Antibody Responses Following Primary Immunization with the Recombinant Herpes Zoster Vaccine (Shingrix®) in VZV Seronegative Immunocompromised Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
2.2. Vaccination Schedule
2.3. Data Extraction
2.4. Analysis of Specific VZV IgG Levels
2.5. Endpoints
2.6. Statistical Analysis
3. Results
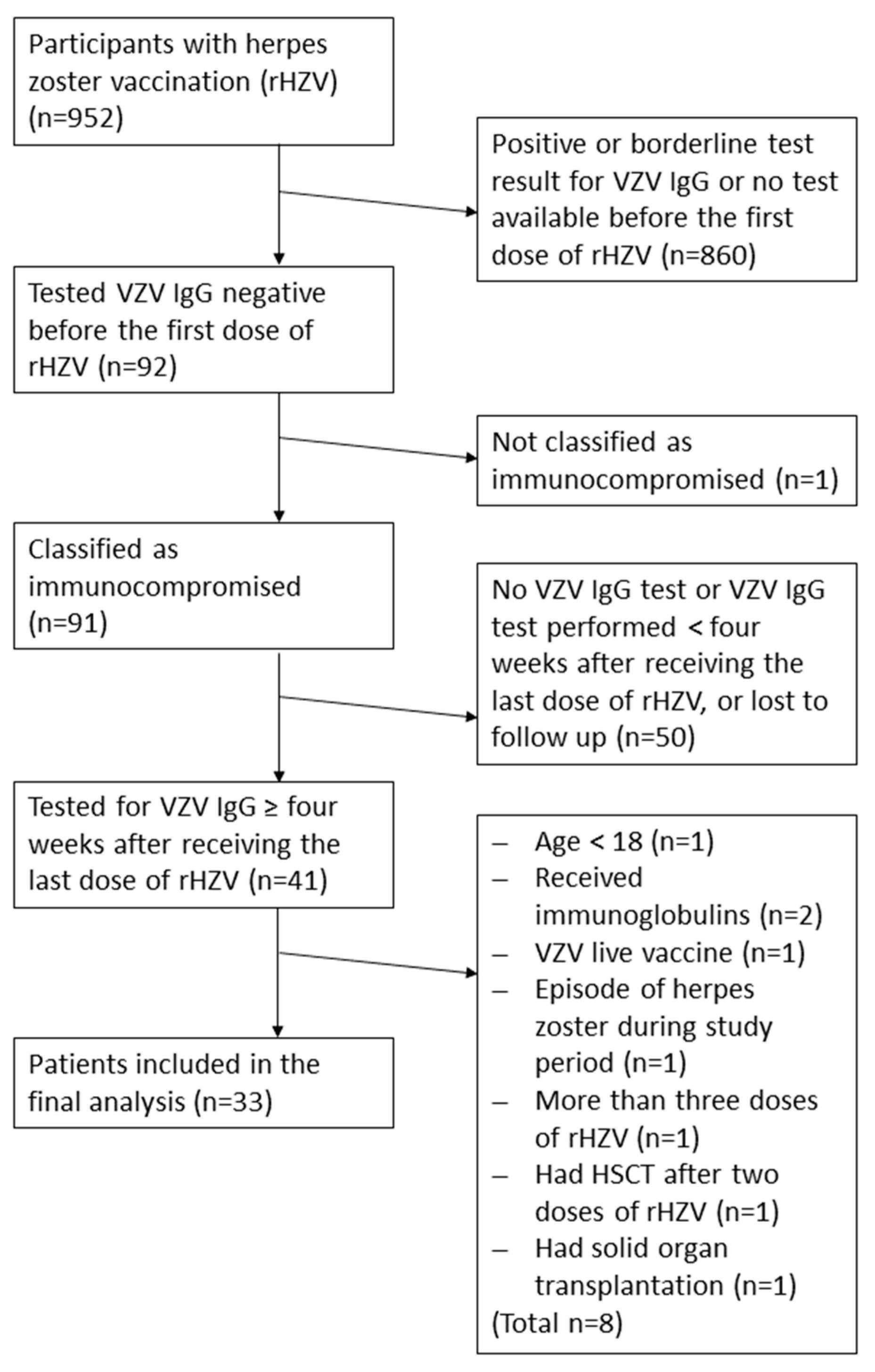
3.1. Study Population and Demographic Data
3.2. Humoral Immune Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feldhoff, C.M.; Balfour, H.H., Jr.; Simmons, R.L.; Najarian, J.S.; Mauer, S.M. Varicella in Children with Renal Transplants. J. Pediatr. 1981, 98, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Fehr, T.; Bossart, W.; Wahl, C.; Binswanger, U. Disseminated Varicella Infection in Adult Renal Allograft Recipients: Four Cases and a Review of the Literature. Transplantation 2002, 73, 608–611. [Google Scholar] [CrossRef]
- Lynfield, R.; Herrin, J.T.; Rubin, R.H. Varicella in Pediatric Renal Transplant Recipients. Pediatrics 1992, 90 Pt 1, 216–220. [Google Scholar] [CrossRef]
- Cullen, G.; Baden, R.P.; Cheifetz, A.S. Varicella Zoster Virus Infection in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2012, 18, 2392–2403. [Google Scholar] [CrossRef]
- L’Huillier, A.G.; Hirzel, C.; Ferreira, V.H.; Ierullo, M.; Ku, T.; Selzner, N.; Schiff, J.; Juvet, S.; Miao, C.; Schmid, D.S.; et al. Evaluation of Recombinant Herpes Zoster Vaccine for Primary Immunization of Varicella-Seronegative Transplant Recipients. Transplantation 2021, 105, 2316–2323. [Google Scholar] [CrossRef] [PubMed]
- López-Fauqued, M.; der Mee, M.C.-V.; Bastidas, A.; Beukelaers, P.; Dagnew, A.F.; Garcia, J.J.F.; Schuind, A.; Tavares-da-Silva, F. Safety Profile of the Adjuvanted Recombinant Zoster Vaccine in Immunocompromised Populations: An Overview of Six Trials. Drug Saf. 2021, 44, 811–823. [Google Scholar] [CrossRef]
- Stadtmauer, E.A.; Sullivan, K.M.; El Idrissi, M.; Salaun, B.; Alonso, A.A.; Andreadis, C.; Anttila, V.J.; Bloor, A.J.; Broady, R.; Cellini, C.; et al. Adjuvanted Recombinant Zoster Vaccine in Adult Autologous Stem Cell Transplant Recipients: Polyfunctional Immune Responses and Lessons for Clinical Practice. Hum. Vaccin. Immunother. 2021, 17, 4144–4154. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; He, L.; Yin, Y. Risk of Herpes Zoster Associated with Jak Inhibitors in Immune-Mediated Inflammatory Diseases: A Systematic Review and Network Meta-Analysis. Front. Pharmacol. 2023, 14, 1241954. [Google Scholar] [CrossRef]
- Schröder, C.; Enders, D.; Schink, T.; Riedel, O. Incidence of Herpes Zoster Amongst Adults Varies by Severity of Immunosuppression. J. Infect. 2017, 75, 207–215. [Google Scholar] [CrossRef]
- Chen, S.Y.; Suaya, J.A.; Li, Q.; Galindo, C.M.; Misurski, D.; Burstin, S.; Levin, M.J. Incidence of Herpes Zoster in Patients with Altered Immune Function. Infection 2014, 42, 325–334. [Google Scholar] [CrossRef]
- Schrauder, A.; Henke-Gendo, C.; Seidemann, K.; Sasse, M.; Cario, G.; Moericke, A.; Schrappe, M.; Heim, A.; Wessel, A. Varicella Vaccination in a Child with Acute Lymphoblastic Leukaemia. Lancet 2007, 369, 1232. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, P.; Forrest, G.N.; Gershon, M.; Zhou, Y.; Chen, J.; LaRussa, P.; Steinberg, S.; Gershon, A.A. Disseminated, Persistent, and Fatal Infection Due to the Vaccine Strain of Varicella-Zoster Virus in an Adult Following Stem Cell Transplantation. Clin. Infect. Dis. 2015, 60, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Sartori, A.M. A Review of the Varicella Vaccine in Immunocompromised Individuals. Int. J. Infect. Dis. 2004, 8, 259–270. [Google Scholar] [CrossRef]
- Alexander, K.E.; Tong, P.L.; Macartney, K.; Beresford, R.; Sheppeard, V.; Gupta, M. Live Zoster Vaccination in an Immunocompromised Patient Leading to Death Secondary to Disseminated Varicella Zoster Virus Infection. Vaccine 2018, 36, 3890–3893. [Google Scholar] [CrossRef]
- GlaxoSmithKline. Summary of Product Characteristics (Sopc) Shingrix, Herpes Zoster Vaccine (Recombinant, Adjuvanted). 2023. Available online: https://www.ema.europa.eu/en/documents/product-information/shingrix-epar-product-information_en.pdf (accessed on 4 July 2025).
- Federal Ministry Republic of Austria for Social Affairs, Health, Care and Consumer Protection. Austrian Vaccination Recommendations 2022. 2022. Available online: https://www.paediatrie.at/media/com_acymailing/upload/impfplan_2022.pdf (accessed on 4 July 2025).
- Lal, H.; Cunningham, A.L.; Godeaux, O.; Chlibek, R.; Diez-Domingo, J.; Hwang, S.J.; Levin, M.J.; McElhaney, J.E.; Poder, A.; Puig-Barberà, J.; et al. Efficacy of an Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults. N. Engl. J. Med. 2015, 372, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.L.; Lal, H.; Kovac, M.; Chlibek, R.; Hwang, S.J.; Díez-Domingo, J.; Godeaux, O.; Levin, M.J.; McElhaney, J.E.; Puig-Barberà, J.; et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N. Engl. J. Med. 2016, 375, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Racine, É.; Gilca, V.; Amini, R.; Tunis, M.; Ismail, S.; Sauvageau, C. A Systematic Literature Review of the Recombinant Subunit Herpes Zoster Vaccine Use in Immunocompromised 18–49 year Old Patients. Vaccine 2020, 38, 6205–6214. [Google Scholar] [CrossRef]
- Vink, P.; Mingorance, I.D.; Alonso, C.M.; Rubio-Viqueira, B.; Jung, K.H.; Moreno, J.F.R.; Grande, E.; Gonzalez, D.M.; Lowndes, S.; Puente, J.; et al. Immunogenicity and Safety of the Adjuvanted Recombinant Zoster Vaccine in Patients with Solid Tumors, Vaccinated before or During Chemotherapy: A Randomized Trial. Cancer 2019, 125, 1301–1312. [Google Scholar] [CrossRef]
- Vink, P.; Torrell, J.M.R.; Fructuoso, A.S.; Kim, S.J.; Kim, S.I.; Zaltzman, J.; Ortiz, F.; Plana, J.M.C.; Rodriguez, A.M.F.; Rodrigo, H.R.; et al. Immunogenicity and Safety of the Adjuvanted Recombinant Zoster Vaccine in Chronically Immunosuppressed Adults Following Renal Transplant: A Phase 3, Randomized Clinical Trial. Clin. Infect. Dis. 2020, 70, 181–190. [Google Scholar] [CrossRef]
- Heineman, T.C.; Cunningham, A.; Levin, M. Understanding the Immunology of Shingrix, a Recombinant Glycoprotein E Adjuvanted Herpes Zoster Vaccine. Curr. Opin. Immunol. 2019, 59, 42–48. [Google Scholar] [CrossRef]
- Bastidas, A.; de la Serna, J.; El Idrissi, M.; Oostvogels, L.; Quittet, P.; López-Jiménez, J.; Vural, F.; Pohlreich, D.; Zuckerman, T.; Issa, N.C.; et al. Effect of Recombinant Zoster Vaccine on Incidence of Herpes Zoster after Autologous Stem Cell Transplantation: A Randomized Clinical Trial. JAMA 2019, 322, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Dagnew, A.F.; Ilhan, O.; Lee, W.S.; Woszczyk, D.; Kwak, J.Y.; Bowcock, S.; Sohn, S.K.; Macías, G.R.; Chiou, T.J.; Quiel, D.; et al. Immunogenicity and Safety of the Adjuvanted Recombinant Zoster Vaccine in Adults with Haematological Malignancies: A Phase 3, Randomised, Clinical Trial and Post-Hoc Efficacy Analysis. Lancet Infect. Dis. 2019, 19, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Winston, D.J.; Mullane, K.M.; Cornely, O.A.; Boeckh, M.J.; Brown, J.W.; Pergam, S.A.; Trociukas, I.; Žák, P.; Craig, M.D.; Papanicolaou, G.A.; et al. Inactivated Varicella Zoster Vaccine in Autologous Haemopoietic Stem-Cell Transplant Recipients: An International, Multicentre, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2018, 391, 2116–2127. [Google Scholar] [CrossRef] [PubMed]
- Feyssaguet, M.; Berthold, V.; Helle, L.; Povey, M.; Ravault, S.; Carryn, S.; Gillard, P.; Di Paolo, E. Comparison of a Glycoprotein E-Based Elisa with a Varicella-Zoster Whole-Virus Elisa for the Quantification of Varicella Vaccine Immune Responses in Young Children. Vaccine 2020, 38, 3300–3304. [Google Scholar] [CrossRef]
- Stadtmauer, E.A.; Sullivan, K.M.; Marty, F.M.; Dadwal, S.S.; Papanicolaou, G.A.; Shea, T.C.; Mossad, S.B.; Andreadis, C.; Young, J.A.; Buadi, F.K.; et al. A Phase 1/2 Study of an Adjuvanted Varicella-Zoster Virus Subunit Vaccine in Autologous Hematopoietic Cell Transplant Recipients. Blood 2014, 124, 2921–2929. [Google Scholar] [CrossRef]
- Katial, R.K.; Ratto-Kim, S.; Sitz, K.V.; Moriarity, R.; Engler, R.J.M. Varicella Immunity: Persistent Serologic Non-Response to Immunization. Ann. Allergy Asthma Immunol. 1999, 82, 431–434. [Google Scholar] [CrossRef]

| Demographics | ||
|---|---|---|
| Patients, n, % | 33 | 100.0 |
| Age at first dose of rHZV in years, median (IQR) | 53.0 (43.0–61.0) | - |
| Female, n, % | 17 | 51.5 |
| Male, n, % | 16 | 48.5 |
| Interval between last dose of rHZV and VZV IgG testing in months, median (IQR) | 9.6 (5.1–13.9) | - |
| Main diagnosis | n | % |
| Hematological disease | 27 | 81.8 |
| MM (multiple myeloma) | 9 | 27.3 |
| ALL (acute lymphoblastic leukemia) | 1 | 3.0 |
| AML (acute myeloid leukemia) | 9 | 27.3 |
| NHL (non-Hodgkin lymphoma) | 1 | 3.0 |
| DLBCL (diffuse large B-cell lymphoma) | 1 | 3.0 |
| MDS (myelodysplastic syndrome) | 3 | 9.1 |
| Osteomyelofibrosis | 2 | 6.1 |
| Plasma cell leukemia | 1 | 3.0 |
| Collagenosis | 1 | 3.0 |
| Multiple sclerosis | 3 | 9.1 |
| Rheumatoid arthritis | 1 | 3.0 |
| Uveitis | 1 | 3.0 |
| Therapy | ||
| HSCT (hematopoietic stem cell transplantation) Δ | 27 | 81.8 |
| Allogenic | 17 | 51.5 |
| Autologous | 10 | 30.3 |
| HSCT with maintenance therapy * | 13 | 39.4 |
| HSCT without maintenance therapy | 14 | 42.4 |
| Immunosuppression other than HSCT | 6 | 18.2 |
| One immunosuppressive drug ** | 2 | 6.1 |
| More than 1 immunosuppressive drug *** | 3 | 9.1 |
| Immunosuppressive medication planned **** | 1 | 3.0 |
| Number of Shingrix® doses | ||
| Two doses of rHZV | 11 | 33.3 |
| Three doses of rHZV | 22 | 66.7 |
| Vaccination history before initial VZV IgG testing | ||
| VZV live vaccine (2 doses) □, patients n, % | 1 | 3.0 |
| rHZV (1 dose) ○, patients n, % | 1 | 3.0 |
| Characteristic | n = 4 |
|---|---|
| Gender | Female (n = 4) |
| Age at first dose of rHZV in years, median (IQR) | 54.0 (35.5–67.3) |
| Number of doses of rHZV | 2 (n = 2), 3 (n = 2) |
| Diagnosis | Acute myeloic leukemia, collagenosis, rheumatoid arthritis, multiple myeloma |
| Interval between last dose of rHZV and VZV IgG testing in months, median (IQR) | 20.9 (12.1–28.8) |
| Therapy ± immunosuppressive medication | Allogenic HSCT plus lenalidomide (n = 1) Autologous HSCT (n = 1) Methotrexate plus tofacitinib plus prednisolone (abatacept—until two weeks before first dose of Shingrix®) (n = 1) Rituximab (starting one month after second dose of Shingrix®) plus prednisolone 50 mg (n = 1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wessely, A.; Zwazl, I.; Poturica, M.; Weseslindtner, L.; Kundi, M.; Wiedermann, U.; Wagner, A. Antibody Responses Following Primary Immunization with the Recombinant Herpes Zoster Vaccine (Shingrix®) in VZV Seronegative Immunocompromised Adults. Vaccines 2025, 13, 737. https://doi.org/10.3390/vaccines13070737
Wessely A, Zwazl I, Poturica M, Weseslindtner L, Kundi M, Wiedermann U, Wagner A. Antibody Responses Following Primary Immunization with the Recombinant Herpes Zoster Vaccine (Shingrix®) in VZV Seronegative Immunocompromised Adults. Vaccines. 2025; 13(7):737. https://doi.org/10.3390/vaccines13070737
Chicago/Turabian StyleWessely, Andrea, Ines Zwazl, Melita Poturica, Lukas Weseslindtner, Michael Kundi, Ursula Wiedermann, and Angelika Wagner. 2025. "Antibody Responses Following Primary Immunization with the Recombinant Herpes Zoster Vaccine (Shingrix®) in VZV Seronegative Immunocompromised Adults" Vaccines 13, no. 7: 737. https://doi.org/10.3390/vaccines13070737
APA StyleWessely, A., Zwazl, I., Poturica, M., Weseslindtner, L., Kundi, M., Wiedermann, U., & Wagner, A. (2025). Antibody Responses Following Primary Immunization with the Recombinant Herpes Zoster Vaccine (Shingrix®) in VZV Seronegative Immunocompromised Adults. Vaccines, 13(7), 737. https://doi.org/10.3390/vaccines13070737







