Development of an Effective Single-Dose PCV2/CSFV Bivalent Subunit Vaccine Against Classical Swine Fever Virus and Porcine Circovirus Type 2
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of Recombinant Proteins CSFV-E2 and PCV2-ORF2
2.2. Preparation of PCV2/CSFV Bivalent Vaccine
2.3. Animals Experiment Design
2.3.1. Vaccination Trial
2.3.2. Challenge Trials
2.3.3. Sample Collection and Analysis
2.4. Clinical Examination After CSFV or PCV2 Challenge
2.5. Serological Test by ELISA and Virus Neutralization Assay
2.6. Viral Detection and Quantification from Sera and Tissue Samples
2.7. Statistical Analysis
3. Results
3.1. ExpiCHOTM Stable Cell Line Expressed Recombinant CSFV-E2 and PCV2-ORF2 Proteins
3.2. Evaluation of Vaccine-Induced Safety and Immune Response After Vaccination
3.3. Clinical Presentation Post-Challenge
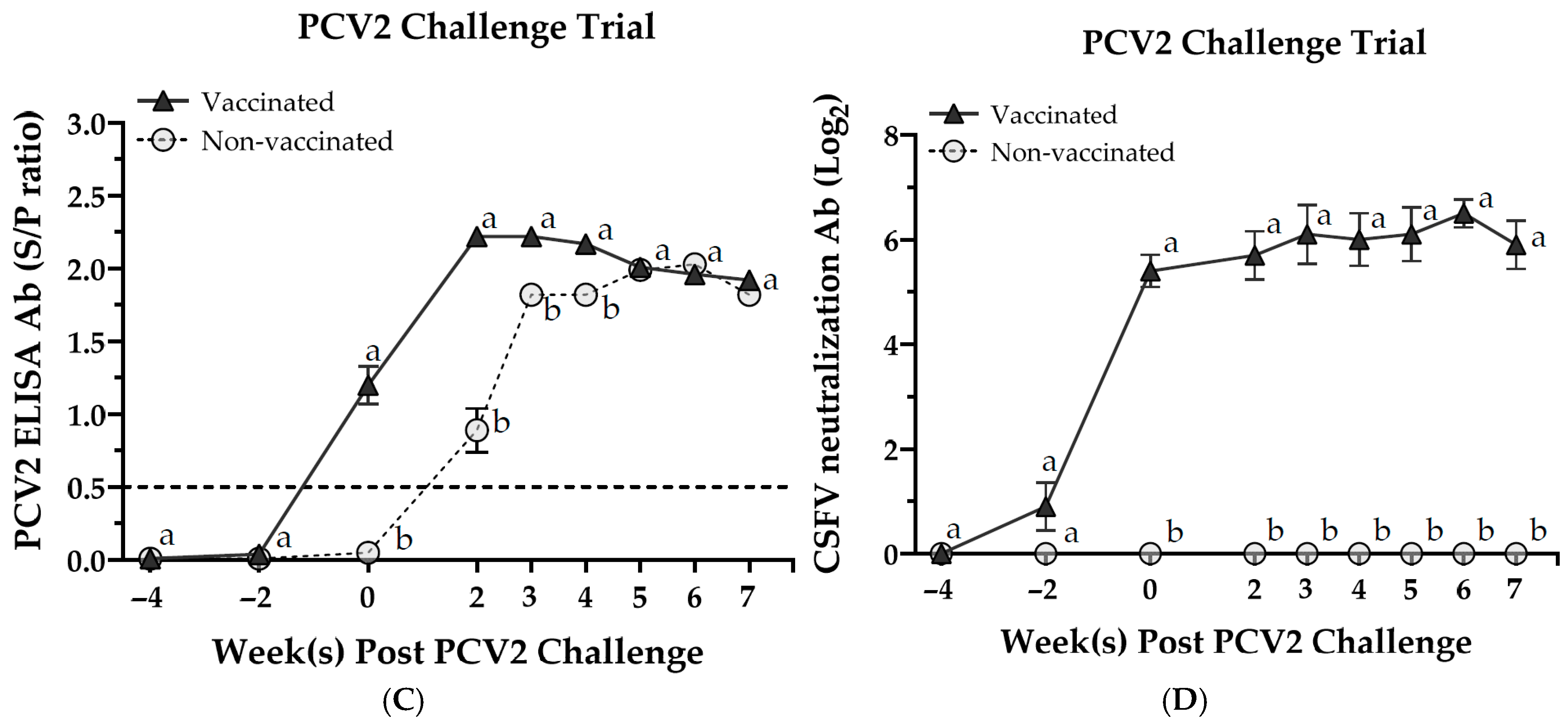
3.4. Serological Responses
3.5. Incidence and Amount of PCV2 or CSFV Viral Loads in Serum and Tissue
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCV2 | Porcine Circovirus Type 2 |
| CSF | Classical Swine Fever |
| CSFV | Classical Swine Fever Virus |
| WOAH/OIE | World Organisation for Animal Health |
| SPF | Specific pathogen-free |
| BSL | Biosafety level |
| HLN | Hilar lymph node |
| MLN | Mesenteric lymph node |
| ILN | Inguinal lymph node |
| IV | Ileocecal valve |
References
- Steiner, E.; Balmelli, C.; Gerber, H.; Summerfield, A.; McCullough, K. Cellular adaptive immune response against porcine circovirus type 2 in subclinically infected pigs. BMC Vet. Res. 2009, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Darwich, L.; Segales, J.; Resendes, A.; Balasch, M.; Plana-Duran, J.; Mateu, E. Transient correlation between viremia levels and IL-10 expression in pigs subclinically infected with porcine circovirus type 2 (PCV2). Res. Vet. Sci. 2008, 84, 194–198. [Google Scholar] [CrossRef]
- Ouyang, T.; Zhang, X.; Liu, X.; Ren, L. Co-Infection of Swine with Porcine Circovirus Type 2 and Other Swine Viruses. Viruses 2019, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Fort, M.; Sibila, M.; Perez-Martin, E.; Nofrarias, M.; Mateu, E.; Segales, J. One dose of a porcine circovirus 2 (PCV2) sub-unit vaccine administered to 3-week-old conventional piglets elicits cell-mediated immunity and significantly reduces PCV2 viremia in an experimental model. Vaccine 2009, 27, 4031–4037. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, Z.; Wang, W.; Tang, D.; Liang, H.; Liu, Z. Establishment and application of a multiplex PCR for rapid and simultaneous detection of six viruses in swine. J. Virol. Methods 2014, 208, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Vlasakova, M.; Leskova, V.; Sliz, I.; Jackova, A.; Vilcek, S. The presence of six potentially pathogenic viruses in pigs suffering from post-weaning multisystemic wasting syndrome. BMC Vet. Res. 2014, 10, 221. [Google Scholar] [CrossRef]
- Perez, L.J.; Diaz de Arce, H.; Percedo, M.I.; Dominguez, P.; Frias, M.T. First report of porcine circovirus type 2 infections in Cuba. Res. Vet. Sci. 2010, 88, 528–530. [Google Scholar] [CrossRef]
- Dvorak, C.M.; Lilla, M.P.; Baker, S.R.; Murtaugh, M.P. Multiple routes of porcine circovirus type 2 transmission to piglets in the presence of maternal immunity. Vet. Microbiol. 2013, 166, 365–374. [Google Scholar] [CrossRef]
- Rajkumar, S.; Arya, R.S.; Narnaware, S.D.; Costa, N.C.; Coutinho, T.J. Pathology and Molecular Diagnosis of Respiratory Disease Outbreak due to PCV-2 in a Pig Farm in Goa, India. Indian J. Anim. Res. 2023, 1, B-5105. [Google Scholar] [CrossRef]
- Albina, E.; Truong, C.; Hutet, E.; Blanchard, P.; Cariolet, R.; L’Hospitalier, R.; Mahe, D.; Allee, C.; Morvan, H.; Amenna, N.; et al. An experimental model for post-weaning multisystemic wasting syndrome (PMWS) in growing piglets. J. Comp. Pathol. 2001, 125, 292–303. [Google Scholar] [CrossRef]
- Patterson, A.R.; Ramamoorthy, S.; Madson, D.M.; Meng, X.J.; Halbur, P.G.; Opriessnig, T. Shedding and infection dynamics of porcine circovirus type 2 (PCV2) after experimental infection. Vet. Microbiol. 2011, 149, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Puvanendiran, S.; Stone, S.; Yu, W.; Johnson, C.R.; Abrahante, J.; Jimenez, L.G.; Griggs, T.; Haley, C.; Wagner, B.; Murtaugh, M.P. Absence of porcine circovirus type 1 (PCV1) and high prevalence of PCV 2 exposure and infection in swine finisher herds. Virus Res. 2011, 157, 92–98. [Google Scholar] [CrossRef]
- Rose, N.; Opriessnig, T.; Grasland, B.; Jestin, A. Epidemiology and transmission of porcine circovirus type 2 (PCV2). Virus Res. 2012, 164, 78–89. [Google Scholar] [CrossRef]
- Opriessnig, T.; Yu, S.; Gallup, J.M.; Evans, R.B.; Fenaux, M.; Pallares, F.; Thacker, E.L.; Brockus, C.W.; Ackermann, M.R.; Thomas, P.; et al. Effect of vaccination with selective bacterins on conventional pigs infected with type 2 porcine circovirus. Vet. Pathol. 2003, 440, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Sarli, G.; Mandrioli, L.; Laurenti, M.; Sidoli, L.; Cerati, C.; Rolla, G.; Marcato, P.S. Immunohistochemical characterisation of the lymph node reaction in pig post-weaning multisystemic wasting syndrome (PMWS). Vet. Immunol. Immunopathol. 2001, 83, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Wu, C.M.; Liao, C.M.; Chen, K.C.; You, C.C.; Wang, Y.W.; Huang, C.; Chien, M.S. The impact of porcine circovirus associated diseases on live attenuated classical swine fever vaccine in field farm applications. Vaccine 2019, 37, 6535–6542. [Google Scholar] [CrossRef]
- Huang, Y.L.; Pang, V.F.; Lin, C.M.; Tsai, Y.C.; Chia, M.Y.; Deng, M.C.; Chang, C.Y.; Jeng, C.R. Porcine circovirus type 2 (PCV2) infection decreases the efficacy of an attenuated classical swine fever virus (CSFV) vaccine. Vet. Res. 2011, 42, 115. [Google Scholar] [CrossRef]
- Huang, Y.L.; Pang, V.F.; Deng, M.C.; Chang, C.Y.; Jeng, C.R. Porcine circovirus type 2 decreases the infection and replication of attenuated classical swine fever virus in porcine alveolar macrophages. Res. Vet. Sci. 2014, 96, 187–195. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wu, C.M.; Chen, Z.W.; Liao, C.M.; Deng, M.C.; Chia, M.Y.; Huang, C.; Chien, M.S. Evaluation of classical swine fever E2 (CSF-E2) subunit vaccine efficacy in the prevention of virus transmission and impact of maternal derived antibody interference in field farm applications. Porc. Health Manag. 2021, 7, 9. [Google Scholar] [CrossRef]
- Ling, Z.; Zhang, H.; Chen, Y.; Sun, L.; Zhao, J. A Subunit Vaccine Based on the VP2 Protein of Porcine Parvovirus 1 Induces a Strong Protective Effect in Pregnant Gilts. Vaccines 2023, 11, 1692. [Google Scholar] [CrossRef]
- Hölzen, P.; Warnck, T.; Hoy, S.; Schlegel, K.; Hennig-Pauka, I.; Gaumann, H. Comparison of Protectivity and Safety of Two Vaccines against Actinobacillus pleuropneumoniae in a Field Study. Agriculture 2021, 11, 1143. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wu, C.M.; Chia, M.Y.; Huang, C.; Chien, M.S. A prospective CSFV-PCV2 bivalent vaccine effectively protects against classical swine fever virus and porcine circovirus type 2 dual challenge and prevents horizontal transmission. Vet. Res. 2023, 54, 57. [Google Scholar] [CrossRef] [PubMed]
- Kayraklioglu, N.; Horuluoglu, B.; Klinman, D.M. CpG Oligonucleotides as Vaccine Adjuvants. Methods Mol. Biol. 2021, 2197, 51–85. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Li, B.; Sun, X.; Pan, Q.; Zheng, Y.; Liu, J.; Zhao, Y.; Wang, J.; Liu, L.; et al. A novel CpG ODN compound adjuvant enhances immune response to spike subunit vaccines of porcine epidemic diarrhea virus. Front. Immunol. 2024, 15, 1336239. [Google Scholar] [CrossRef] [PubMed]
- Hansoongnern, P.; Phecharat, N.; Wasanasuk, K.; Tommeurd, W.; Chankeeree, P.; Lekcharoensuk, C.; Semkum, P.; Pinitkiatisakul, S.; Lekcharoensuk, P. Encapsidated-CpG ODN enhances immunogenicity of porcine circovirus type 2 virus-like particles. Vet. Microbiol. 2022, 275, 109583. [Google Scholar] [CrossRef]
- Burakova, Y.; Madera, R.; McVey, S.; Schlup, J.R.; Shi, J. Adjuvants for Animal Vaccines. Viral Immunol. 2018, 31, 11–22. [Google Scholar] [CrossRef]
- Shirota, H.; Klinman, D.M. Recent progress concerning CpG DNA and its use as a vaccine adjuvant. Expert Rev. Vaccines 2014, 13, 299–312. [Google Scholar] [CrossRef]
- Chaung, H.C. CpG oligodeoxynucleotides as DNA adjuvants in vertebrates and their applications in immunotherapy. Int. Immunopharmacol. 2006, 6, 1586–1596. [Google Scholar] [CrossRef]
- Narita, M.; Kawashima, K.; Kimura, K.; Mikami, O.; Shibahara, T.; Yamada, S.; Sakoda, Y. Comparative immunohistopathology in pigs infected with highly virulent or less virulent strains of hog cholera virus. Vet. Pathol. 2000, 37, 402–408. [Google Scholar] [CrossRef]
- Mittelholzer, C.; Moser, C.; Tratschin, J.D.; Hofmann, M.A. Analysis of classical swine fever virus replication kinetics allows differentiation of highly virulent from avirulent strains. Vet. Microbiol. 2000, 74, 293–308. [Google Scholar] [CrossRef]
- Segales, J. Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012, 164, 10–19. [Google Scholar] [CrossRef]
- Hoffmann, B.; Beer, M.; Schelp, C.; Schirrmeier, H.; Depner, K. Validation of a real-time RT-PCR assay for sensitive and specific detection of classical swine fever. J. Virol. Methods 2005, 130, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.B.; Chan, W.H.; Chaung, H.C.; Lien, Y.; Wu, C.C.; Huang, Y.L. Real-time PCR for quantitation of porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 in naturally-infected and challenged pigs. J. Virol. Methods 2005, 124, 11–19. [Google Scholar] [CrossRef]
- Feng, H.; Segales, J.; Fraile, L.; Lopez-Soria, S.; Sibila, M. Effect of high and low levels of maternally derived antibodies on porcine circovirus type 2 (PCV2) infection dynamics and production parameters in PCV2 vaccinated pigs under field conditions. Vaccine 2016, 34, 3044–3050. [Google Scholar] [CrossRef]
- Martelli, P.; Saleri, R.; Ferrarini, G.; De Angelis, E.; Cavalli, V.; Benetti, M.; Ferrari, L.; Canelli, E.; Bonilauri, P.; Arioli, E.; et al. Impact of maternally derived immunity on piglets’ immune response and protection against porcine circovirus type 2 (PCV2) after vaccination against PCV2 at different age. BMC Vet. Res. 2016, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Fraile, L.; Sibila, M.; Nofrarias, M.; Lopez-Jimenez, R.; Huerta, E.; Llorens, A.; Lopez-Soria, S.; Perez, D.; Segales, J. Effect of sow and piglet porcine circovirus type 2 (PCV2) vaccination on piglet mortality, viraemia, antibody titre and production parameters. Vet. Microbiol. 2012, 161, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Cheng, X.; Zhang, J.; Tong, T.; Lin, W.; Liao, M.; Fan, H. Induction of robust immunity response in mice by dual-expression-system-based recombinant baculovirus expressing the capsid protein of porcine circovirus type 2. Virol. J. 2013, 10, 316. [Google Scholar] [CrossRef]
- Fort, M.; Sibila, M.; Allepuz, A.; Mateu, E.; Roerink, F.; Segales, J. Porcine circovirus type 2 (PCV2) vaccination of conventional pigs prevents viremia against PCV2 isolates of different genotypes and geographic origins. Vaccine 2008, 26, 1063–1071. [Google Scholar] [CrossRef]
- Gong, W.; Li, J.; Wang, Z.; Sun, J.; Mi, S.; Xu, J.; Cao, J.; Hou, Y.; Wang, D.; Huo, X.; et al. Commercial E2 subunit vaccine provides full protection to pigs against lethal challenge with 4 strains of classical swine fever virus genotype 2. Vet. Microbiol. 2019, 237, 108403. [Google Scholar] [CrossRef]
- Zhang, H.; Wen, W.; Zhao, Z.; Wang, J.; Chen, H.; Qian, P.; Li, X. Enhanced protective immunity to CSFV E2 subunit vaccine by using IFN-gamma as immunoadjuvant in weaning piglets. Vaccine 2018, 36, 7353–7360. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Ma, H.; Ren, X.; Hao, G.; Zhang, H.; Zhao, Z.; Fang, K.; Li, X.; Rong, Z.; et al. Efficient mucosal vaccination of a novel classical swine fever virus E2-Fc fusion protein mediated by neonatal Fc receptor. Vaccine 2020, 38, 4574–4583. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.T.; Truong, D.A.; Ly, V.D.; Vu, H.T.; Hoang, T.V.; Nguyen, C.T.; Chu, N.T.; Nguyen, V.T.; Nguyen, D.T.; Miyazawa, K.; et al. The potential efficacy of the E2-subunit vaccine to protect pigs against different genotypes of classical swine fever virus circulating in Vietnam. Clin. Exp. Vaccine Res. 2020, 9, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Madera, R.; Gong, W.; Wang, L.; Burakova, Y.; Lleellish, K.; Galliher-Beckley, A.; Nietfeld, J.; Henningson, J.; Jia, K.; Li, P.; et al. Pigs immunized with a novel E2 subunit vaccine are protected from subgenotype heterologous classical swine fever virus challenge. BMC Vet. Res. 2016, 12, 197. [Google Scholar] [CrossRef]
- Zhou, P.; Huang, J.; Li, Y.; Chen, H.; Wu, Y.; Fu, X.; Hao, X.; Li, Q.; Zeng, R.; Zhang, G. Efficiency Comparison of a Novel E2 Subunit Vaccine and a Classic C-Strain Vaccine against Classical Swine Fever. Vet. Sci. 2021, 8, 148. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lee, C.Y.; Wu, C.C.; Kao, P.L.; Chen, T.A.; Huang, Y.; Chung, W.B.; Kuo, T.Y.; Chen, C. Efficacy evaluation of a bivalent subunit vaccine against classical swine fever virus and porcine circovirus type 2. Sci. Rep. 2024, 14, 2997. [Google Scholar] [CrossRef]
- Hoffmann, F.; Rinas, U. Stress induced by recombinant protein production in Escherichia coli. Adv. Biochem. Eng. Biotechnol. 2004, 89, 73–92. [Google Scholar] [CrossRef]
- Baneyx, F.; Mujacic, M. Recombinant protein folding and misfolding in Escherichia coli. Nat. Biotechnol. 2004, 22, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Fahnert, B.; Lilie, H.; Neubauer, P. Inclusion bodies: Formation and utilisation. Adv. Biochem. Eng. Biotechnol. 2004, 89, 93–142. [Google Scholar] [CrossRef]
- Walsh, G.; Jefferis, R. Post-translational modifications in the context of therapeutic proteins. Nat. Biotechnol. 2006, 24, 1241–1252. [Google Scholar] [CrossRef]
- Wurm, F.M. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat. Biotechnol. 2004, 22, 1393–1398. [Google Scholar] [CrossRef]
- Gasser, B.; Saloheimo, M.; Rinas, U.; Dragosits, M.; Rodriguez-Carmona, E.; Baumann, K.; Giuliani, M.; Parrilli, E.; Branduardi, P.; Lang, C.; et al. Protein folding and conformational stress in microbial cells producing recombinant proteins: A host comparative overview. Microb. Cell Factories 2008, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.K.; Magoola, M. Advances in Escherichia coli-Based Therapeutic Protein Expression: Mammalian Conversion, Continuous Manufacturing, and Cell-Free Production. Biologics 2023, 3, 380–401. [Google Scholar] [CrossRef]
- He, M.; Zhou, X.; Wang, X. Glycosylation: Mechanisms, biological functions and clinical implications. Signal Transduct. Target. Ther. 2024, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, Y.; Han, G.; Fang, W.; He, F. Identification of E2 with improved secretion and immunogenicity against CSFV in piglets. BMC Microbiol. 2020, 20, 26. [Google Scholar] [CrossRef]
- van Oers, M.M.; Thomas, A.A.; Moormann, R.J.; Vlak, J.M. Secretory pathway limits the enhanced expression of classical swine fever virus E2 glycoprotein in insect cells. J. Biotechnol. 2001, 86, 31–38. [Google Scholar] [CrossRef]
- Lopez-Vidal, J.; Gomez-Sebastian, S.; Barcena, J.; Nunez Mdel, C.; Martinez-Alonso, D.; Dudognon, B.; Guijarro, E.; Escribano, J.M. Improved Production Efficiency of Virus-Like Particles by the Baculovirus Expression Vector System. PLoS ONE 2015, 10, e0140039. [Google Scholar] [CrossRef]
- Hua, R.H.; Huo, H.; Li, Y.N.; Xue, Y.; Wang, X.L.; Guo, L.P.; Zhou, B.; Song, Y.; Bu, Z.G. Generation and efficacy evaluation of recombinant classical swine fever virus E2 glycoprotein expressed in stable transgenic mammalian cell line. PLoS ONE 2014, 9, e106891. [Google Scholar] [CrossRef]
- Sanchez, O.; Barrera, M.; Rodriguez, M.P.; Frias, M.T.; Figueroa, N.E.; Naranjo, P.; Montesino, R.; Farnos, O.; Castell, S.; Venereo, A.; et al. Classical swine fever virus E2 glycoprotein antigen produced in adenovirally transduced PK-15 cells confers complete protection in pigs upon viral challenge. Vaccine 2008, 26, 988–997. [Google Scholar] [CrossRef]
- Luo, Q.; Zhou, J.; Tang, W.; Jiang, P.; Wan, X.; Ahmed, W.; Mohsin, A.; Zhuang, Y.; Guo, M. Investigation and development of transient production process for porcine circovirus Type-2 (PCV2) capsid protein in HEK293F cells. Protein Expr. Purif. 2023, 208–209, 106293. [Google Scholar] [CrossRef]
- Mao, Q.; Zhang, W.; Ma, S.; Qiu, Z.; Li, B.; Xu, C.; He, H.; Fan, S.; Wu, K.; Chen, J.; et al. Fusion Expression and Immune Effect of PCV2 Cap Protein Tandem Multiantigen Epitopes with CD154/GM-CSF. Vet. Sci. 2021, 8, 211. [Google Scholar] [CrossRef]
- Huynh, L.T.; Sohn, E.J.; Park, Y.; Kim, J.; Shimoda, T.; Hiono, T.; Isoda, N.; Hong, S.H.; Lee, H.N.; Sakoda, Y. Development of a dual immunochromatographic test strip to detect E2 and E(rns) antibodies against classical swine fever. Front. Microbiol. 2024, 15, 1383976. [Google Scholar] [CrossRef]
- Wei, Q.; Bai, Y.; Song, Y.; Liu, Y.; Yu, W.; Sun, Y.; Wang, L.; Deng, R.; Xing, G.; Zhang, G. Generation and immunogenicity analysis of recombinant classical swine fever virus glycoprotein E2 and E(rns) expressed in baculovirus expression system. Virol. J. 2021, 18, 44. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, F.; Yang, D.; Li, Q.; Yan, L.; Ou, J.; Zhang, L.; Liu, Y.; Zhan, Q.; Li, R.; et al. Rice-produced classical swine fever virus glycoprotein E2 with herringbone-dimer design to enhance immune responses. Plant Biotechnol. J. 2023, 21, 2546–2559. [Google Scholar] [CrossRef]
- Opriessnig, T.; Meng, X.J.; Halbur, P.G. Porcine Circovirus Type 2–Associated Disease: Update on Current Terminology, Clinical Manifestations, Pathogenesis, Diagnosis, and Intervention Strategies. J. Vet. Diagn. Investig. 2007, 19, 591–615. [Google Scholar] [CrossRef]
- Ju, L.; You, S.H.; Lee, M.A.; Jayaramaiah, U.; Jeong, Y.J.; Lee, H.S.; Hyun, B.H.; Lee, N.; Kang, S.J. Selection and Evaluation of Porcine circovirus (PCV) 2d Vaccine Strains to Protect against Currently Prevalent PCV2. Vaccines 2023, 11, 1447. [Google Scholar] [CrossRef]
- Kang, S.J.; Bae, S.M.; Lee, H.J.; Jeong, Y.J.; Lee, M.A.; You, S.H.; Lee, H.S.; Hyun, B.H.; Lee, N.; Cha, S.H. Porcine Circovirus (PCV) Genotype 2d-Based Virus-like Particles (VLPs) Induced Broad Cross-Neutralizing Antibodies against Diverse Genotypes and Provided Protection in Dual-Challenge Infection of a PCV2d Virus and a Type 1 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV). Pathogens 2021, 10, 1145. [Google Scholar] [CrossRef]
- Madera, R.F.; Wang, L.; Gong, W.; Burakova, Y.; Buist, S.; Nietfeld, J.; Henningson, J.; Cino-Ozuna, A.G.; Tu, C.; Shi, J. Toward the development of a one-dose classical swine fever subunit vaccine: Antigen titration, immunity onset, and duration of immunity. J. Vet. Sci. 2018, 19, 393–405. [Google Scholar] [CrossRef]

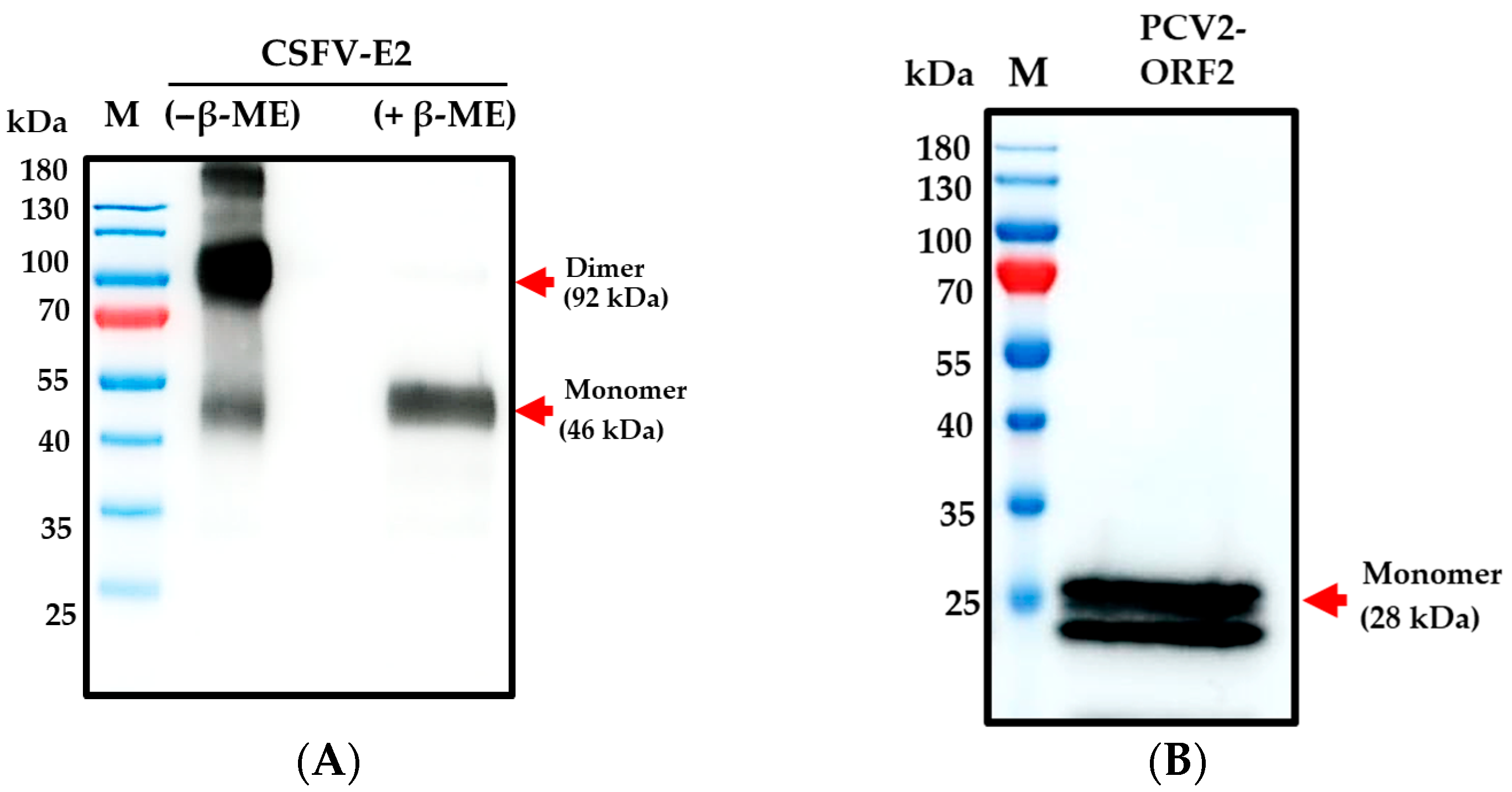


| Group (n) * | Vaccinated (5) | Non-Vaccinated (5) | ||
|---|---|---|---|---|
| Ct | Log10 TCID50/mL | Ct | Log10 TCID50/mL | |
| Day(s) Post CSFV Challenge (dpv) | ||||
| −28 dpv | (−) | (−) a | (−) | (−) a |
| −14 dpv | (−) | (−) a | (−) | (−) a |
| 0 dpv | (−) | (−) a | (−) | (−) a |
| 4 dpv | 32.0 ⁘ | 0.5 ± 0.5 a | 25.2 ± 0.8 | 4.6 ± 0.2 b |
| 7 dpv | (−) | (−) a | 16.9 ± 0.6 | 7.2 ± 0.2 b |
| 10 dpv | (−) | (−) a | 13.8 ± 0.8 | 8.1 ± 0.2 b |
| 14 dpv | (−) | (−) a | 14.7 ± 0.4 | 7.8 ± 0.1 b |
| Group (n) * | Vaccinated (5) | Non-Vaccinated (5) | ||
|---|---|---|---|---|
| Ct | Log Copies/mL | Ct | Log Copies/mL | |
| Week(s) Post PCV2 Challenge (wpv) | ||||
| −4 wpv | N/A | (−) a | N/A | (−) a |
| −2 wpv | N/A | (−) a | N/A | (−) a |
| 0 wpv | N/A | (−) a | N/A | (−) a |
| 2 wpv | N/A | (−) a | 32.8 ± 0.8 | 5.2 ± 0.2 b |
| 3 wpv | N/A | (−) a | 31.7±0.6 | 6.0 ± 0.2 b |
| 4 wpv | 35 ⁘ | 0.9 ± 0.9 a | 31.4 ± 1.3 | 5.6 ± 0.4 b |
| 5 wpv | N/A | (−) a | 35.2 ± 0.8 | 5.7 ± 0.2 b |
| 6 wpv | 37 ⁘ | 1.0 ± 1.0 a | 38.7 ± 0.3 | 1.8 ± 1.1 a |
| 7 wpv | N/A | (−) | 39 ⁘ | 0.9 ± 0.9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Chung, W.-B.; Chaung, H.-C.; Huang, Y.-L.; Chen, C.-C.; Ke, G.-M. Development of an Effective Single-Dose PCV2/CSFV Bivalent Subunit Vaccine Against Classical Swine Fever Virus and Porcine Circovirus Type 2. Vaccines 2025, 13, 736. https://doi.org/10.3390/vaccines13070736
Chen Y-C, Chung W-B, Chaung H-C, Huang Y-L, Chen C-C, Ke G-M. Development of an Effective Single-Dose PCV2/CSFV Bivalent Subunit Vaccine Against Classical Swine Fever Virus and Porcine Circovirus Type 2. Vaccines. 2025; 13(7):736. https://doi.org/10.3390/vaccines13070736
Chicago/Turabian StyleChen, Yu-Chieh, Wen-Bin Chung, Hso-Chi Chaung, Yen-Li Huang, Chi-Chih Chen, and Guan-Ming Ke. 2025. "Development of an Effective Single-Dose PCV2/CSFV Bivalent Subunit Vaccine Against Classical Swine Fever Virus and Porcine Circovirus Type 2" Vaccines 13, no. 7: 736. https://doi.org/10.3390/vaccines13070736
APA StyleChen, Y.-C., Chung, W.-B., Chaung, H.-C., Huang, Y.-L., Chen, C.-C., & Ke, G.-M. (2025). Development of an Effective Single-Dose PCV2/CSFV Bivalent Subunit Vaccine Against Classical Swine Fever Virus and Porcine Circovirus Type 2. Vaccines, 13(7), 736. https://doi.org/10.3390/vaccines13070736





