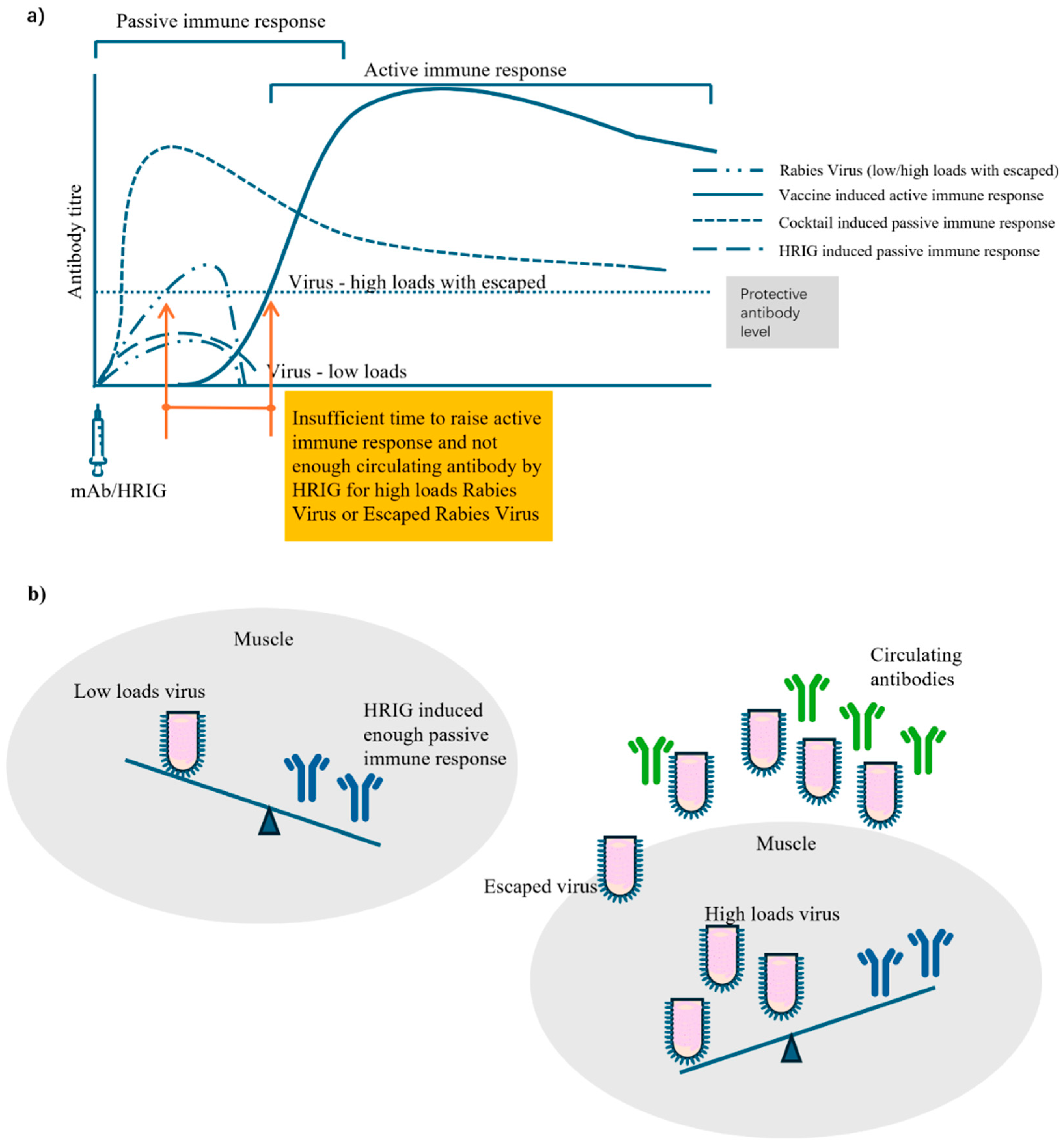
3.3. Passive Immunity in PEP
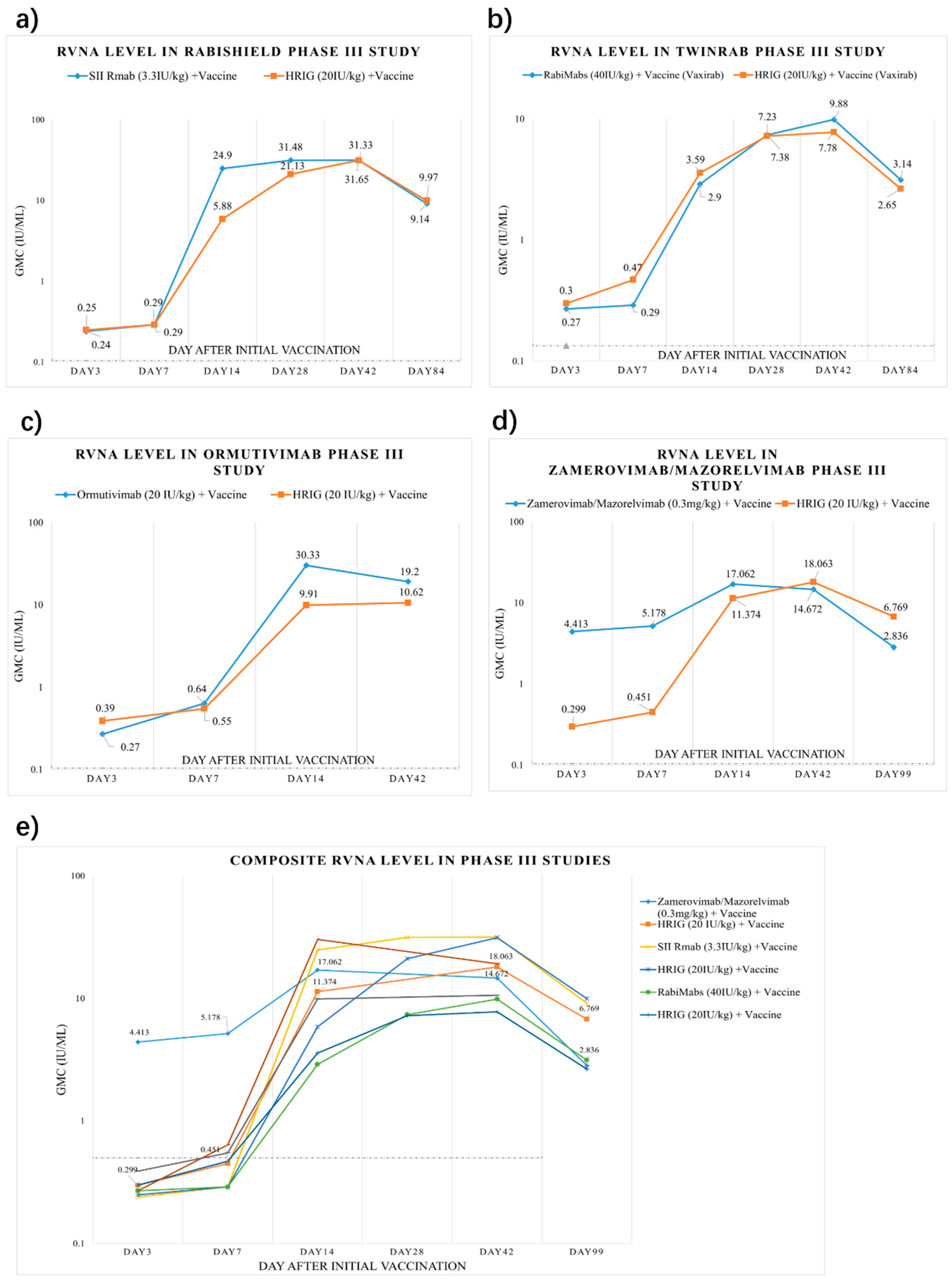
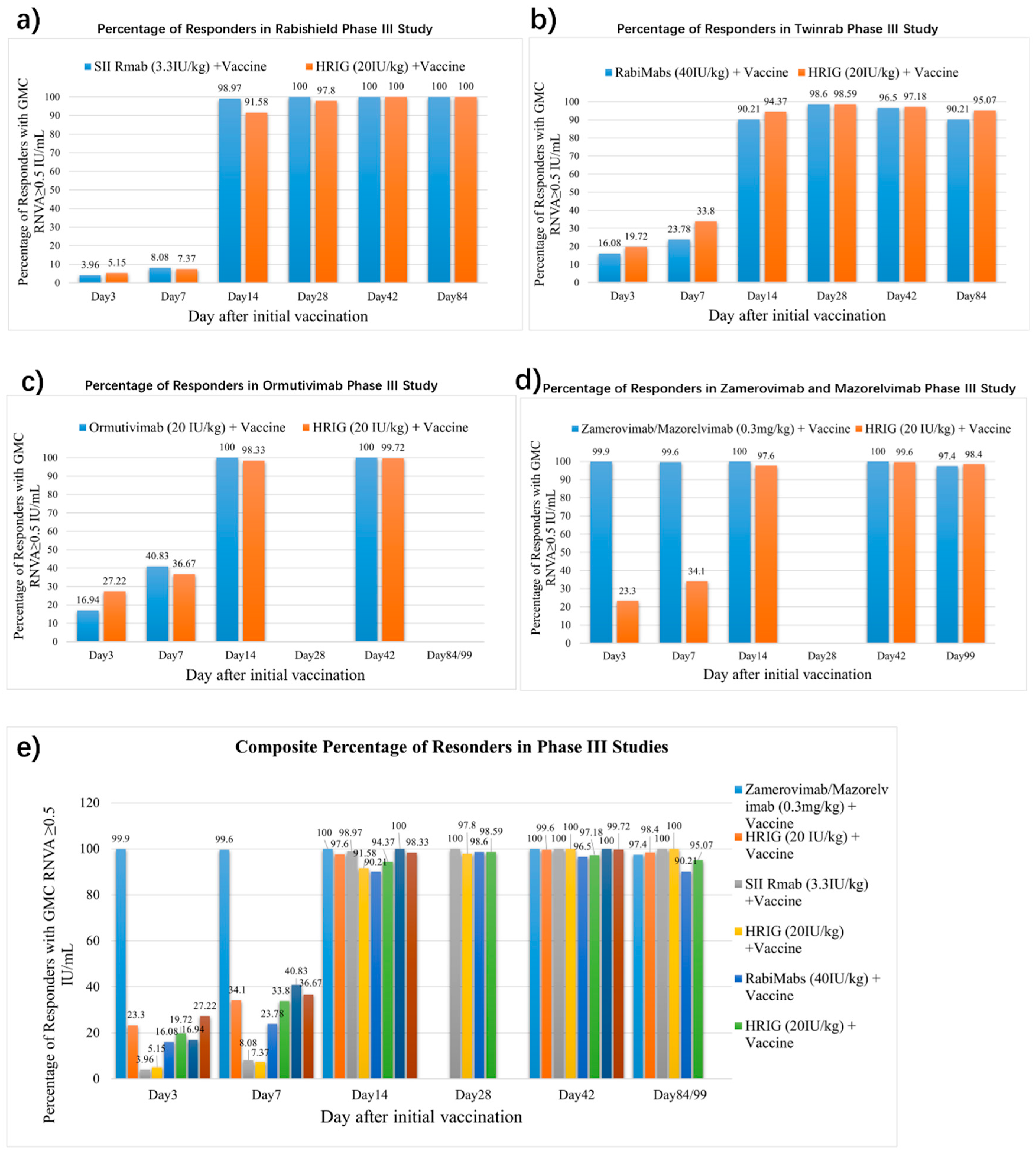
3.4. Circulating Antibody
3.5. PEP Failure, Non-Response, Fatal Breakthrough Infection, and Possible Attribution
3.6. Guidelines for Clinical Trial Design
3.7. Approved and Under Developing mAb (Cocktails)
3.8. mAbs Rapidly Attain Higher Post-Exposure Circulating Antibody Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Rabies. Available online: https://www.who.int/news-room/fact-sheets/detail/rabies (accessed on 1 June 2025).
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar] [CrossRef]
- Rupprecht, C.E.; Briggs, D.; Brown, C.M.; Franka, R.; Katz, S.L.; Kerr, H.D.; Lett, S.; Levis, R.; Meltzer, M.I.; Schaffner, W.; et al. Evidence for a 4-dose vaccine schedule for human rabies post-exposure prophylaxis in previously non-vaccinated individuals. Vaccine 2009, 27, 7141–7148. [Google Scholar] [CrossRef] [PubMed]
- Habel, K.; Koprowski, H. Laboratory data supporting the clinical trial of anti-rabies serum in persons bitten by a rabid wolf. Bull. World Health Organ. 1955, 13, 773–779. [Google Scholar] [PubMed]
- Manning, S.E.; Rupprecht, C.E.; Fishbein, D.; Hanlon, C.A.; Lumlertdacha, B.; Guerra, M.; Meltzer, M.I.; Dhankhar, P.; Vaidya, S.A.; Jenkins, S.R.; et al. Human rabies prevention—United States, 2008: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm. Rep. 2008, 57, 1–28. [Google Scholar] [PubMed]
- World Health Organization. Rabies vaccines: WHO position paper, April 2018—Recommendations. Vaccine 2018, 36, 5500–5503. [Google Scholar] [CrossRef] [PubMed]
- WHO. Protocol for a Well-Performed Rabies Post-Exposure Prophylaxis Delivery: To Read Along with the Decision Trees 1—Wound Risk Assessment and 2—PEP Risk Assessment. Available online: https://www.who.int/publications/i/item/B09018 (accessed on 1 June 2025).
- Gogtay, N.; Thatte, U.; Kshirsagar, N.; Leav, B.; Molrine, D.; Cheslock, P.; Kapre, S.V.; Kulkarni, P.S. Safety and pharmacokinetics of a human monoclonal antibody to rabies virus: A randomized, dose-escalation phase 1 study in adults. Vaccine 2012, 30, 7315–7320. [Google Scholar] [CrossRef] [PubMed]
- Gogtay, N.J.; Munshi, R.; Ashwath Narayana, D.H.; Mahendra, B.J.; Kshirsagar, V.; Gunale, B.; Moore, S.; Cheslock, P.; Thaker, S.; Deshpande, S.; et al. Comparison of a Novel Human Rabies Monoclonal Antibody to Human Rabies Immunoglobulin for Postexposure Prophylaxis: A Phase 2/3, Randomized, Single-Blind, Noninferiority, Controlled Study. Clin. Infect. Dis. 2018, 66, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Kansagra, K.; Parmar, D.; Mendiratta, S.K.; Patel, J.; Joshi, S.; Sharma, N.; Parihar, A.; Bhoge, S.; Patel, H.; Kalita, P.; et al. A Phase 3, Randomized, Open-label, Noninferiority Trial Evaluating Anti-Rabies Monoclonal Antibody Cocktail (TwinrabTM) Against Human Rabies Immunoglobulin (HRIG). Clin. Infect. Dis. 2021, 73, e2722–e2728. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Y.; Bai, Y.; Li, G.; Zhang, J.; Yang, L.; Zhao, W.; Zhao, W.; Luo, F.; Zhao, Q.; et al. Neutralizing antibody activity, safety and immunogenicity of human anti-rabies virus monoclonal antibody (Ormutivimab) in Chinese healthy adults: A phase IIb randomized, double-blind, parallel-controlled study. Vaccine 2022, 40, 6153–6162. [Google Scholar] [CrossRef] [PubMed]
- Quiambao, B.P.; Payumo, R.A.; Roa, C.; Borja-Tabora, C.F.; Emmeline Montellano, M.; Reyes, M.R.L.; Zoleta-De Jesus, L.; Capeding, M.R.; Solimen, D.P.; Barez, M.Y.; et al. A phase 2b, Randomized, double blinded comparison of the safety and efficacy of the monoclonal antibody mixture SYN023 and human rabies immune globulin in patients exposed to rabies. Vaccine 2024, 42, 126018. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Zha, Y.X.; Wang, Z.X.; Jiang, Y.; Zhang, X.Y.; Guo, J.S.; Li, J.Y.; Liu, X.Q. Effects of Zamerovimab/Mazorelvimab on the rabies virus neutralizing antibody level in the grade III rabies post exposure subjects. Chin. J. Exp. Clin. Virol. 2024, 38, 7. [Google Scholar]
- Holzbauer, S.M.; Schrodt, C.A.; Prabhu, R.M.; Asch-Kendrick, R.J.; Ireland, M.; Klumb, C.; Firestone, M.J.; Liu, G.; Harry, K.; Ritter, J.M.; et al. Fatal Human Rabies Infection With Suspected Host-Mediated Failure of Post-Exposure Prophylaxis Following a Recognized Zoonotic Exposure-Minnesota, 2021. Clin. Infect. Dis. 2023, 77, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.; Nguyen, C.; Taha, M.; Taylor, R.S. Older adults and non-response to rabies post-exposure prophylaxis: Challenges and approaches. Can. Commun. Dis. Rep. 2023, 49, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, E.R.; Mandra, A.; Bonwitt, J.; Beasley, E.A.; Taliano, J.; Rao, A.K. Human rabies despite post-exposure prophylaxis: A systematic review of fatal breakthrough infections after zoonotic exposures. Lancet Infect. Dis. 2023, 23, e167–e174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.J.; Liu, R.Q. Analysis of the reasons for immunization failure of rabies vaccine in 67 cases. J. Jining Med. Coll. 1992, 15, 2. [Google Scholar]
- China News Week. A 3-Year-Old Boy Passed Away After Being Bitten by a Dog, and the Dog’s Owner Has Not Been Found to This Day. 2024. Available online: https://news.cnr.cn/native/gd/20240515/t20240515_526706229.shtml (accessed on 1 June 2025).
- Qing, R.; Huang, L. Xi’an Woman, 32, Succumbs to Rabies After Dog Bite Despite Receiving Four Vaccine Shots. Huashang Newspaper, 19 July 2017. Available online: http://ehsb.hspress.net/shtml/hsb/20170719/653034.shtml (accessed on 1 June 2025).
- Wu, Y. Xi’an Health Commission Claims Rabies Vaccine Administered in Fatal Case Was Compliant, Family to Sue Hospital. News Peach Uptide, 4 August 2017. Available online: https://m.thepaper.cn/newsDetail_forward_1752446#topper.cn (accessed on 1 June 2025).
- Wuhu Boy Dies of Rabies After Being Bitten by Dog. Xinhuanet, 7 August 2018. Available online: https://baijiahao.baidu.com/s?id=1608105911661302067&wfr=spider&for=pc (accessed on 1 June 2025).
- Boy Dies 13 Days After Dog Bite Despite Three Vaccine Doses: Why Did Rabies Still Strike? CCTV.com. 2018. Available online: https://jingji.cctv.com/2018/09/29/ARTIsSG2R4Zg3Fn4T1HpHIpQ180929.shtml (accessed on 1 June 2025).
- Knobel, D.L.; Cleaveland, S.; Coleman, P.G.; Fèvre, E.M.; Meltzer, M.I.; Miranda, M.E.; Shaw, A.; Zinsstag, J.; Meslin, F.X. Re-evaluating the burden of rabies in Africa and Asia. Bull. World Health Organ. 2005, 83, 360–368. [Google Scholar] [PubMed]
- Gan, H.; Hou, X.; Wang, Y.; Xu, G.; Huang, Z.; Zhang, T.; Lin, R.; Xue, M.; Hu, H.; Liu, M.; et al. Global burden of rabies in 204 countries and territories, from 1990 to 2019: Results from the Global Burden of Disease Study 2019. Int. J. Infect. Dis. 2023, 126, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Hemachudha, T.; Laothamatas, J.; Rupprecht, C.E. Human rabies: A disease of complex neuropathogenetic mechanisms and diagnostic challenges. Lancet Neurol. 2002, 1, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Hemachudha, T.; Ugolini, G.; Wacharapluesadee, S.; Sungkarat, W.; Shuangshoti, S.; Laothamatas, J. Human rabies: Neuropathogenesis, diagnosis, and management. Lancet Neurol. 2013, 12, 498–513. [Google Scholar] [CrossRef] [PubMed]
- Karliner, J.S.; Belaval, G.S. Incidence of Reactions Following Administration of Antirabies Serum; Study of 526 Cases. JAMA 1965, 193, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Hafkin, B.; Alls, M.E.; Baer, G.M. Human rabies globulin and human diploid vaccine dose determinations. Dev. Biol. Stand. 1978, 40, 121–127. [Google Scholar] [PubMed]
- Nicholson, K.G.; Turner, G.S. Studies with human diploid cell strain rabies vaccine and human antirabies immunoglobulin in man. Dev. Biol. Stand. 1978, 40, 115–120. [Google Scholar] [PubMed]
- Sparrow, E.; Torvaldsen, S.; Newall, A.T.; Wood, J.G.; Sheikh, M.; Kieny, M.P.; Abela-Ridder, B. Recent advances in the development of monoclonal antibodies for rabies post exposure prophylaxis: A review of the current status of the clinical development pipeline. Vaccine 2019, 37 (Suppl. S1), A132–A139. [Google Scholar] [CrossRef] [PubMed]
- WHO. Report of the Sixth WHO Consultation on Monoclonal Antibodies in Rabies Diagnosis and Research; The Wistar Institute: Philadelphia, PA, USA, 1990. [Google Scholar]
- Nicholson, K.G. Modern vaccines. Rabies. Lancet 1990, 335, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- WHO. Expert Consultation on Rabies. Second Report; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 2013; pp. 1–139, back cover. [Google Scholar]
- WHO. WHO Expert Consultation on Rabies: Third Report; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- FDA. Rabies: Developing Monoclonal Antibody Cocktails for the Passive Immunization Omponent of Post-Exposure Prophylaxis Guidance for Industry. Available online: https://www.fda.gov/media/151102/download (accessed on 1 June 2025).
- NMPA. Technical Guidelines for Clinical Trials of New Monoclonal Antibody Drugs Against Rabies Virus. Available online: https://www.cde.org.cn/zdyz/domesticinfopage?zdyzIdCODE=b8d20904bd51aa02b508c87fb81af65d (accessed on 1 June 2025).
- Chao, T.Y.; Ren, S.; Shen, E.; Moore, S.; Zhang, S.F.; Chen, L.; Rupprecht, C.E.; Tsao, E. SYN023, a novel humanized monoclonal antibody cocktail, for post-exposure prophylaxis of rabies. PLoS Negl. Trop. Dis. 2017, 11, e0006133. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.J.; Dong, G.M.; Liu, X.Q.; Liu, S.; Liu, C.; Chen, Q.J.; Yin, W.W.; Wang, C.L. Progress and prospect of clinical application of anti-rabies virus monoclonal antibody preparation. Zhonghua Yi Xue Za Zhi 2023, 103, 2475–2479. [Google Scholar] [CrossRef] [PubMed]
- A Randomized, Double-Blind, Placebo Controlled Multicentric Phase I/II Study to Evaluate the Safety, Tolerability and Neutralizing Activity of Rabimabs (A Murine Anti-Rabies Monoclonal Antibody Cocktails) Against Rabies Virus in Healthy Subjects (CTRI/2012/12/003225). Available online: https://ctri.nic.in/Clinicaltrials/pmaindet2.php?EncHid=NTcxNg==&Enc=&userName=CTRI/2012/12/003225 (accessed on 1 June 2025).
- Wang, M.X.; Jia, M.; Jin, M.; Han, J.; Duan, J.; Wang, L.Q.; Jin, R.H.; Li, N.; Yao, J.L.; Li, Y.F.; et al. Safety of a single injection with recombinant human rabies immunoglobin at various dosages in humans. Chin. J. Biol. 2013, 26, 5. [Google Scholar]
- Zhang, J.N.; Meng, Y.J.; Bai, Y.H.; Li, Y.F.; Yang, L.Q.; Shi, N.M.; Han, H.X.; Gao, J.; Zhu, L.J.; Li, S.P.; et al. Rabies Virus Neutralizing Activity, Safety, and Immunogenicity of Recombinant Human Rabies Antibody Compared with Human Rabies Immunoglobulin in Healthy Adults. Biomed. Environ. Sci. 2022, 35, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Li, J.; Zhou, J.; Guo, J.; Pu, Y.; Jiang, Y.; Zhou, Y.; Jiang, Z.; Shu, Q.; et al. Comparing recombinant human rabies monoclonal antibody (ormutivimab) with human rabies immunoglobulin (HRIG) for postexposure prophylaxis: A phase III, randomized, double-blind, non-inferiority trial. Int. J. Infect. Dis. 2023, 134, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wu, M.; Zhang, H.; Zhu, X.; Hu, Y.; Li, X.; Liu, J.; Tsao, E.; Liu, M.; Li, C. Safety, pharmacokinetics and pharmacodynamics of SYN023 alone or in combination with a rabies vaccine: An open, parallel, single dose, phase 1 bridging study in healthy Chinese subjects. Antivir. Res. 2020, 184, 104956. [Google Scholar] [CrossRef] [PubMed]
- McClain, J.B.; Chuang, A.; Moore, S.M.; Tsao, E. Safety, Pharmacokinetics, and Neutralizing Activity of SYN023, a Mixture of Two Novel Antirabies Monoclonal Antibodies Intended for Use in Postrabies Exposure Prophylaxis. Clin. Pharmacol. Drug Dev. 2021, 10, 807–817. [Google Scholar] [CrossRef] [PubMed]
- McClain, J.B.; Chuang, A.; Reid, C.; Moore, S.M.; Tsao, E. Rabies virus neutralizing activity, pharmacokinetics, and safety of the monoclonal antibody mixture SYN023 in combination with rabies vaccination: Results of a phase 2, randomized, blinded, controlled trial. Vaccine 2021, 39, 5822–5830. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A. Rabies Vaccines: Duration of Pep from 90 Days to 7 Days. Int. J. Clin. Rep. Stud. 2024, 3. Available online: https://www.clinicsearchonline.org/article/rabies-vaccines-duration-of-pep-from-90-days-to-7-days (accessed on 1 June 2025).
- HS, R.; Khobragade, A.; Satapathy, D.; Gupta, M.; Kumar, S.; Bhomia, V.; V, R.; Desai, M.; Agrawal, A.D. Safety and Immunogenicity of a novel three-dose recombinant nanoparticle rabies G protein vaccine administered as simulated post exposure immunization: A randomized, comparator controlled, multicenter, phase III clinical study. Hum. Vaccin. Immunother. 2021, 17, 4239–4245. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, C.; Liu, C.; Xie, K.; Li, Y.; Li, H.C. A study on the outcome-based training method of rabies passive immunization injection. Chin. J. Emerg. Resusc. Disaster Med. 2021, 16, 3. [Google Scholar]
- Zhu, Z.; Huang, S.; Lu, S.; Zhang, M.; Meng, S.; Hu, Q.; Fang, Y. Severe multiple rabid dog bite injuries in a child in central China: Continuous 10-year observation and analysis on this case. Hum. Vaccin. Immunother. 2020, 16, 904–906. [Google Scholar] [CrossRef] [PubMed]
- Scholand, S.J.; Quiambao, B.P.; Rupprecht, C.E. Time to Revise the WHO Categories for Severe Rabies Virus Exposures-Category IV? Viruses 2022, 14, 1111. [Google Scholar] [CrossRef] [PubMed]
- Banović, P.; Mijatović, D.; Simin, V.; Vranješ, N.; Meletis, E.; Kostoulas, P.; Obregon, D.; Cabezas-Cruz, A. Real-world evidence of rabies post-exposure prophylaxis in Serbia: Nation-wide observational study (2017–2019). Travel. Med. Infect. Dis. 2024, 58, 102697. [Google Scholar] [CrossRef] [PubMed]
| mAbs | Stage of Development | Isoform | Target | Developer | Product |
|---|---|---|---|---|---|
| Rabishield®(SII Rmab, 17C7) | Approved in India in December 2016 | Single human IgG1-type mAb | Glycoprotein, antigenic site III | Serum Institute of India PVT. LTD. (SIIPL), Pune, India | 10 mL vials with a minimal potency of 300 IU/mL |
| Twinrab® (RabiMabs, Docaravimab, and Miromavimab) | Approved in India in September 2019 | Two murine IgG1/lgG2b mAb cocktails | Glycoprotein, antigenic sites II and III | Zydus Lifesciences, Ahmedabad, India | 10 mL vials with a minimal potency of 300 IU/mL |
| Ormutivimab (NM57) | Approved in China in January 2022 (adults) and May 2024 (>2 years children) | Full human IgG1-type mAb | Glycoprotein, antigenic site I | North China Pharmaceutical Group New Drug Research and Development, Shijiazhuang, China | 200 IU (1 mL)/vial |
| Zamerovimab and Mazorelvimab Injection (SYN023/CTB011 and CTB012) | Approved in China in June 2024 | Two humanized monoclonal human IgG1 kappa antibodies | Glycoprotein, antigenic sites III and G5 | Synermore Biologics (Suzhou) Co., Ltd., Suzhou, China | 6 mg/2 mL/vial |
| mAbs | Stage of Development | Clinical Trial Title | Sponsor/Institute | Trial No. |
|---|---|---|---|---|
| GR1801 | Phase III | Study to Evaluate GR1801’s Efficacy and Safety | Genrix (Shanghai) Biopharmaceutical Co., Ltd., Shanghai, China | NCT05846568 |
| CBB 1 | Phase I | Safety, Pharmacokinetics, and Pharmacodynamic Testing of Rabies mAb CBB 1 | Changchun BCHT Biotechnology Co. Ltd., Changchun, China | NCT05832073 |
| R172 (RAB1-RAB2) and R173 (RAB1-CR57) | Preclinical | N/A | MassBiologics of the University of Massachusetts Chan Medical School, Worcester, Massachusetts, United States | N/A |
| NP-19-9 and 11B6 | Preclinical | N/A | Department of Research and Development, Celltrion, INC, Incheon, Republic of Korea | N/A |
| RVC20 and RVC58 | Preclinical | N/A | Italian Ministry of Health, Rome, Italy | N/A |
| CR57, RV08, and RV3A5 | Preclinical | N/A | Chinese CDC, Beijing, China | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Cai, L.; Lv, X.; Liu, S.; Liu, C.; Liu, J.; Liu, X.; Yin, W.; Wang, C.; Zhu, Z. Circulating Antibody’s Role During Post-Exposure Prophylaxis, and Beyond for Rabies: A Review. Vaccines 2025, 13, 775. https://doi.org/10.3390/vaccines13070775
Chen Q, Cai L, Lv X, Liu S, Liu C, Liu J, Liu X, Yin W, Wang C, Zhu Z. Circulating Antibody’s Role During Post-Exposure Prophylaxis, and Beyond for Rabies: A Review. Vaccines. 2025; 13(7):775. https://doi.org/10.3390/vaccines13070775
Chicago/Turabian StyleChen, Qingjun, Li Cai, Xinjun Lv, Si Liu, Cheng Liu, Jiayang Liu, Xiaoqiang Liu, Wenwu Yin, Chuanlin Wang, and Zhenggang Zhu. 2025. "Circulating Antibody’s Role During Post-Exposure Prophylaxis, and Beyond for Rabies: A Review" Vaccines 13, no. 7: 775. https://doi.org/10.3390/vaccines13070775
APA StyleChen, Q., Cai, L., Lv, X., Liu, S., Liu, C., Liu, J., Liu, X., Yin, W., Wang, C., & Zhu, Z. (2025). Circulating Antibody’s Role During Post-Exposure Prophylaxis, and Beyond for Rabies: A Review. Vaccines, 13(7), 775. https://doi.org/10.3390/vaccines13070775





