Immune Durability and Breakthrough Infections 15 Months After SARS-CoV-2 Boosters in People over 65: The IMMERSION Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
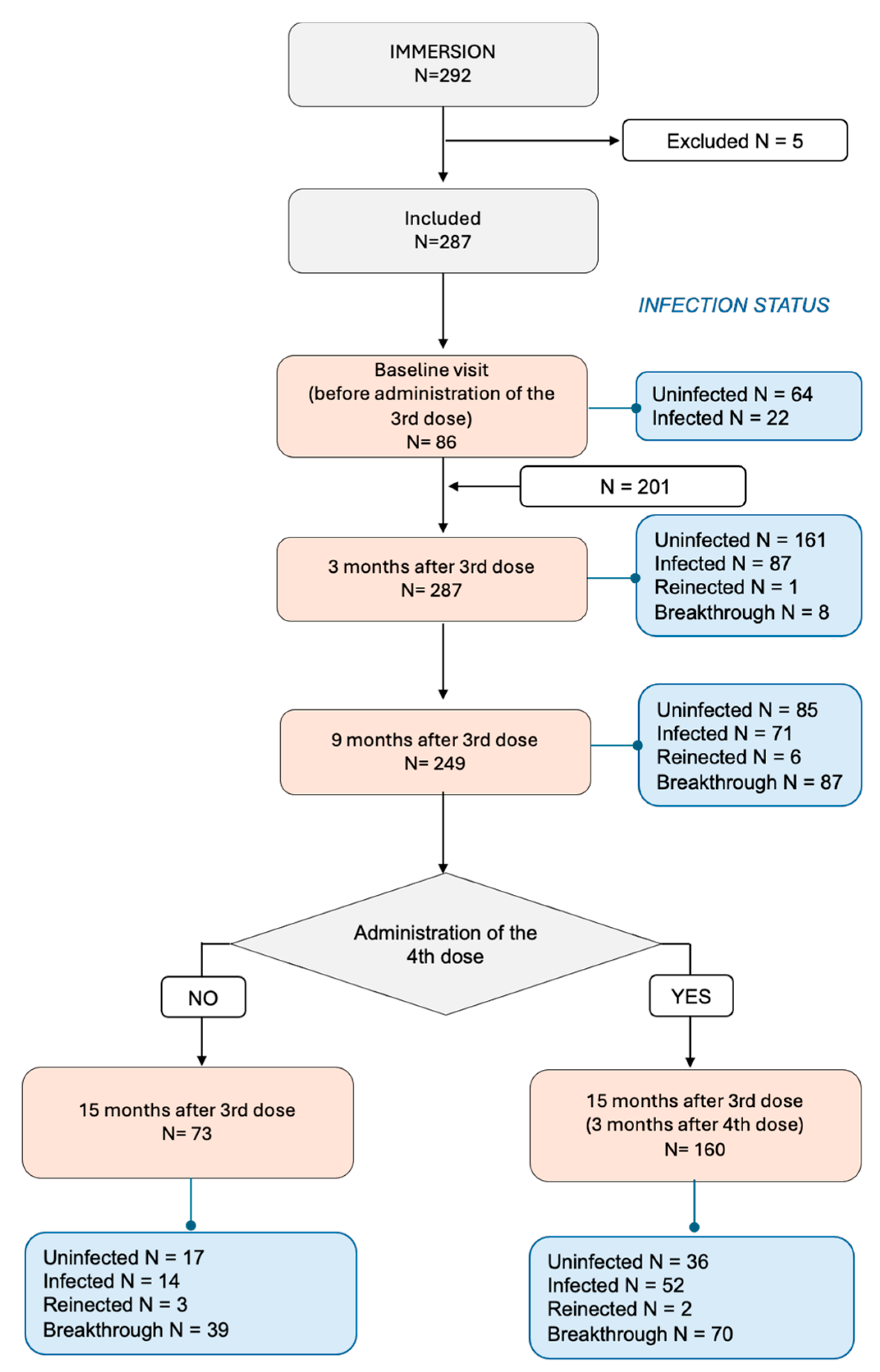
2.2. Participant Recruitment, Follow-Up
2.3. Study Outcomes
2.4. Covariables
2.5. Determination of Total Anti-SARS-CoV-2 Antibodies, Neutralization Titers, and T-Cell Responses
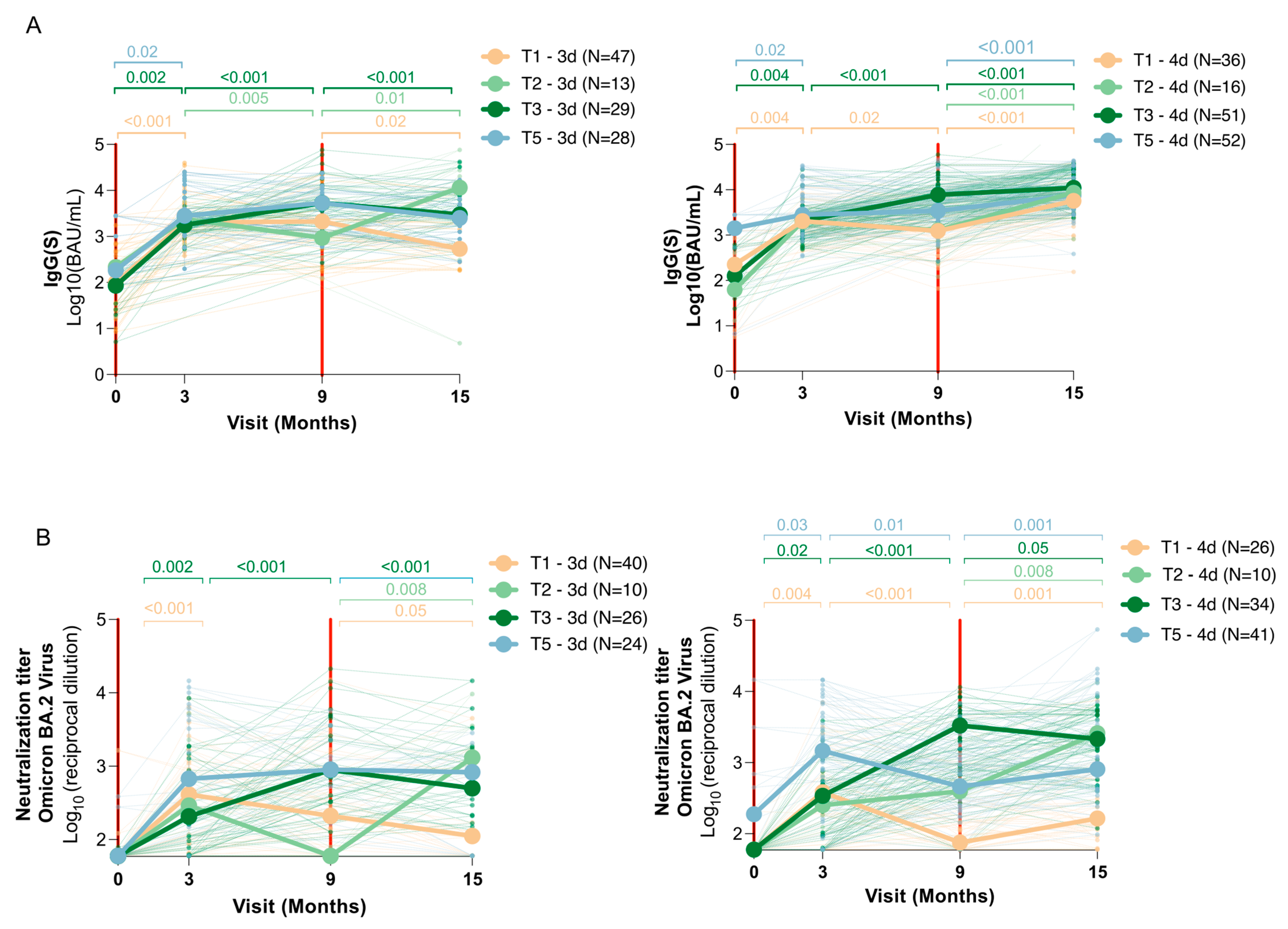
2.6. Determination of Different Trajectories According to Infected or Uninfected Status at Baseline
2.7. Sample Size and Statistical Power
2.8. Statistical Analysis
3. Results
3.1. Participant Characteristics
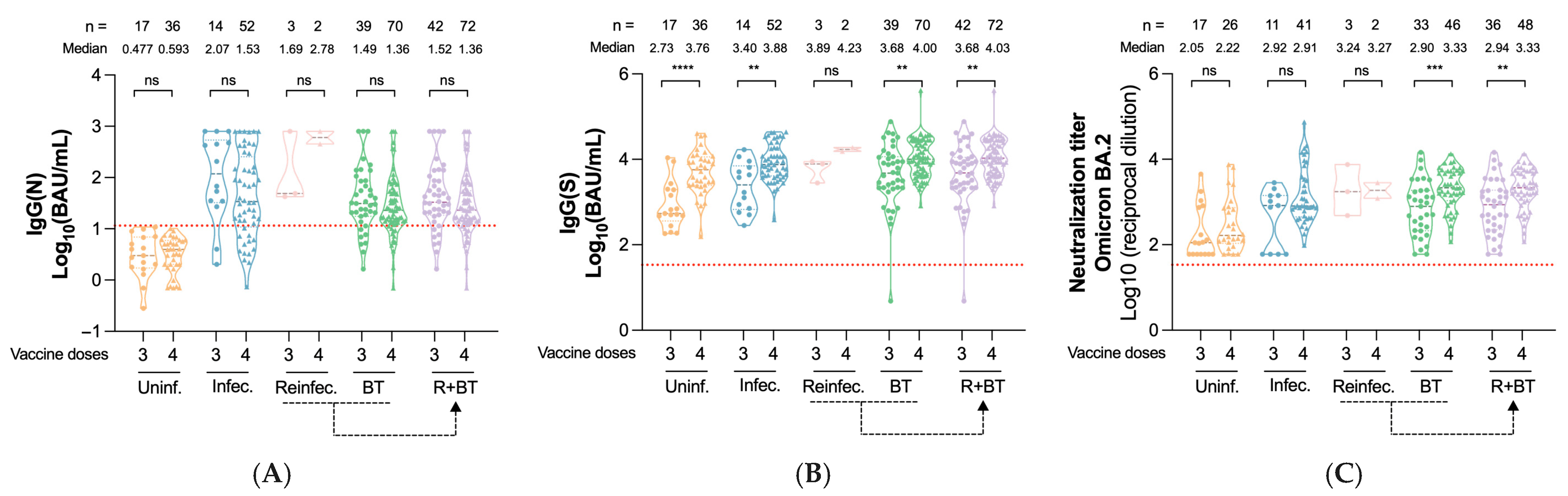
3.2. Impact of Prior Infection on Immunity After the Third Vaccine Dose
3.3. Vaccine Dose and Immune Response Dynamics (Trajectories)
3.4. Risk of Reinfection and Breakthrough Infections
3.5. Cellular Immune Response Differences by Sex
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| COVID-19 | Coronavirus disease |
| IgG(N) | Anti-nucleocapsid IgG |
| IgG(S) | Anti-spike IgG |
| NAb | Neutralizing antibodies |
| RT-PCR | Real-Time polymerase chain reaction |
| INF-γ | Interferon gamma |
| OR | Odds ratio |
| CI | Confidence interval |
| BAU | Binding antibody units |
Appendix A
Appendix A.1
Appendix A.1.1. Determination of Anti-SARS-CoV-2 Antibodies
Appendix A.1.2. Pseudovirus Neutralization Assay
Appendix A.1.3. SARS-CoV-2-Specific Cellular Responses
Appendix A.2. Determination of Different Trajectories According to Infected or Uninfected Status at Baseline
| Trajectory Group | Baseline | 3 Months | 9 Months | 15 Months |
| 1 | No infection | No infection | No infection | No infection |
| 2 | No infection | No infection | No infection | Breakthrough |
| 3 | No infection | No infection | Breakthrough | Breakthrough |
| 4 | No infection | Breakthrough | Breakthrough | Breakthrough |
| 5 | Infected | Infected | Infected | Infected |
| 6 | Infected | Infected | Infected | Reinfected |
| 7 | Infected | Infected | Reinfected | Reinfected |
| 8 | Infected | Reinfected | Reinfected | Reinfected |
References
- European Centre for Disease Prevention and Control (ECDC). SARS-CoV-2 Variants of Concern as of 28 February 2025. 2025. Available online: https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 24 May 2025).
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Català, M.; Coma, E.; Alonso, S.; Andrés, C.; Blanco, I.; Antón, A.; Bordoy, A.E.; Cardona, P.-J.; Fina, F.; Martró, E.; et al. Transmissibility, hospitalization, and intensive care admissions due to omicron compared to delta variants of SARS-CoV-2 in Catalonia: A cohort study and ecological analysis. Front. Public Health 2022, 10, 961030. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Staying Up to Date with COVID-19 Vaccines. 2025. Available online: https://www.cdc.gov/covid/vaccines/stay-up-to-date.html (accessed on 24 May 2025).
- Trigueros, M.; Pradenas, E.; Palacín, D.; Muñoz-López, F.; Ávila-Nieto, C.; Trinité, B.; Bonet-Simó, J.M.; Isnard, M.; Moreno, N.; Marfil, S.; et al. Reduced humoral response 3 months following BNT162b2 vaccination in SARS-CoV-2 uninfected residents of long-term care facilities. Age Ageing 2022, 51, afac101. [Google Scholar] [CrossRef]
- Ferreira, I.A.; Lee, C.Y.; Foster, W.S.; Abdullahi, A.; Dratva, L.M.; Tuong, Z.K.; Stewart, B.J.; Ferdinand, J.R.; Guillaume, S.M.; Potts, M.O.; et al. Atypical B cells and impaired SARS-CoV-2 neutralization following heterologous vaccination in the elderly. Cell Rep. 2023, 42, 112991. [Google Scholar] [CrossRef]
- Romero-Olmedo, A.J.; Schulz, A.R.; Hochstätter, S.; Das Gupta, D.; Virta, I.; Hirseland, H.; Staudenraus, D.; Camara, B.; Münch, C.; Hefter, V.; et al. Induction of robust cellular and humoral immunity against SARS-CoV-2 after a third dose of BNT162b2 vaccine in previously unresponsive older adults. Nat. Microbiol. 2022, 7, 195–199. [Google Scholar] [CrossRef]
- Newman, J.; Thakur, N.; Peacock, T.P.; Bialy, D.; Elrefaey, A.M.E.; Bogaardt, C.; Horton, D.L.; Ho, S.; Kankeyan, T.; Carr, C.; et al. Neutralizing antibody activity against 21 SARS-CoV-2 variants in older adults vaccinated with BNT162b2. Nat. Microbiol. 2022, 7, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; Selemon, A.; Whelan, M.; Premji, Z.; Issa, H.; et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis. 2023, 23, 556–567. [Google Scholar] [CrossRef]
- Birhane, M.; Bressler, S.; Chang, G.; Clark, T.; Dorough, L.; Fischer, M.; Watkins, L.F.; Goldstein, J.M.; Kugeler, K.; CDC COVID-19 Vaccine Breakthrough Case Investigations Team; et al. COVID-19 Vaccine Breakthrough Infections Reported to CDC—United States, January 1–April 30, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 792–793. [Google Scholar]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.S.; Ash, N.; Alroy-Preis, S.; Huppert, A.; Milo, R. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N. Engl. J. Med. 2022, 386, 2201–2212. [Google Scholar] [CrossRef]
- Barouch, D.H. Covid-19 Vaccines—Immunity, Variants, Boosters. N. Engl. J. Med. 2022, 387, 1011–1031. [Google Scholar] [CrossRef]
- Teran, R.A.; Walblay, K.A.; Shane, E.L.; Xydis, S.; Gretsch, S.; Gagner, A.; Samala, U.; Choi, H.; Zelinski, C.; Black, S.R. Postvaccination SARS-CoV-2 infections among skilled nursing facility residents and staff members—Chicago, Illinois, December 2020–March 2021. Am. J. Transplant. 2021, 21, 2290–2297. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, S.; Pilishvili, T.; Derado, G.; Soe, M.M.; Dollard, P.; Wu, H.; Li, Q.; Bagchi, S.; Dubendris, H.; Link-Gelles, R.; et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant—National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1163–1166. [Google Scholar] [PubMed]
- European Centre for Disease Prevention and Control (ECDC); European Medicines Agency (EMA). ECDC-EMA Statement on Booster Vaccination with Omicron Adapted Bivalent COVID-19 Vaccines; European Medicine Agency: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Britton, A.; Jacobs Slifka, K.M.; Edens, C.; Nanduri, S.A.; Bart, S.M.; Shang, N.; Harizaj, A.; Armstrong, J.; Xu, K.; Ehrlich, H.Y.; et al. Effectiveness of the Pfizer-BioNTech COVID-19 Vaccine Among Residents of Two Skilled Nursing Facilities Experiencing COVID-19 Outbreaks—Connecticut, December 2020–February 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 396–401. [Google Scholar] [CrossRef]
- Eliakim-Raz, N.; Leibovici-Weisman, Y.; Stemmer, A.; Ness, A.; Awwad, M.; Ghantous, N.; Stemmer, S.M. Antibody Titers Before and After a Third Dose of the SARS-CoV-2 BNT162b2 Vaccine in Adults Aged ≥ 60 Years. JAMA 2021, 326, 2203–2204. [Google Scholar] [CrossRef]
- Falsey, A.R.; Frenck, R.W.; Walsh, E.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Bailey, R.; Swanson, K.A.; Xu, X.; et al. SARS-CoV-2 Neutralization with BNT162b2 Vaccine Dose 3. N. Engl. J. Med. 2021, 385, 1627–1629. [Google Scholar] [CrossRef]
- Mwimanzi, F.; Lapointe, H.R.; Cheung, P.K.; Sang, Y.; Yaseen, F.; Kalikawe, R.; Datwani, S.; Burns, L.; Young, L.; Leung, V.; et al. Impact of Age and Severe Acute Respiratory Syndrome Coronavirus 2 Breakthrough Infection on Humoral Immune Responses After Three Doses of Coronavirus Disease 2019 mRNA Vaccine. Open Forum Infect. Dis. 2023, 10, ofad073. [Google Scholar] [CrossRef] [PubMed]
- Virk, A.; Johnson, M.G.; Roellinger, D.L.; Scott, C.G.; Sampathkumar, P.; Breeher, L.E.; Swift, M. Hybrid Immunity Provides Protective Advantage Over Vaccination or Prior Remote Coronavirus Disease 2019 Alone. Open Forum Infect. Dis. 2023, 10, ofad161. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef]
- Zheng, H.; Wu, S.; Chen, W.; Cai, S.; Zhan, M.; Chen, C.; Lin, J.; Xie, Z.; Ou, J.; Ye, W. Meta-analysis of hybrid immunity to mitigate the risk of Omicron variant reinfection. Front. Public Health 2024, 12, 1457266. [Google Scholar] [CrossRef]
- Fernández-Rivas, G.; Barallat, J.; Quirant-Sánchez, B.; González, V.; Doladé, M.; Martinez-Caceres, E.; Piña, M.; Matllo, J.; Blanco, I.; Cardona, P.-J. Follow up of the Humoral Response in Healthcare Workers after the Administration of Two Dose of the Anti SARS-CoV-2 Vaccines—Effectiveness in Delta Variant Breakthrough Infections. Viruses 2022, 14, 1385. [Google Scholar] [CrossRef]
- Crotty, S. Hybrid immunity. COVID-19 vaccine responses provide insights into how the immune system perceives threats. Science (1979) 2021, 372, 1392–1393. [Google Scholar]
- Stamatatos, L.; Czartoski, J.; Wan, Y.-H.; Homad, L.J.; Rubin, V.; Glantz, H.; Neradilek, M.; Seydoux, E.; Jennewein, M.F.; MacCamy, A.J.; et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science (1979) 2021, 372, 1413–1418. [Google Scholar] [CrossRef]
- Samanovic, M.I.; Cornelius, A.R.; Gray-Gaillard, S.L.; Allen, J.R.; Karmacharya, T.; Wilson, J.P.; Hyman, S.W.; Tuen, M.; Koralov, S.B.; Mulligan, M.J.; et al. Robust immune responses are observed after one dose of BNT162b2 mRNA vaccine dose in SARS-CoV-2-experienced individuals. Sci. Transl. Med. 2022, 14, 8961. [Google Scholar] [CrossRef]
- Wratil, P.R.; Stern, M.; Priller, A.; Willmann, A.; Almanzar, G.; Vogel, E.; Feuerherd, M.; Cheng, C.-C.; Yazici, S.; Christa, C.; et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat. Med. 2022, 28, 496–503. [Google Scholar] [CrossRef]
- Trinité, B.; Tarrés-Freixas, F.; Rodon, J.; Pradenas, E.; Urrea, V.; Marfil, S.; de la Concepción, M.L.R.; Ávila-Nieto, C.; Aguilar-Gurrieri, C.; Barajas, A.; et al. SARS-CoV-2 infection elicits a rapid neutralizing antibody response that correlates with disease severity. Sci. Rep. 2021, 11, 2608. [Google Scholar] [CrossRef]
- Pradenas, E.; Trinité, B.; Urrea, V.; Marfil, S.; Tarrés-Freixas, F.; Ortiz, R.; Rovirosa, C.; Rodon, J.; Vergara-Alert, J.; Segalés, J.; et al. Clinical course impacts early kinetics, magnitude, and amplitude of SARS-CoV-2 neutralizing antibodies beyond 1 year after infection. Cell Rep. Med. 2022, 3, 100523. [Google Scholar] [CrossRef] [PubMed]
- Pradenas, E.; Marfil, S.; Urrea, V.; Trigueros, M.; Pidkova, T.; Pons-Grífols, A.; Ortiz, R.; Rovirosa, C.; Tarrés-Freixas, F.; Aguilar-Gurrieri, C.; et al. Impact of hybrid immunity booster vaccination and Omicron breakthrough infection on SARS-CoV-2 VOCs cross-neutralization. iScience 2023, 26, 106457. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Knabl, L.; Moliva, J.I.; Werner, A.P.; Boyoglu-Barnum, S.; Kapferer, S.; Pateter, B.; Walter, M.; Sullivan, N.J.; Furth, P.A.; et al. mRNA vaccination in octogenarians 15 and 20 months after recovery from COVID-19 elicits robust immune and antibody responses that include Omicron. Cell Rep. 2022, 39, 110680. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alós, L.; Hansen, C.B.; Armenteros, J.J.A.; Madsen, J.R.; Heftdal, L.D.; Hasselbalch, R.B.; Pries-Heje, M.M.; Bayarri-Olmos, R.; Jarlhelt, I.; Hamm, S.R.; et al. Previous immunity shapes immune responses to SARS-CoV-2 booster vaccination and Omicron breakthrough infection risk. Nat. Commun. 2023, 14, 5624. [Google Scholar] [CrossRef]
- Murphy, P. Individuals with Less Severe Manifestations of SARS-CoV-2 Infection May Not Develop Long-Lasting Humoral Immunity. Am. J. Clin. Pathol. 2021, 155, 320. [Google Scholar] [CrossRef]
- Cheetham, N.J.; Kibble, M.; Wong, A.; Silverwood, R.J.; Knuppel, A.; Williams, D.M.; Hamilton, O.K.; Lee, P.H.; Staatz, C.B.; Di Gessa, G.; et al. Antibody levels following vaccination against SARS-CoV-2: Associations with post-vaccination infection and risk factors in two UK longitudinal studies. Elife 2023, 12, e80428. [Google Scholar] [CrossRef]
- Goyani, P.; Christodoulou, R.; Vassiliou, E. Immunosenescence: Aging and Immune System Decline. Vaccines 2024, 12, 1314. [Google Scholar] [CrossRef]
- Arnold, C.G.; Libby, A.; Vest, A.; Hopkinson, A.; Monte, A.A. Immune mechanisms associated with sex-based differences in severe COVID-19 clinical outcomes. Biol. Sex Differ. 2022, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Ravinayagam, V.; Nahvi, I.; Aldossary, H.; Al-Shammari, M.; Amiri, M.S.A.; Kishore, U.; Al-Suhaimi, E.A. Immunity, Sex Hormones, and Environmental Factors as Determinants of COVID-19 Disparity in Women. Front. Immunol. 2021, 12, 680845. [Google Scholar] [CrossRef] [PubMed]
- Ortona, E.; Malorni, W. Long COVID: To investigate immunological mechanisms and sex/gender related aspects as fundamental steps for tailored therapy. Eur. Respir. J. 2022, 59, 2102245. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, K.S.; Jiwrajka, N.; Lovell, C.D.; Toothacre, N.E.; Anguera, M.C. The conneXion between sex and immune responses. Nat. Rev. Immunol. 2024, 24, 487–502. [Google Scholar] [CrossRef]
- Ouyang, Q.; Cicek, G.; Westendorp, R.; Cools, H.; der Klis, R.-J.; Remarque, E. Reduced IFN-γ production in elderly people following in vitro stimulation with influenza vaccine and endotoxin. Mech. Ageing Dev. 2001, 121, 131–137. [Google Scholar] [CrossRef]
- Ho, V.W.T.; Boon, L.H.; Cui, J.; Juequn, Z.; Shunmuganathan, B.; Gupta, R.; Tan, N.Y.J.; Qian, X.; Purushotorman, K.; SCOPE Cohort Study Group; et al. Relative deficiency in interferon-γ-secreting CD4+ T cells is strongly associated with poorer COVID-19 vaccination responses in older adults. Aging Cell 2024, 23, e14099. [Google Scholar] [CrossRef]

| Sex | Baseline (N = 86) | 3-Month Visit (N = 287) | 9-Month Visit (N = 249) | 15-Month Visit (N = 233) | ||||
|---|---|---|---|---|---|---|---|---|
| Female (n = 52) | Male (n = 34) | Female (n = 152) | Male (n = 135) | Female (n = 134) | Male (n = 115) | Female (n = 123) | Male (n = 110) | |
| Sociodemographic characteristics | ||||||||
| Age, years, median [Q1; Q3] | 69.0 [67.0; 76.0] | 68.0 [66.0; 73.8] | 75.0 [70.0; 81.2] | 77.0 [72.5; 84.0] | 75.0 [70.0; 81.0] | 77.0 [72.0; 83.0] | 74.0 [70.0; 80.0] | 76.0 [72.0; 82.0] |
| Age, years (categorical), n (%) | ||||||||
| 63–74 | 37 (71.2) | 27 (79.4) | 71 (46.7) | 49 (36.3) | 65 (48.5) | 45 (39.1) | 64 (52.0) | 45 (40.9) |
| 75–84 | 13 (15.0) | 5 (14.7) | 60 (39.5) | 61 (45.2) | 51 (38.1) | 50 (43.5) | 49 (39.8) | 48 (43.6) |
| >84 | 2 (3.8) | 2 (5.9) | 21 (13.8) | 25 (18.5) | 18 (13.4) | 20 (17.4) | 10 (8.1) | 17 (15.5) |
| Health problems 1 | ||||||||
| Number of diseases, n (%) | ||||||||
| 0 | 1 (1.9) | 1 (3.0) | 1 (0.7) | 2 (1.5) | 1 (0.8) | 2 (1.8) | 1 (0.8) | 2 (1.9) |
| 1–2 | 5 (9.8) | 7 (21.2) | 13 (8.7) | 17 (12.9) | 11 (8.4) | 16 (14.3) | 10 (8.3) | 16 (14.9) |
| 3–5 | 13 (25.5) | 4 (12.1) | 31 (20.8) | 22 (16.7) | 26 (19.8) | 17 (15.2) | 24 (20.0) | 18 (16.8) |
| 6–9 | 11 (21.6) | 11 (33.3) | 37 (24.8) | 45 (34.1) | 34 (26.0) | 39 (34.8) | 33 (27.5) | 34 (31.8) |
| >9 | 21 (41.2) | 10 (30.3) | 67 (45.0) | 46 (34.8) | 59 (45.0) | 38 (33.9) | 52 (43.3) | 37 (34.6) |
| Vaccination status | ||||||||
| Number of vaccine doses, n (%) | ||||||||
| 2 | 52 (100) | 34 (100) | . | . | . | . | . | . |
| 3 | . | . | 152 (100) | 135 (100) | 134 (100) | 115 (100) | 42 (34.1) | 31 (28.1) |
| 4 | . | . | . | . | . | . | 81 (65.9) | 79 (71.8) |
| Vaccination strategy 2, n (%) | ||||||||
| Heterologous | 27 (51.9) | 19 (55.9) | 27 (17.8) | 20 (14.8) | 25 (18.7) | 18 (15.7) | 25 (20.3) | 18 (16.4) |
| Homologous | 25 (48.1) | 15 (44.1) | 125 (82.2) | 115 (85.2) | 109 (81.3) | 97 (84.3) | 98 (79.7) | 92 (83.6) |
| SARS-CoV-2 infection | ||||||||
| Infection status, n (%) | ||||||||
| Uninfected | 43 (82.7) | 21 (61.8) | 108 (71.1) | 83 (61.5) | 49 (36.6) | 36 (31.3) | 30 (24.4) | 23 (20.9) |
| Infected | 9 (17.3) | 13 (38.2) | 37 (24.3) | 50 (37.0) | 29 (21.6) | 42 (36.5) | 27 (22.0) | 39 (35.5) |
| Reinfected | 0 (0) | 0 (0) | 1 (0.7) | 0 (0) | 4 (3.0) | 2 (1.7) | 3 (2.4) | 2 (1.8) |
| Breakthrough | 0 (0) | 0 (0) | 6 (3.9) | 2 (1.5) | 52 (38.8) | 35 (30.4) | 63 (51.2) | 46 (41.8) |
| Humoral immune response | ||||||||
| IgG(N), Log10 (BAU/mL), mean (SD) | 0.33 (0.85) | 0.66 (0.97) | 0.61 (0.65) | 0.77 (0.73) | 1.14 (0.91) | 1.21 (0.85) | 1.27 (0.78) | 1.43 (0.82) |
| IgG(S), Log10 (BAU/mL), mean (SD) | 2.16 (0.72) | 2.30 (0.75) | 3.39 (0.40) | 3.42 (0.47) | 3.48 (0.61) | 3.54 (0.58) | 3.71 (0.67) | 3.82 (0.52) |
| Neutralization capacity 3, Log10 (reciprocal dilution), mean (SD) | 1.90 (0.43) (n = 47) | 2.05 (0.56) (n = 32) | 2.75 (0.62) (n = 116) | 2.70 (0.72) (n = 109) | 2.73 (0.68) (n = 101) | 2.81 (0.72) (n = 95) | 2.88 (0.66) (n = 90) | 2.97 (0.72) (n = 89) |
| Trajectory 1 Uninfected in All Visits | IgG(S), Log10 (BAU/mL), Median [IQR] | Neutralization Titers, Log10 (Reciprocal Dilution), Median [IQR] | ||
|---|---|---|---|---|
| T1 month 0, all participants | 2.260 [1.418, 2.688] | n = 31 | 1.778 [1.778, 1.778] | n = 26 |
| T1 month 3 all participants | 3.307 [3.083, 3.474] | n = 83 | 2.602 [2.052, 3.081] | n = 66 |
| T1 month 9, all participants | 3.146 [2.795, 3.547] | n = 59 | 2.094 [1.778, 2.866] | n = 48 |
| T1 month 15, 3 doses | 2.731 [2.559, 3.293] | n = 17 | 2.048 [1.778, 2.891] | n = 17 |
| T1 month 15, 4 doses | 3.756 [3.393, 4.060] | n = 36 | 2.215 [2.033, 2.865] | n = 26 |
| p-values, Wilcoxon | ||||
| T1, Month 0 vs. month 3 | <0.001 | ⇧ | <0.001 | ⇧ |
| T1, Month 3 vs. month 9 | 0.02 | ⇩ | 0.02 | ⇩ |
| T1, Month 9 vs. month 15, 3 doses | 0.02 | ⇩ | 0.05 | ⇩ |
| T1, Month 9 vs. month 15, 4 doses | <0.001 | ⇧ | 0.008 | ⇧ |
| Trajectory 2 Breakthrough infection between month 9 and 15 | IgG(S), Log10 (BAU/mL), median [IQR] | Neutralization Titers, Log10 (reciprocal dilution), median [IQR] | ||
| T2 month 0, all participants | 1.815 [1.258, 2.444] | n = 7 | 1.778 [1.778, 1.778] | n = 7 |
| T2 month 3 all participants | 3.299 [3.109, 3.451] | n = 29 | 2.436 [1.975, 2.843] | n = 20 |
| T2 month 9, all participants | 2.971 [2.757, 3.459] | n = 27 | 1.811 [1.778, 2.979] | n = 19 |
| T2 month 15, 3 doses | 4.061 [3.681, 4.478] | n = 13 | 3.117 [2.618, 3.539] | n = 10 |
| T2 month 15, 4 doses | 3.938 [3.629, 4.524] | n = 16 | 3.414 [3.090, 3.880] | n = 9 |
| p-values, Wilcoxon | ||||
| T2, Month 0 vs. month 3 | 0.02 | ⇧ | 0.02 | ⇧ |
| T2, Month 3 vs. month 9 | 0.002 | ⇩ | 0.13 | |
| T2, Month 9 vs. month 15, 3 doses | 0.01 | ⇧ | 0.008 | ⇧ |
| T2, Month 9 vs. month 15, 4 doses | <0.001 | ⇧ | 0.008 | ⇧ |
| Trajectory 3 Breakthrough infection between month 3 and 9 | IgG(S), Log10 (BAU/mL), median [IQR] | Neutralization Titers, Log10 (reciprocal dilution), median [IQR] | ||
| T3 month 0, all participants | 1.945 [1.540, 2.134] | n = 19 | 1.778 [1.778, 1.778] | n = 19 |
| T3 month 3 all participants | 3.293 [3.110, 3.447] | n = 80 | 2.473 [1.981, 2.787] | n = 60 |
| T3 month 9, all participants | 3.860 [3.484, 4.119] | n = 80 | 3.346 [2.869, 3.688] | n = 60 |
| T3 month 15, 3 doses | 3.479 [3.307, 3.924] | n = 22 | 2.700 [2.333, 3.239] | n = 19 |
| T3 month 15, 4 doses | 4.045 [3.742, 4.391] | n = 51 | 3.333 [3.013, 3.673] | n = 34 |
| p-values, Wilcoxon | ||||
| T3, Month 0 vs. month 3 | <0.001 | ⇧ | <0.001 | ⇧ |
| T3, Month 3 vs. month 9 | <0.001 | ⇧ | <0.001 | ⇧ |
| T3, Month 9 vs. month 15, 3 doses | 0.007 | ⇩ | <0.001 | ⇩ |
| T3, Month 9 vs. month 15, 4 doses | <0.001 | ⇧ | 0.05 | ⇧ |
| Trajectory 5 Infected, without reinfections | IgG(S), Log10 (BAU/mL), median [IQR] | Neutralization Titers, Log10 (reciprocal dilution), median [IQR] | ||
| T5 month 0, all participants | 2.865 [1.948, 3.447] | n = 18 | 1.842 [1.778,2.620] | n = 17 |
| T5 month 3 all participants | 3.447 [3.197, 3.950 | n = 80 | 3.049 [2.650, 3.634] | n = 65 |
| T5 month 9, all participants | 3.563 [3.272, 3.898] | n = 69 | 2.741 [2.397, 3.074] | n = 56 |
| T5 month 15, 3 doses | 3.401 [2.827, 3.850] | n = 14 | 2.918 [1.778, 3.151] | n = 11 |
| T5 month 15, 4 doses | 3.876 [3.612, 4.260] | n = 52 | 2.908 [2.651, 3.560] | n = 41 |
| p-values, Wilcoxon | ||||
| T5, Month 0 vs. month 3 | <0.001 | ⇧ | <0.001 | ⇧ |
| T5, Month 3 vs. month 9 | 0.78 | = | 0.01 | ⇩ |
| T5, Month 9 vs. month 15, 3 doses | 0.27 | = | 0.64 | = |
| T5, Month 9 vs. month 15, 4 doses | <0.001 | ⇧ | 0.001 | ⇧ |
| A | B | C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 3–9 Months (N = 197) | 3–15 Months (N = 181) | 0–15 Months (N = 76) | |||||||
| OR | 95%CI | p-Value | OR | 95%CI | p-Value | OR | 95%CI | p-Value | |
| Previous infection (Ref. uninfected) | 0.077 | [0.021; 0.213] | <0.001 | 0.043 | [0.012; 0.120] | <0.001 | 0.048 | [0.004; 0.276] | 0.003 |
| IgG(S) * | 1.419 | [0.446; 4.645] | 0.556 | 1.691 | [0.498; 5.969] | 0.404 | 0.825 | [0.340; 1.973] | 0.663 |
| Neutralizing antibodies * | 0.638 | [0.286; 1.391] | 0.263 | 0.524 | [0.216; 1.242] | 0.147 | 3.224 | [0.539; 24.94] | 0.195 |
| Age (Ref. 63–74 years) | |||||||||
| 75–84 | 0.660 | [0.293; 1.452] | 0.306 | 0.483 | [0.205; 1.111] | 0.089 | 0.293 | [0.075; 1.033] | 0.062 |
| >84 | 1.365 | [0.550; 3.404] | 0.500 | 1.128 | [0.361; 3.664] | 0.838 | 0 | 0.990 | |
| Sex (Ref. female) | 0.850 | [0.423; 1.700] | 0.645 | 1.010 | [0.472; 2.179] | 0.980 | 1.084 | [0.348; 3.574] | 0.891 |
| Vaccine doses (Ref. 3 doses) | - | - | - | 0.950 | [0.443; 2.036] | 0.896 | 0.911 | [0.316; 2.643] | 0.862 |
| Sex | 3-Month Visit | 9-Month Visit | 15-Month Visit | |||
|---|---|---|---|---|---|---|
| Female (n = 20) | Male (n = 11) | Female (n = 13) | Male (n = 11) | Female (n = 14) | Male (n = 10) | |
| Cellular immune response | ||||||
| Cellular response, pg/mL, median [Q1;Q3] | 90.0 [36.1; 254] | 42.8 [21.6; 76.2] | 178 [120; 272] | 33.8 [19.8; 71.2] | 118 [72.2; 161] | 47.9 [11.4; 87.4] |
| Cellular status, n (%) | ||||||
| Positive | 16 (80.0) | 9 (81.8) | 13 (100) | 11 (100) | 14 (100) | 7 (70.0) |
| Negative | 0 (0) | 1 (9.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Undetermined | 2 (10.0) | 1 (9.1) | 0 (0) | 0 (0) | 0 (0) | 3 (30.0) |
| Not valuable | 2 (10.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Violán, C.; Quirant-Sánchez, B.; Palau-Antoja, M.; Palacin, D.; Pradenas, E.; Trigueros, M.; Pera, G.; Molist, G.; Fernández-Rivas, G.; Boigués, M.; et al. Immune Durability and Breakthrough Infections 15 Months After SARS-CoV-2 Boosters in People over 65: The IMMERSION Study. Vaccines 2025, 13, 738. https://doi.org/10.3390/vaccines13070738
Violán C, Quirant-Sánchez B, Palau-Antoja M, Palacin D, Pradenas E, Trigueros M, Pera G, Molist G, Fernández-Rivas G, Boigués M, et al. Immune Durability and Breakthrough Infections 15 Months After SARS-CoV-2 Boosters in People over 65: The IMMERSION Study. Vaccines. 2025; 13(7):738. https://doi.org/10.3390/vaccines13070738
Chicago/Turabian StyleViolán, Concepció, Bibiana Quirant-Sánchez, Maria Palau-Antoja, Dolors Palacin, Edwards Pradenas, Macedonia Trigueros, Guillem Pera, Gemma Molist, Gema Fernández-Rivas, Marc Boigués, and et al. 2025. "Immune Durability and Breakthrough Infections 15 Months After SARS-CoV-2 Boosters in People over 65: The IMMERSION Study" Vaccines 13, no. 7: 738. https://doi.org/10.3390/vaccines13070738
APA StyleViolán, C., Quirant-Sánchez, B., Palau-Antoja, M., Palacin, D., Pradenas, E., Trigueros, M., Pera, G., Molist, G., Fernández-Rivas, G., Boigués, M., Isnard, M., Prat, N., Carmona-Cervelló, M., Lamonja-Vicente, N., León-Gómez, B. B., Martínez-Cáceres, E. M., Cardona, P. J., Blanco, J., Massanella, M., & Torán-Monserrat, P. (2025). Immune Durability and Breakthrough Infections 15 Months After SARS-CoV-2 Boosters in People over 65: The IMMERSION Study. Vaccines, 13(7), 738. https://doi.org/10.3390/vaccines13070738








